Abstract
Tandem duplications are concentrated within the Pcdh cluster throughout vertebrate evolution and as copy number variations (CNVs) in human populations, but the effects of tandem duplication in the Pcdh cluster remain elusive. To investigate the effects of tandem duplication in the Pcdh cluster, here we generated and analyzed a new line of the Pcdh cluster mutant mice. In the mutant allele, a 218-kb region containing the Pcdh-α2 to Pcdh-αc2 variable exons with their promoters was duplicated and the individual duplicated Pcdh isoforms can be disctinguished. The individual duplicated Pcdh-α isoforms showed diverse expression level with stochastic expression manner, even though those have an identical promoter sequence. Interestingly, the 5′-located duplicated Pcdh-αc2, which is constitutively expressed in the wild-type brain, shifted to stochastic expression accompanied by increased DNA methylation. These results demonstrate that tandem duplication in the Pcdh cluster expands the stochastic expression repertoire irrespective of sequence divergence.
Genetic variations play critical roles in animal evolution and human diseases1,2,3. These variations involve single nucleotide polymorphisms, small insertions/deletions, and large rearrangements, including inversions, translocations, and copy number variations (CNVs). CNVs involve either a gain (duplication) or a loss (deletion) of DNA segments. Tandem duplications, which are generated by unequal crossover, are frequent within tandemly arrayed gene clusters, which are adjacent groups of paralogous genes. Such clustered genes represent about 14% of vertebrate genes4 and are involved in a variety of important physiological and biochemical functions. They include, for instance, the immunoglobulin and T-cell receptor genes5, HOX genes6, zinc finger genes7, α– and β–globin genes8,9, and olfactory receptor genes10.
Although detailed analyses of these gene clusters have revealed their sophisticated gene regulation mechanisms, for example, somatic DNA rearrangements in the immunoglobulin and T-cell receptor clusters5, collinear expression in the HOX cluster6, and monoallelic and exclusive expression in the olfactory receptor cluster10, the evolutionary events by which these sophisticated gene regulations were acquired remain unclear.
Recently, the protocadherin (Pcdh) cluster was shown to be rich in tandem duplications3 and to exhibit sophisticated gene regulation mechanisms11,12. The Pcdh cluster has been identified in wide range of vertebrate species13,14,15. The mammalian Pcdh cluster genes are further classified into three subfamilies: Pcdh-α, Pcdh-β, and Pcdh-γ16. The Pcdh cluster genes probably arose by the tandem duplication and sequence divergence of existing Pcdh genes at some undetermined point in vertebrate evolution17,18. In fact, several mammalian Pcdh-α isoforms (α1-α12 in mouse and α1-α13 in human) are orthologous to four coelacanth Pcdh-α isoforms (α11–α14), and numerous mammalian Pcdh-β isoforms (β1–β22 in mouse and β1–β16 in human) are orthologous to four coelacanth Pcdh-β isoforms (β1–β4), suggesting that the common ancestors of those Pcdh-α and -β isoforms have become highly expanded in the mammalian lineage via tandem duplications19. The intriguing point about these expansions is that the Pcdh repertoire in mammals is more diverse than that in coelacanth. Furthermore, current human populations show a large number of CNVs in the PCDH locus3,20. However, the relevant ancestral material and human material could not be assessed, thus calling for the development of animal models of tandem duplication in the Pcdh cluster.
The diversity and sophisticated gene regulation exhibited by the Pcdh cluster genes are important for normal development of the nervous system11,12,21,22. The Pcdh cluster genes, which encode a group of diverse cadherin-related transmembrane proteins, are expressed mainly in the nervous system, and gene regulation mechanisms in the Pcdh clusters include both constitutive and stochastic expression in single neurons23,24,25,26,27. Gene ablation studies showed that Pcdh-α and Pcdh-γ are required for neuronal survival, synapse formation, axonal targeting, dendritic arborization, and self-avoidance of dendrites11,12,28,29,30. These findings led to the suggestion that the Pcdh cluster genes are likely candidates for the individualization of neurons in the vertebrate brain, which would be generated through the stochastic expression of these genes11,12,21,22. Although these findings suggest that stochastic expression in the Pcdh cluster is important in neurodevelopment, the evolutionary origin of the stochastic expression in the Pcdh cluster has remained a mystery.
To better understand the evolutionary origin of the stochastic expression and CNVs' effect in the Pcdh cluster, here we focused on the effect of tandem duplication in the mouse Pcdh-α cluster. In the present study, we engineered a targeted tandem duplication within the mouse Pcdh-α cluster, a situation somewhat comparable to that occurring during vertebrate evolution and in current human populations. The individual Pcdh-α isoforms transcribed from each duplicate exon can be distinguished in the mutant mice, enabling us to determine the manner by which their expression was regulated. The individual duplicated Pcdh-α isoforms showed diverse expression level with stochastic expression manner, even though those have an identical promoter sequence. Surprisingly, the duplicated Pcdh-αc2 isoform, which shows constitutive expression in the wild-type allele, shifted to stochastic expression accompanied by increased DNA methylation. Our results demonstrate that tandem duplication in the Pcdh cluster expands the stochastic expression repertoire irrespective of sequence divergence.
Results
Targeted tandem duplication in the mouse Pcdh-α cluster
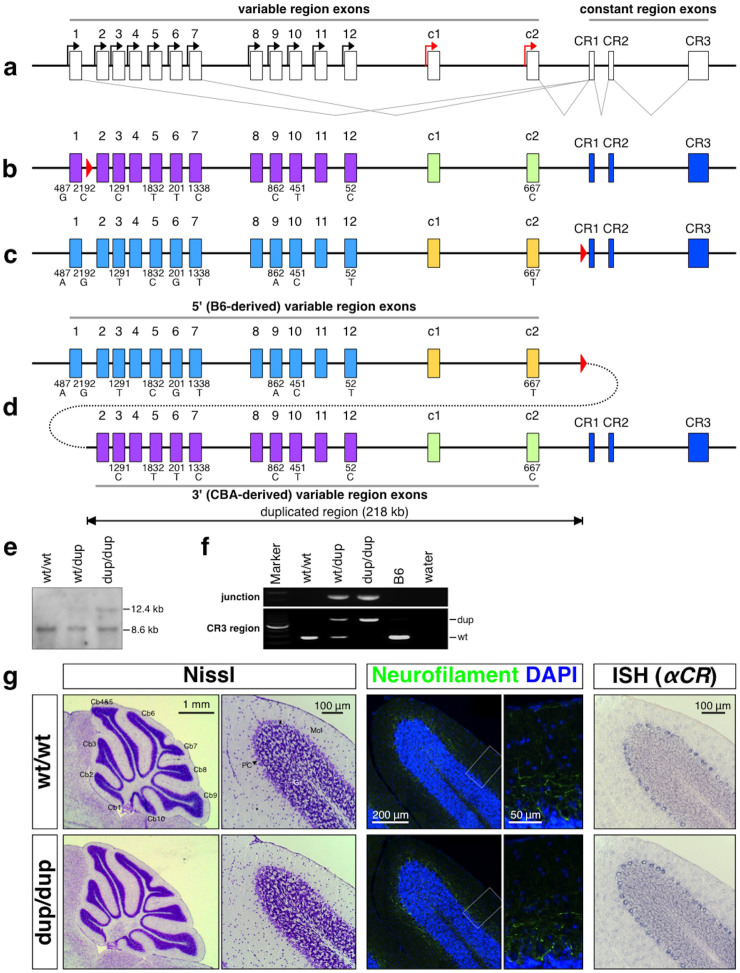
To study the consequences of tandem duplication in the Pcdh gene cluster, we generated a targeted tandem duplication in the mouse Pcdh-α cluster using the inter-strain targeted meiotic recombination (iTAMERE) system31. The wild-type mouse Pcdh-α cluster contains 14 large ‘variable’ exons, each of which encodes a cadherin-like type I membrane protein consisting of extracellular domains, a transmembrane domain, and a proximal cytoplasmic domain. Each variable exon is expressed from its own promoter and spliced to three short ‘constant’ exons, which encode a shared, 152-amino acid C-terminal domain (Fig. 1a)16. In the mutant allele [hereafter called dup(2-c2) or simply dup], a 218-kb region containing the Pcdh-α2 to Pcdh-αc2 variable exons with their promoters was duplicated; the 5′-located duplicate was derived from the C57BL/6J (B6) strain and the 3′-located one was derived from the CBA strain. We selected this particular region for duplication, because it contains both stochastically and constitutively expressed Pcdh-α isoforms, and because several of the isoforms involve a single nucleotide polymorphism (SNP) between the B6 and CBA strains in the coding region (Fig. 1b, c, d). The isoforms with a SNP between the B6 and CBA strains are Pcdh-α3, Pcdh-α5, Pcdh-α6, Pcdh-α7, Pcdh-α9, Pcdh-α10, Pcdh-α12, and Pcdh-αc2, and among these isoforms, only Pcdh-α12 has a polymorphism in its promoter region. Although the individual duplicated genes in the previous Pcdh-α duplication lines cannot be distinguished25, the individual duplicated genes derived from these eight Pcdh-αs in the dup(2-c2) allele can be distinguished from each other. Therefore, the dup(2-c2) mice for the first time enables the expressional analysis of the individual duplicated isoforms in the mouse Pcdh-α cluster.
Figure 1. Targeted tandem duplication in the Pcdh-α cluster.
(a) Genomic structure of the Pcdh-α wild-type allele. The Pcdh-α allele consists of variable region exons (1–12, c1 and c2) and constant region exons (CR1–CR3). Each variable region exon is transcribed from its own promoter in a stochastic (black arrows) or constitutive (red arrows) manner. A Pcdh-α transcript is produced from one of the variable region exons and the set of constant region exons by splicing. (b) The G16Neo allele: a loxP site was inserted between Pcdh-α1 and Pcdh-α2. The loxP site is shown as a red triangle. (c) The SR allele: a loxP site was inserted between Pcdh-αc2 and CR1. (d) The dup(2-c2) allele: duplication of Pcdh-α2-Pcdh-αc2. The dup(2-c2) allele was produced by Cre-loxP-mediated trans-allelic meiotic recombination between the G16neo and SR alleles. The duplicated segments are shown under the position of the original segments. Importantly, each duplicate Pcdh-α3, Pcdh-α5, Pcdh-α6, Pcdh-α7, Pcdh-α9, Pcdh-α10, Pcdh-α12, and Pcdh-αc2 could be distinguished by SNP analysis; the 5′ genes were from B6 and the 3′ ones were from CBA. (e & f) Confirmation of the duplication allele by Southern blotting (e) and PCR (f). G, Histological analysis of the cerebellum of 4-week-old wild-type and Pcdhαdup(2-c2)/dup(2-c2) mice. Nissl staining (left), immunostaining for neurofilament (middle), and in situ hybridization using a Pcdh-α CR probe (right). Cb1-10, 1st – 10th lobule of the cerebellum; Gra, granule cell layer; Mol, molecular layer; Pur, Purkinje cell layer.
Before using the iTAMERE system, we individually inserted two loxP sites into the variable region of the Pcdh-α cluster with the same orientation. First, the “G16Neo” allele25 was generated by inserting a loxP site between the Pcdh-α1 and Pcdh-α2 exons of the CBA allele in the TT2 ES embryonic stem (ES) cell line, which is on a CBAxB6 F1 genetic background (Fig. 1b). Second, a loxP site was inserted between the Pcdh-αc2 exon and the first exon of the constant region (CR1) in the Pcdh-α cluster to generate the “SR” allele (Fig. 1c and Supplementary Fig. S1). To insert this loxP site into the B6 allele, we used the RENKA ES cell line, which is on a pure B6 genetic background32.
To duplicate the sequence between the loxP site of the G16Neo allele and that of the SR allele using the Cre-loxP system, we obtained male mice that possessed the G16Neo and SR alleles (G16Neo/SR) and the Sycp1-Cre transgene, which elicits Cre recombinase expression specifically in the testis25. The male mice were crossed with B6 female mice, and the genotypes of the F1 pups were analyzed by PCR. The minority of F1 pups carried the dup(2-c2) allele, in which exons Pcdh-α2 to Pcdh-αc2 were duplicated (Fig. 1d), or the del(2-c2) allele, in which exons Pcdh-α2 to Pcdh-αc2 were deleted (data not shown). F1 pups carrying these duplication or deletion alleles were obtained at 5.8% (4 of 69 pups) and 1.4% (1 of 69 pups), respectively. We then analyzed the tail DNA of the duplication-containing mice by PCR to detect the Cre-mediated duplication alleles, and sequenced the PCR products to confirm the presence of the predicted junction sequences generated by the Cre-mediated site-specific recombination events. Animals homozygous for the duplicated allele (Pcdhαdup(2-c2)/dup(2-c2)) were obtained by crossing heterozygous (Pcdhαwt/dup(2-c2)) parents.
The Pcdhαdup(2-c2)/dup(2-c2) pups were born with the expected Mendelian distribution (Supplementary Table S1), developed normally to adulthood, and were fertile. Histochemical analysis with Nissl staining revealed an apparently normal gross anatomy of the Pcdhαdup(2-c2)/dup(2-c2) mouse brain (Fig. 1g left and Supplementary Fig. S2a). This finding was further supported by cytochrome oxidase staining showing a normal barrel structure in the Pcdhαdup(2-c2)/dup(2-c2) mice (Supplementary Fig. S2a). Furthermore, neural pathway and serotonergic axon analyses by anti-neurofilament and anti-SERT staining, respectively, showed no obvious differences between the genotypes (Fig. 1g middle and Supplementary Fig. S2b). Finally, the distribution of c-fos mRNA, a well-known marker for neuronal activity, was also similar between the genotypes (Supplementary Fig. S2c). These findings suggested that the tandem duplication of exons Pcdh-α2 to Pcdh-αc2 does not result in any deleterious effects on mouse development or brain morphogenesis.
Tandem duplication maintains the expression level of neighboring genes
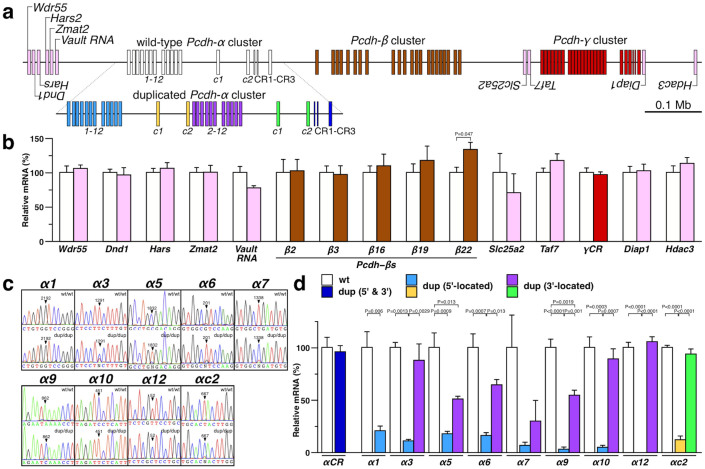
Previous studies have indicated that tandem duplication may alter not only the expression of genes within the duplication boundaries but also of genes located in their genomic neighborhoods33. Prompted by these observations, we first quantified the expression levels of transcripts in the vicinity of the Pcdh-α cluster in the cerebellum of 4-week-old Pcdhαdup(2-c2)/dup(2-c2) mice. The analyzed non-Pcdh genes included Wdr55, Dnd1, Hars, Zmat2, and Vault, located about 130-kb ~ 170-kb upstream from the duplication's 5′ boundary and Slc25a2, Taf7, Diap1, and Hdac3, located about 480-kb ~ 780-kb downstream from the duplication's 3′ boundary (Fig. 2a). We found that the duplication did not alter the expression of the Pcdh-β, Pcdh-γ, or non-Pcdh genes, except for a small effect on Pcdh-β22 (Fig. 2b). These results indicated that the tandem duplication of exons Pcdh-α2 to Pcdh-αc2 did not exert long-range effects on the regulation of neighboring genes.
Figure 2. Expressional re-allocation in the duplicated Pcdh-α cluster, but no alteration in neighboring gene expression.
(a) Genomic structure of the Pcdh-α cluster (upper; wild-type, lower; duplicated) and neighboring genes. (b) qRT-PCR analysis of neighboring genes in the cerebellum of 4-week-old wild-type and Pcdhαdup(2-c2)/dup(2-c2) mice. wt (n = 3) and dup (n = 3). (c) SNP analysis of transcripts for Pcdh-α1, Pcdh-α3, Pcdh-α5, Pcdh-α6, Pcdh-α7, Pcdh-α9, Pcdh-α10, Pcdh-α12, and Pcdh-αc2 in the cerebellum of 4-week-old wild-type and Pcdhαdup(2-c2)/dup(2-c2) mice. (d) Expression levels of wild-type and 5′- and 3′-located duplicated isoforms of Pcdh-α1, Pcdh-α3, Pcdh-α5, Pcdh-α6, Pcdh-α7, Pcdh-α9, Pcdh-α10, Pcdh-α12, and Pcdh-αc2 in the cerebellum of 4-week-old wild-type and Pcdhαdup(2-c2)/dup(2-c2) mice. wt/wt (n = 3), dup/dup (n = 3).
Tandem duplication re-allocates the manner of Pcdh-α expression
We next examined the distribution of Pcdh transcripts in the cerebellum of 4-week-old Pcdhαdup(2-c2)/dup(2-c2) mice by in situ hybridization (ISH). We analyzed the expression of all the Pcdh-α genes (using Pcdh-αCR probe), Pcdh-α1, Pcdh-α3, Pcdh-αc2, Pcdh-β22, and all the Pcdh-γ genes (using Pcdh-γCR probe). Similar positive signals were observed for all the genes examined between the wild-type and the Pcdhαdup(2-c2)/dup(2-c2) cerebellum (Fig. 1g right and Supplementary Fig. S3). These results suggested that the gene regulatory mechanisms governing the spatial distribution patterns of the Pcdh transcript were maintained in the dup(2-c2) allele.
We next examined whether both the 5′- and 3′-located duplicated Pcdh-αs were expressed. Since each duplicate of Pcdh-α3, Pcdh-α5, Pcdh-α6, Pcdh-α7, Pcdh-α9, Pcdh-α10, Pcdh-α12, and Pcdh-αc2 could be distinguished by single nucleotide polymorphism (SNP) analysis, we focused on these exons and on Pcdh-α1, which was not duplicated. We amplified the cDNA fragments of these Pcdh-α genes using specific primer combinations, and the resultant amplicons were sequenced directly (Fig. 2c). The analysis detected most of the 5′- and 3′-located duplicated Pcdh-α transcripts.
Next, the expression levels of these duplicated Pcdh-αs were quantified by qRT-PCR and cloning-mediated SNP analysis, which is highly sensitive and yields quantitative data (Fig. 2d). The expression level of the spliced CR transcripts, which are common to all the 5′-located and 3′-located duplicated Pcdh-αs, was unchanged in the Pcdhαdup(2-c2)/dup(2-c2) mice. There were no significant differences in the expression levels of most of the 3′-located duplicated Pcdh-αs (Pcdh-α3, Pcdh-α6, Pcdh-α7, Pcdh-α10, Pcdh-α12, and Pcdh-αc2) compared to wild-type. In contrast, the expression levels of all the 5′-located duplicated Pcdh-αs and the 3′-located duplicated Pcdh-α5 and Pcdh-α9 genes were significantly reduced compared to wild-type. These observations revealed that the expression level of the total Pcdh-α genes was maintained, while that of individual isoforms was altered, indicating that expressional re-allocation occurred in the dup(2-c2) allele.
Interestingly, the expression levels of the 5′-located duplicated Pcdh-αs were significantly lower than those of their duplicated 3′-located counterparts. This observation suggested that, despite their identical promoter sequences, each 5′-located and 3′-located duplicated Pcdh-α receives distinct gene-regulation influences. Taken together, these findings indicate that tandem duplication alters the manner of gene regulation in the Pcdh-α cluster.
Stochastic expression of duplicate Pcdh-α genes with identical promoter sequences
To investigate whether the duplicated Pcdh-αs retained their stochastic expression at the single-neuron level, we performed single-cell RT-PCR and SNP analysis on Purkinje cells of the PcdhαJF1/dup(2-c2) mice. The PcdhαJF1/dup(2-c2) mice were F1 mice from a JF1 × Pcdhαdup(2-c2)/dup(2-c2) cross. To distinguish the 5′-located exons (B6) and 3′-located exons (CBA) in the dup(2-c2) allele, and exons in the wild-type allele (JF1) by SNP analysis, we focused on Pcdh-α3, Pcdh-α5, Pcdh-α7 (Fig. 3).
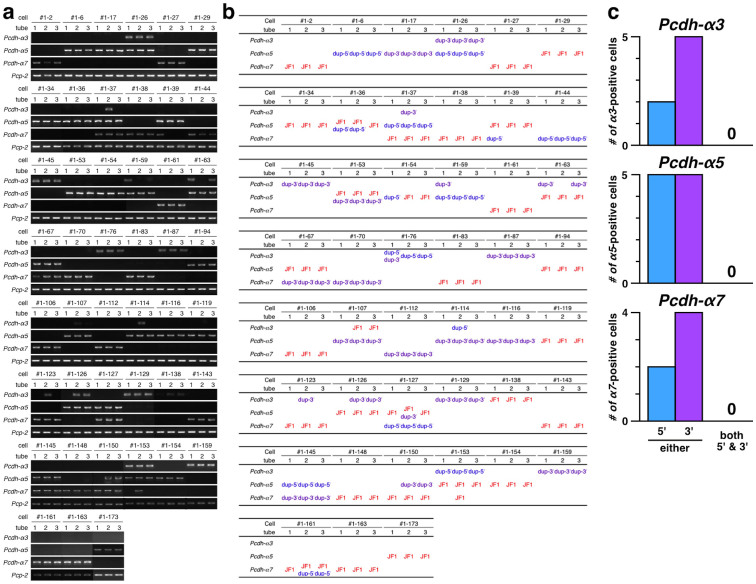
Figure 3. Stochastic expression of duplicated Pcdh-α3, Pcdh-α5, and Pcdh-α7 in single Purkinje cells.
(a) Electrophoresis results of single-cell RT-PCR for the Pcdh-α3, Pcdh-α5, or Pcdh-α7 and Pcp-2 genes in individual Purkinje cells. The numbers #1-2 ~ #1-173 designate individual cells. 1–3, tubes into which the cDNA from an individual Purkinje cell was divided; independent PCRs were performed for each tube. This figure shows only the cells that gave PCR products for Pcdh-α3, Pcdh-α5, or Pcdh-α7. (b) SNP analysis to distinguish between Pcdh-α transcripts from the 5′-located (shown as dup-5′) and 3′-located (shown as dup-3′) isoforms on the dup(2-c2) allele and those from the wild-type allele (designated JF1). (c) Classification of Pcdh-α3, Pcdh-α5, or Pcdh-α7 expressed from the dup(2-c2) allele in individual Purkinje cells. Blue and purple bars indicate the number of Purkinje cells expressing the 5′-located and 3′-located isoforms on the dup(2-c2) allele, respectively. The cells expressing one or two of three transcripts were excluded from the analysis.
To analyze the expression of Pcdh-α3, Pcdh-α5, and Pcdh-α7 in the dup(2-c2) allele, single Purkinje cells from 4-week-old PcdhαJF1/dup(2-c2) mice were picked up by glass capillary. Complementary DNA of Pcdh-α3, Pcdh-α5, Pcdh-α7, and Pcp-2 (a marker for Purkinje cells) was synthesized from the single-cell samples in the same tube, and the resulting cDNA was then divided into three tubes and subjected to separate, first-round multiplex PCR analysis. The second round of PCR amplification was carried out individually for each tube and used nested primers for the Pcdh-α3, Pcdh-α5, Pcdh-α7 genes, and for Pcp-2. Finally, each PCR product was subjected to direct sequencing to determine from which exon the transcript was derived: i.e., the wild-type (JF1) allele or the 5′-located (dup-5′) or 3′-located (dup-3′) exons in the dup(2-c2) allele.
Of the 163 single Purkinje cells analyzed, 45 yielded PCR amplicons of Pcdh-α3, Pcdh-α5, or Pcdh-α7 from the same exons in all three tubes, and all 45 cells were positive for Pcp-2, confirming that they were differentiated Purkinje cells. In addition to three of three specific transcripts from the same exon, some cells showed one or two of three transcripts (for example, Pcdh-α3 in cell #1-37); these findings suggested that the amounts of corresponding transcripts in these cells were low, and therefore we excluded these cells from the following analysis.
For Pcdh-α3, the transcripts were derived from the wild-type exon, 5′-located exon, and 3′-located exon in 1 cell, 2 cells, and 5 cells, respectively: the wild-type (#1-138), 5′-located (#1-76, and #1-153), and 3′-located exon (#1-26, #1-45, #1-87, #1-129, and #1-159). For Pcdh-α5, the transcripts were derived from the wild-type exon, 5′-located exon, and 3′-located exon in 14 cells, 5 cells, and 5 cells, respectively: the wild-type (#1-29, #1-34, #1-36, #1-39, #1-53, #1-63, #1-67, #1-94, #1-119, #1-126, #1-127, #1-153, #1-154, and #1-173), 5′-located (#1-6, #1-26, #1-37, #1-59, and #1-145), and 3′-located exon (#1-17, #1-53, #1-107, #1-114, and #1-116). For Pcdh-α7, the transcripts were derived from the wild-type exon, 5′-located exon, and 3′-located exon in 13 cells, 2 cells, and 4 cells, respectively: the wild-type (#1-2, #1-27, #1-37, #1-38, #1-61, #1-83, #1-106, #1-123, #1-143, #1-148, #1-150, #1-161, and #1-163), 5′-located (#1-44, and #1-127), and 3′-located exon (#1-67, #1-70, #1-112, and #1-145). Only one cell expressed both the wild-type and the 3′-located exon for the same Pcdh-α isoform (Pcdh-α5 in cell #1-53) simultaneously, indicating that Pcdh-α genes are not always expressed monoallelically. Importantly, although it cannot rule out the possibility of small number of analyzed cells and possible lower expression level of the 5′-located exons may reduce the chance of detection, no cell expressed both the 5′-located and the 3′-located exon for the same Pcdh-α isoform simultaneously. These results strongly suggested that tandem duplication maintained the stochastic expression of the Pcdh-α isoforms, as seen in the wild-type brain.
The 5′-located Pcdh-αc2 acquired stochastic expression upon tandem duplication
To investigate whether the 5′-located and 3′-located duplicated Pcdh-αc2 retained its constitutive expression at the single-neuron level, single-cell RT-PCR and SNP analysis was carried out (Fig. 4). Of the 16 single Purkinje cells analyzed, all showed the Pcdh-αc2 transcript from the wild-type (JF1) and 3′-located duplicated exon in all three tubes. They were also all positive for Pcp-2, confirming that they were differentiated Purkinje cells. These results suggested that the Pcdh-αc2 wild-type exon (JF1) and 3′-located duplicated exon were expressed constitutively in differentiated Purkinje cells.
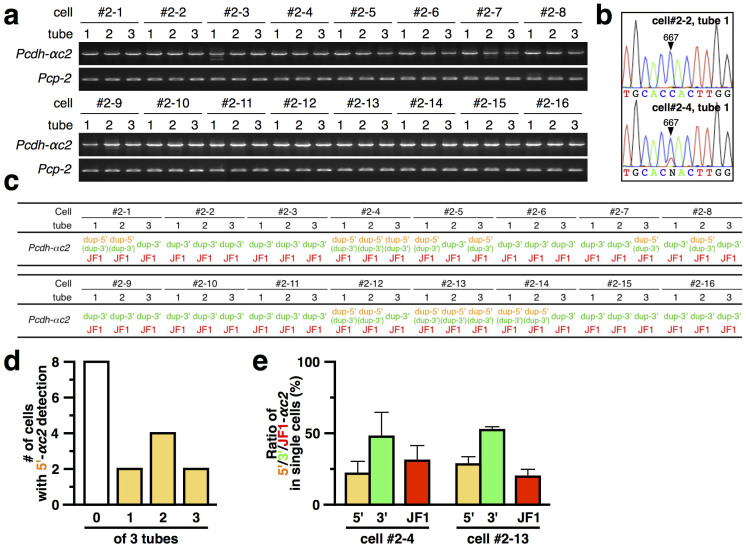
Figure 4. Stochastic expression of 5′-located Pcdh-αc2, but constitutive expression of 3′-located Pcdh-αc2 in single Purkinje cells.
(a) Electrophoresis results of the split single-cell RT-PCR for the Pcdh-αc2 and Pcp-2 genes in individual Purkinje cells. The numbers #2-1 ~ #2-16 designate individual cells. 1–3, tubes into which the cDNA from an individual Purkinje cell was divided; independent PCRs were performed for each tube. (b) Example chromatograms showing the absence (upper) and presence (lower) of the 5′-located duplicated Pcdh-αc2 transcript. (c) SNP analysis to distinguish between Pcdh-αc2 transcripts from the 5′-located (dup-5′) and 3′-located (dup-3′) isoforms on the dup(2-c2) allele and from the wild-type allele (JF1). (d) Number of Purkinje cells showing the 5′-located duplicated Pcdh-αc2 transcript in 0, 1, 2, or 3 tubes. (e) Comparison of the Pcdh-αc2 expression levels in individual Purkinje cells (cells #2-4 and #2-13). Yellow, green, and red bars represent the expression ratio from the 5′- and 3′-located Pcdh-αc2 exons on the dup(2-c2) allele, and the wild-type (JF1) exon, respectively. Error bars indicate ±S.E.M of the averages from 3 tubes.
To our surprise, the Pcdh-αc2 transcript from the 5′-located duplicated exon was detected in three tubes for only two of the 16 cells (#2-4 and #2-13). Notably, 8 of the 16 cells (#2-2, #2-3, #2-6, #2-9, #2-10, #2-11, #2-15 and #2-16) showed no Pcdh-αc2 transcript from the 5′-located duplicated exon (Fig. 4d). For those that did express it, the quantity expressed from the 5′-located duplicated exon was comparable to that from the wild-type (JF1) and 3′-located duplicated exons (Fig. 4e). These results strongly suggested that the expression of the 5′-located duplicated Pcdh-αc2 changed from constitutive to stochastic.
Tandem duplication masks cluster-structure dependent DNA hypomethylation
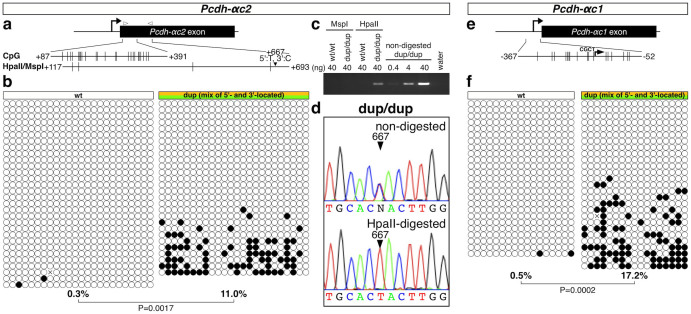
The 5′-located Pcdh-αc2 was down-regulated in the Pcdhαdup(2-c2)/dup(2-c2) mouse cerebellum (Fig. 2) and acquired a stochastic expression pattern (Fig. 4), suggesting that the regulation was different between the 5′-located and the 3′-located Pcdh-αc2 in the dup(2-c2) allele. Since Pcdh-αc2 is extensively hypomethylated in the wild-type mouse brain, and higher mosaic DNA methylation levels are correlated with a lower transcription of stochastically expressed Pcdh genes34,35,36,37, we first examined the DNA methylation of Pcdh-αc2 in the Pcdhαdup(2-c2)/dup(2-c2) mouse cerebellum using bisulfite sequencing (Fig. 5a and 5b). The results clearly showed higher mosaic DNA methylation of Pcdh-αc2 in the Pcdhαdup(2-c2)/dup(2-c2) mouse (11.0%) compared with the wild-type mouse (0.3%).
Figure 5. Tandem duplication increases the DNA methylation of Pcdh-αc2 and Pcdh-αc1.
(a) Schematic representation of Pcdh-αc2; the positions of CpGs and HpaII/MspI sites are shown to scale by vertical lines. (b) Results of bisulfite sequencing. Each circle represents a methylated (black) or unmethylated (white) CpG dinucleotide. Each row represents a single clone. A primer set was designed to amplify the region corresponding to the 5′ region of the exon, which has the same sequence in the 5′- and 3′-located duplicated Pcdh-αc2 isoforms. The percentage below each methylation pattern indicates the CpG methylation rate for the region. C, D, Discrimination of the DNA methylation between the 5′- and 3′-located duplicated Pcdh-αc2 isoforms by HpaII digestion-mediated analysis. (c) Electrophoresis results. D, Example chromatograms showing that the HpaII-resistant fraction contained the region of the 5′-located duplicated Pcdh-αc2 exon. E, Schematic representation of Pcdh-αc1; positions of CpGs are shown to scale by vertical lines. F, Results of bisulfite sequencing. A primer set was designed to amplify the region corresponding to the Pcdh-αc1 promoter, which has the same sequence in the 5′- and 3′-located Pcdh-αc1 duplicates. The percentage below each methylation pattern indicates the CpG methylation rate for the region.
Because bisulfite sequencing was unable to discriminate between the two Pcdh-αc2s in the dup(2-c2) allele, we further analyzed the DNA methylation using HpaII digestion-mediated DNA methylation analysis (Fig. 5c and 5d). The HpaII-resistant fraction, containing methylated CCGG, predominantly included the 5′-located duplicated Pcdh-αc2 genomic DNA. The DNA methylation level on the 5′-located duplicated Pcdh-αc2 was higher than that on the 3′-located and wild-type Pcdh-αc2.
We further examined the DNA methylation of Pcdh-αc1 in the Pcdhαdup(2-c2)/dup(2-c2) mouse cerebellum. Similar to Pcdh-αc2, bisulfite sequencing showed higher DNA methylation of Pcdh-αc1 in the Pcdhαdup(2-c2)/dup(2-c2) mouse (17.2%) than in the wild-type mouse (0.5%) (Fig. 5f). Taken together, these results suggested that the DNA hypomethylation in Pcdh-αc1 and Pcdh-αc2, which are constitutively expressed in the wild-type brain, is, at least in part, dependent on the cluster structure, and that tandem duplication directly increases the DNA methylation in the 5′-located Pcdh-αc1 and Pcdh-αc2.
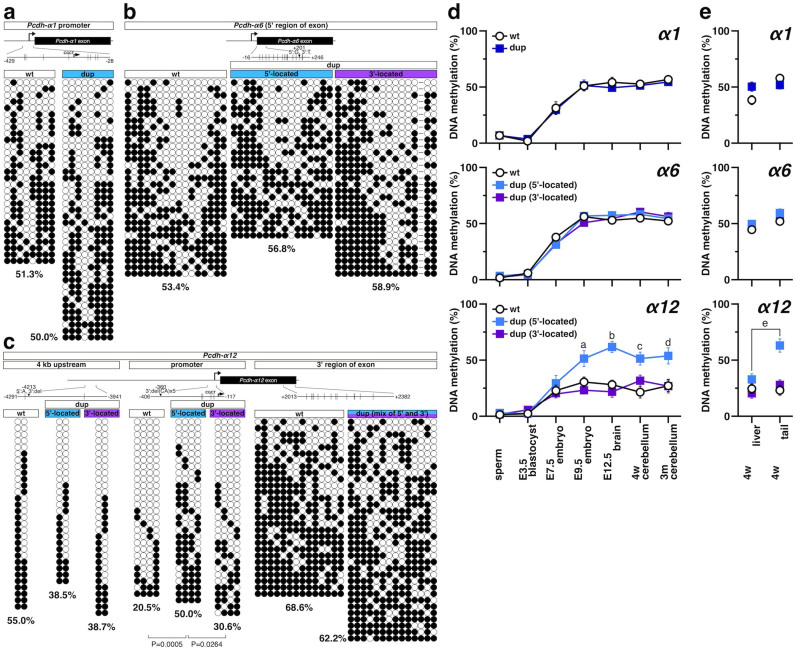
We next extended the DNA methylation analysis to the stochastically expressed Pcdh-α isoforms. To distinguish the 5′-located from the 3′-located exons in the dup(2-c2) allele by SNP analysis after the bisulfite reaction, we focused on the Pcdh-α1 (promoter), Pcdh-α6 (exon 5′-region), and Pcdh-α12 (promoter) (Fig. 6a–6c). A mosaic methylation pattern was observed for all three, Pcdh-α1, Pcdh-α6, and Pcdh-α12, and similar DNA methylation levels, around 50%, were observed for Pcdh-α1 and Pcdh-α6 in the wild-type and the dup(2-c2) allele. However, in the promoter region of the 5′-located Pcdh-α12, we found a higher methylation level (50.0%) than in the wild-type (20.5%) or 3′-located Pcdh-α12 (30.6%).
Figure 6. Tandem duplication increases DNA methylation of the Pcdh-α12 promoter, but not of Pcdh-α1 and Pcdh-α6.
DNA methylation patterns on Pcdh-α1 (a), Pcdh-α6 (b), and Pcdh-α12 (c) in the cerebellum from 4-week-old wild-type and Pcdhαdup(2-c2)/dup(2-c2) mice. (Top) Schematic representations of Pcdh-α1, 6, and 12; positions of CpGs are shown to scale by vertical lines. (bottom) Results of bisulfite sequencing. Each circle represents a methylated (black) or unmethylated (white) CpG dinucleotide. Each row represents a single clone. A primer set was designed to amplify the region corresponding to the promoter (Pcdh-α1 and Pcdh-α12) or 5′ region of the exon (Pcdh-α6) containing the SNP. In addition, the 4-kb upstream region and 3′ region of Pcdh-α12 were analyzed. The percentage below each methylation pattern indicates the CpG methylation rate for each region. (d) Changes in DNA methylation levels during development. DNA methylation levels of the Pcdh-α1 promoter (top), 5′ region of the Pcdh-α6 exon (middle), and Pcdh-α12 promoter (bottom) were analyzed by bisulfite sequencing of sperm and of mice at E3.5, E7.5, E9.5, E12.5, 4-weeks old, and 3-months old. aP = 0.0071 compared with wt, P = 0.0002 compared with the 3′-located Pcdh-α12, bP < 0.0001 compared with wt, P < 0.0001 compared with the 3′-located Pcdh-α12, cP = 0.0005 compared with wt, P = 0.0264 compared with the 3′-located Pcdh-α12, dP = 0.0167 compared with wt, P = 0.0086 compared with the 3′-located Pcdh-α12. (e) DNA methylation levels in the liver and tail of 4-week-old mice. DNA methylation levels at the Pcdh-α1 promoter (top), 5′ region of the Pcdh-α6 exon (middle), and Pcdh-α12 promoter (bottom) were analyzed by bisulfite sequencing. eP = 0.0003 compared liver with tail of the 5′-located Pcdh-α12.
To identify the region with increased DNA methylation more precisely, DNA methylation around the 5′-located Pcdh-α12 promoter was examined (Fig. 6c). No significant differences in the DNA methylation level were observed for the 4-kb upstream region (38.5% ~ 55.0%, for the wild-type, the 5′-located, and 3′-located exon) or for the 3′-region exon (around 65%, for wild-type and the mixture of the 5′-located and 3′-located exon), suggesting that the increase in DNA methylation was specific for the promoter. Therefore, as in Pcdh-αc1 and Pcdh-αc2, DNA hypomethylation in the Pcdh-α12 promoter is dependent on the cluster structure, and tandem duplication directly increased the DNA methylation of the 5′-located Pcdh-α12.
To gain further insight into the cluster-dependent regulation of the DNA methylation, we next examined the methylation during development (Fig. 6d). Interestingly, while the DNA methylation level on Pcdh-α1 and Pcdh-α6 showed similar for all the loci, the DNA methylation level on the 5′-located Pcdh-α12 promoter was higher than that on the wild-type and 3′-located Pcdh-α12 promoter. The DNA methylation level on the 5′-located Pcdh-α12 resembled that of Pcdh-α1 and Pcdh-α6 throughout development. The increased DNA methylation on the 5′-located Pcdh-α12 promoter was also observed in the tail but not in the liver (Fig. 6e), suggesting that an organ-dependent DNA methylation regulator influenced the DNA methylation on the 5′-located Pcdh-α12 promoter.
Taken together, these results suggest that 3′-located genes in the wild-type Pcdh-α cluster, Pcdh-α12, Pcdh-αc1, and Pcdh-αc2, are hypomethylated in a cluster-structure dependent manner, and that these DNA hypomethylations were masked by position shift to 5′-location, which is caused by tandem duplication in the dup(2-c2) allele. This mechanism probably underlies the lower expression level of the the 5′-located Pcdh-α12 duplicate and the stochastic expression of the 5′-located Pcdh-αc2 duplicate in the dup(2-c2) allele.
Discussion
Tandem duplications are concentrated within the Pcdh cluster throughout vertebrate evolution and as CNVs in human populations3,19,20, but the effects of tandem duplication in the Pcdh cluster remain elusive. Here we revealed a critical role for tandem duplication in the Pcdh cluster gene regulation. The individual duplicated Pcdh-α isoforms showed diverse expression level with stochastic expression manner, even though those have an identical promoter sequence. Interestingly, the 5′-located duplicated Pcdh-αc2, which is constitutively expressed in the wild-type brain, shifted to stochastic expression upon tandem duplication, accompanied by increased DNA methylation. These observations suggest that tandem duplication has been beneficial for the acquisition of the stochastic expression and the expansion of its repertoire through vertebrate evolution and in human populations (Fig. 7).
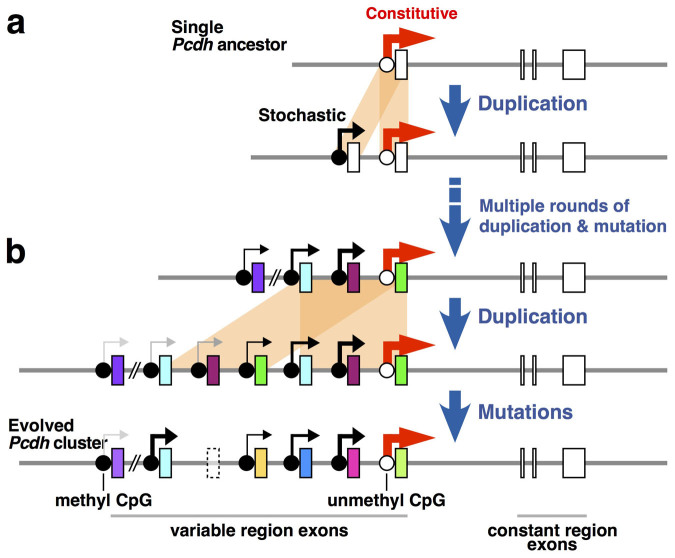
Figure 7. Model for the expansion of stochastic Pcdh-α expression via tandem duplication.
(a) Hypothetical evolutionary history from an ancestral single Pcdh gene. (b) Hypothetical evolutionary history from clustered Pcdh-α genes. Tandem duplication (orange shading) creates duplicated exons in the Pcdh-α cluster. Both of the duplicate stochastically expressed Pcdh-α isoforms (those with black-grey arrows) retain their stochastic expression. In contrast, from constitutively expressed isoforms (thick red arrow), the 3′-located duplicated Pcdh-α maintains its constitutive expression, but the 5′-located one shifts to stochastic expression accompanied by higher DNA methylation. Mutations in exons and/or regulatory sequences generate diverse coding exons, pseudogenes, and altered gene regulatory systems. Boxes, Pcdh-α exons. Dotted box, pseudogene.
What are the mechanisms by which individual duplicated Pcdh-αs, each of which has an identical promoter sequence, are expressed stochastically? The present data are consistent with our previous findings, which is that the stochastic expression of Pcdh-α cluster genes is governed by enhancer and promoter DNA methylation25,38. The enhancers for the Pcdh-α genes, named HS5-1 and HS7, are located downstream of the Pcdh-α cluster39,40,41, and the present data suggest that the HS5-1 and/or HS7 enhancers in the duplicated allele are still effective at even greater distances than in the wild-type allele (distance between the HS5-1 enhancer and the most distal promoter (Pcdh-α1) in the wild-type allele was ~280 kb; in the dup(2-c2) allele, it was ~500 kb). However, the present finding of lower expression of the 5′-located duplicated Pcdh-α genes than the 3′-located duplicated Pcdh-α genes in the dup(2-c2) allele indicates that the longer distance between the HS5-1 enhancer and the promoter lowers the probability of expression. The present data strongly support the “enhancer sharing and stochastic promoter competition” model, in which a single enhancer stochastically governs the expression of the Pcdh cluster genes25.
Stochastic promoter competition has also been suggested for other gene clusters, such as the olfactory receptor MOR28 cluster10,42 and the primate red and green-pigment genes43. Furthermore, non-stochastic promoter competition has been suggested for other gene clusters, such as the Hoxd gene cluster44, α– and β–globin gene cluster8,9, and zebrafish red opsin genes45; these gene clusters show temporally and spatially organized expression. Thus, promoter competition is widely distributed through gene clusters. We argue that the characteristics of the enhancer and/or promoter add further sophistication to gene regulatory systems.
Here we found that the 5′-located duplicated Pcdh-αc2, which is constitutively expressed in the wild-type brain, acquired stochastic expression upon tandem duplication, accompanied by increased DNA methylation. These results provide supportive evidence for previous findings that mosaic DNA methylation states are correlated with the stochastic expression of Pcdh-α isoforms in wild-type mouse brain36. Previous studies described the suppression of promoter DNA methylation, which locates the region 3′ proximal to the Pcdh-α cluster (Pcdh-α12, Pcdh-αc1 and Pcdh-αc2 in wild-type mouse brain and PCDHA13, PCDHAC1, and PCDHAC2 in normal human kidney and human kidney tumor)34,36,38. Our recent results suggested that the establishment of mosaic DNA methylation patterns in the Pcdh clusters is cooperatively regulated by the specificity of Dnmt3b, the gene cluster structure, the enhancer element, and the sequence features38. Another possible mechanism is an altered enhancer-promoter interaction40,46. Collectively, the mechanism underlying the shift of the 5′-located duplicated Pcdh-αc2 to stochastic expression is an important question for future study.
The experiments described here reveal some role of tandem duplication on gene regulatory differentiation, that include expression level divergences of the duplicated Pcdh-α genes and changes from constitutive to stochastic manner of the 5′-located duplicated Pcdh-αc2 gene. The data suggest that the current state of the Pcdh-α cluster in mammals, in terms of how it is expressed, was shaped by tandem duplication and distance from promoter to enhancer. Furthermore, although it is not clear that Pcdh-α gene number or stochastic expression have been strongly selected during vertebrate evolution, the present results may imply the evolutionary history of Pcdh cluster gene regulation. Previous reports have suggested that Pcdh cluster evolution included successive tandem duplications and sequence divergences16,17,19. Here, we propose a model in which stochastic expression of the duplicated Pcdh genes is immediately acquired after tandem duplication, which precedes sequence divergence (Fig. 7).
The human PCDH cluster is particularly rich in CNVs, including duplications and deletions3,20,47 (see Database of Genome Variants: http://dgv.tcag.ca/gb2/gbrowse/dgv2_hg18/?name=chr5:140050001..141050000). The present study showed that the tandem duplication resulted in healthy mice with a macroscopically normal brain. This result can be explained in part by the maintenance of the expression levels and distribution patterns of the total Pcdh-α transcript, by the re-allocation of Pcdh-α isoform expression, and by the maintenance of the expression levels of neighboring genes, upon duplication. Thus, it is likely that following various CNV events in the human PCDH cluster, the total expression level, dual gene-regulatory mechanisms, and stochastic expression of the human PCDH genes are maintained. This robustness may provide the predominant reason for the frequent CNVs in the human PCDH cluster. One study reported that there is no phenotypic link between a CNV in the human PCDH cluster, a 16.7-kb deletion affecting PCDHA8-A10, and psychiatric disorders47. Recently, a de novo gene disruption in PCDHA13 was reported in autism48. Furthermore, Anitha A. et al. reported strong genetic evidence of PCDHA as a potential candidate gene for autism49. The PCDHA cluster is also a candidate locus for bipolar disorder50. Furthermore, deletion of PCDHA1-PCDHA9 is associated with higher brain function, such as music perception51. It will be interesting to investigate the effects of these genetic mutations on the PCDHA expression and neural circuit formation.
Recent human genome analyses revealed that tandem duplications contribute to human phenotypes, including many psychiatric disorders, color vision, Parkinson's disease, and Rheumatoid arthritis1,3,52. For example, duplications of 7q36.3, which contains the vasoactive intestinal peptide receptor gene VIPR2, confer significant risk for schizophrenia, and VIPR2 mRNA levels are increased differently among duplication carriers53. These findings suggest the importance of conducting detailed investigations addressing the effects of duplications on gene regulation. The iTAMERE approach enables careful analyses directed toward understanding the etiology of CNV-associated human disorders.
The vertebrate Pcdh cluster shows remarkable similarity to the Drosophila Dscam1 gene, within which tandem duplication is frequent throughout its evolutionary history54. Importantly, recent studies demonstrated that the stochastic gene regulation in Pcdh and Dscam1 play important roles in neural circuit development by providing a source for cell surface diversity11,12. These findings suggest essential roles for tandem duplications in the evolution of vertebrate and invertebrate nervous systems. Further studies aimed at dissecting fine-scale neural circuits in the Pcdhαdup(2-c2)/dup(2-c2) mice will improve our understanding how tandem duplication in the Pcdhα cluster contribute to brain function.
Methods
Animals
B6 mice were purchased from Charles River Japan. The wild-type mouse strain JF1 was obtained from the National Institute for Genetics (Mishima, Shizuoka, Japan). All animals were maintained in a specific pathogen-free space under a 12-h light/dark regimen. Experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the Science Council of Japan and approved by the Animal Experiment Committee of Gunma University and Osaka University.
Generation of Pcdhαdup(2-c2)/dup(2-c2) mice
By crossing SR mice (see Supplemental Experimental Procedures) with Sycp1-Cre transgenic mice25, we generated SR mice carrying the Sycp1-Cre transgene. We crossed these mice with mice bearing the G16Neo allele, then selected male offspring bearing the SR allele, G16Neo allele, and the Sycp1-Cre transgene (PcdhαSR/G16Neo, Sycp1-Cre). We then crossed PcdhαSR/G16Neo, Sycp1-Cre male and B6 female, and genotyped the pups using genomic DNA extracted from the tail. Some of these pups carried the duplicated [dup(2-c2)] or deleted [del(2-c2)] allele as a result of TAMERE in the testis31. To identify the Pcdhαwt/dup(2-c2) mice, we performed Southern blotting and PCR analyses (Fig. 1e and 1f). The BamHI-digested genomic DNA from the tail was subjected to Southern blotting using probe A (the same probe used to identify the SR allele). A band at 8.6 kb indicated the wild-type allele, and a band at 12.4 kb indicated the dup(2-c2) allele. Pcdhαdup(2-c2)/dup(2-c2) mice were obtained by crossing Pcdhαwt/dup(2-c2) parents, which were backcrossed with B6 for more than three generations.
In situ hybridization
In situ hybridization (ISH) was performed essentially as described previously27. The details are provided in the Supplemental Experimental Procedures.
Expression analysis in cerebellum
The cerebellum was dissected from 4-week-old mice and immediately frozen in liquid nitrogen. The tissue was homogenized with a Polytron homogenizer. Total RNA was isolated using RNeasy (Qiagen), according to the supplier's recommendations. To obtain cDNA, 2.5 μg of the total RNA was treated with DNase I (Takara) and reverse transcribed with SuperscriptIII reverse transcriptase (Invitrogen) using random primers in a 40-μl reaction volume.
For the SNP analyses, PCR for each Pcdh-α isoform was performed using 0.4 μl of cDNA from the cerebellum of a 4-week-old mouse as a template. The primer sequences used for the SNP analyses are shown in Supplementary Table S2. For direct SNP analyses, the PCR products were sequenced using a standard method. For the cloning-mediated SNP analysis, the PCR products were cloned into pT7-Blue (Novagen), white colonies were randomly picked, and individual clones were sequenced using a standard method. The SNPs used for the direct sequencing analysis are shown in Supplementary Table S3.
The qRT-PCR was performed with SYBR Premix ExTaq II (Takara) using the 7500Fast Real-Time PCR system (Applied Biosystems) or LightCycler480 (Roche). The primer sequences used for qRT-PCR are shown in Supplementary Table S2. All data shown are normalized to beta 2 microglobulin. The efficiency of all the primer pairs was confirmed by performing reactions with serially diluted samples. The specificity of all the primer pairs was confirmed by analyzing the dissociation curve.
Split single-cell RT-PCR
Single-cell RT-PCR was performed essentially as described previously, with small modifications24,55. The details are provided in the Supplemental Experimental Procedures.
DNA methylation analysis by bisulfite sequencing and HpaII digestion
The genomic DNA was prepared with the QIAamp DNA Micro Kit (Qiagen) from the cerebellum of a 4-week-old or 3-month-old mouse, or from sperm, or with the EpiTect Plus LyseAll Kit (Qiagen) from the brain of an E12.5 embryo, head of an E9.5 embryo, E7.5 whole embryo, or blastocyst (E3.5), according to the supplier's recommendations. Sperm was obtained from the cauda epididymides of adult male mice. Embryos were obtained by the natural breeding of Pcdhαwt/dup(2-c2) parents. The morning of the vaginal plug was designated E0.5. Bisulfite conversion was performed with the EpiTect Plus DNA Bisulfite Kit (Qiagen), according to the supplier's recommendations. We used “MethPrimer” (http://www.urogene.org/methprimer/) to design primers for use on bisulfite-treated DNA56. The primers used for DNA amplification are listed in Supplementary Table S2. The first PCR program consisted of 95°C for 3 min, 25 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s, and a final extension of 72°C for 7 min. The second PCR was carried out using the same program as the first, except that 32 cycles were performed. To obtain the methylation profile from the acquired data, we used the web-based tool, “QUMA” (http://quma.cdb.riken.jp/)57.
In the HpaII digestion-mediated DNA methylation analysis, HpaII was used to distinguish between methylated (undigested) and unmethylated (digested) HpaII/MspI sites, whereas MspI digested both methylated and unmethylated HpaII/MspI sites. The HpaII/MspI-digested DNA was subjected to PCR analysis to amplify the SNP-containing regions. We mixed 500 ng of DNA with 10 U HpaII or 10 U MspI, and restriction buffer in a 10-μl reaction volume. Samples were incubated at 37°C overnight, and we used 40 ng of the digested DNA for the PCR reaction. The primers are listed in Supplementary Table S2. The PCR program consisted of 95°C for 3 min, 30 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 1 min, and a final extension of 72°C for 7 min. The PCR products were sequenced using a standard method. The results shown are representative of two independent experiments.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Version 6.0 (GraphPad Software, La Jolla, CA, USA) and was performed using one-way ANOVA followed by Bonferroni's post hoc test, or using an unpaired two-tailed Student's t test if applicable. All data are expressed as the mean ± S.E.M.
Author Contributions
R.K. and T.Y. conceived and designed the experiments, analyzed the data and wrote the manuscript. R.K. and M.A. performed research; R.K., M.A., T.H., A.U., K.S. and Y.Y. contributed unpublished reagents/analytic tools.
Supplementary Material
Supplementary information
Acknowledgments
We are grateful to Dr. T. Shiroishi (The National Institute of Genetics, Japan) for providing the JF1 mouse strain, Dr. M. Mishina (University of Tokyo, Japan) for providing the FLP66 mouse strain, and Dr. J. Takeda (Osaka University, Japan) for providing the pPE7neoW-F2LF plasmid. We thank members of the Yagi laboratory, Yanagawa laboratory, and Drs. A. Oue, H. Hata for suggestions and discussion during the course of this work. We appreciate Mses. R. Natsume, C. Choji, A. Kinjo, Y. Hidaka, T. Kakinuma, S. Sato, and A. Morita for their technical support and Ms. J. Shuto for their excellent secretarial assistance. This work was supported by Grant-in-Aid for Young Scientists (B) (JSPS) (No. 19770147, 22700326), Grant-in-Aid for Scientific Research (C) (JSPS) (No. 24500375), the Life Science Foundation of Japan, and the Takeda Science Foundation (Ryosuke Kaneko), and Grant-in-Aid for Scientific Research (S) (JSPS) (No. 19100006), Innovative Areas “Mesoscopic Neurocircuitry” (No. 23115513 and No. 25115720) and (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports, and Culture of Japan (MEXT), and JST-CREST (Takeshi Yagi).
References
- Hurles M. E., Dermitzakis E. T. & Tyler-Smith C. The functional impact of structural variation in humans. Trends Genet 24, 238–245 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Evolution by Gene Duplication. (Springer-Verlag, Berlin-Heidelberg-New York; 1970). [Google Scholar]
- Cooper G. M., Nickerson D. A. & Eichler E. E. Mutational and selective effects on copy-number variants in the human genome. Nat Genet 39, S22–29 (2007). [DOI] [PubMed] [Google Scholar]
- Pan D. & Zhang L. Tandemly arrayed genes in vertebrate genomes. Comp Funct Genomics 545269 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman G. W. et al. Phylogenetic diversification of immunoglobulin genes and the antibody repertoire. Mol Biol Evol 10, 60–72 (1993). [DOI] [PubMed] [Google Scholar]
- Duboule D. The rise and fall of Hox gene clusters. Development 134, 2549–2560 (2007). [DOI] [PubMed] [Google Scholar]
- Shannon M., Hamilton A. T., Gordon L., Branscomb E. & Stubbs L. Differential expansion of zinc-finger transcription factor loci in homologous human and mouse gene clusters. Genome Res 13, 1097–1110 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lower K. M. et al. Adventitious changes in long-range gene expression caused by polymorphic structural variation and promoter competition. Proc Natl Acad Sci U S A 106, 21771–21776 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer D. & de Laat W. Joining the loops: beta-globin gene regulation. IUBMB Life 60, 824–833 (2008). [DOI] [PubMed] [Google Scholar]
- Sakano H. Neural map formation in the mouse olfactory system. Neuron 67, 530–542 (2010). [DOI] [PubMed] [Google Scholar]
- Yagi T. Molecular codes for neuronal individuality and cell assembly in the brain. Front Mol Neurosci 5, 45 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky S. L. & Sanes J. R. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell 143, 343–353 (2010). [DOI] [PubMed] [Google Scholar]
- Jiang X. J., Li S., Ravi V., Venkatesh B. & Yu W. P. Identification and comparative analysis of the protocadherin cluster in a reptile, the green anole lizard. PLoS One 4, e7614 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. P. et al. Elephant shark sequence reveals unique insights into the evolutionary history of vertebrate genes: A comparative analysis of the protocadherin cluster. Proc Natl Acad Sci U S A 105, 3819–3824 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C., Huang W., Ying G. & Wu Q. Sequence analysis and expression mapping of the rat clustered protocadherin gene repertoires. Neuroscience 144, 579–603 (2007). [DOI] [PubMed] [Google Scholar]
- Wu Q. & Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 97, 779–790 (1999). [DOI] [PubMed] [Google Scholar]
- Lajoie M., Bertrand D. & El-Mabrouk N. Inferring the evolutionary history of gene clusters from phylogenetic and gene order data. Mol Biol Evol 27, 761–772 (2010). [DOI] [PubMed] [Google Scholar]
- Noonan J. P., Grimwood J., Schmutz J., Dickson M. & Myers R. M. Gene conversion and the evolution of protocadherin gene cluster diversity. Genome Res 14, 354–366 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan J. P. et al. Coelacanth genome sequence reveals the evolutionary history of vertebrate genes. Genome Res 14, 2397–2405 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz J. et al. The DNA sequence and comparative analysis of human chromosome 5. Nature 431, 268–274 (2004). [DOI] [PubMed] [Google Scholar]
- Chen W. V. & Maniatis T. Clustered protocadherins. Development 140, 3297–3302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. A. & Jontes J. D. Protocadherins, not prototypical: a complex tale of their interactions, expression, and functions. Front Mol Neurosci 6, 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi S. et al. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet 37, 171–176 (2005). [DOI] [PubMed] [Google Scholar]
- Kaneko R. et al. Allelic gene regulation of Pcdh-alpha and Pcdh-gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. J Biol Chem 281, 30551–30560 (2006). [DOI] [PubMed] [Google Scholar]
- Noguchi Y. et al. Total expression and dual gene-regulatory mechanisms maintained in deletions and duplications of the Pcdha cluster. J Biol Chem 284, 32002–32014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Su H. & Bradley A. Molecular mechanisms governing Pcdh-gamma gene expression: evidence for a multiple promoter and cis-alternative splicing model. Genes Dev 16, 1890–1905 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K. et al. Single-neuron diversity generated by Protocadherin-beta cluster in mouse central and peripheral nervous systems. Front Mol Neurosci 5, 90 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A. M., Schreiner D., Lobas M. A. & Weiner J. A. gamma-protocadherins control cortical dendrite arborization by regulating the activity of a FAK/PKC/MARCKS signaling pathway. Neuron 74, 269–276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre J. L., Kostadinov D., Chen W. V., Maniatis T. & Sanes J. R. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature 488, 517–521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katori S. et al. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas. J Neurosci 29, 9137–9147 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herault Y., Rassoulzadegan M., Cuzin F. & Duboule D. Engineering chromosomes in mice through targeted meiotic recombination (TAMERE). Nat Genet 20, 381–384 (1998). [DOI] [PubMed] [Google Scholar]
- Mishina M. & Sakimura K. Conditional gene targeting on the pure C57BL/6 genetic background. Neurosci Res 58, 105–112 (2007). [DOI] [PubMed] [Google Scholar]
- Henrichsen C. N. et al. Segmental copy number variation shapes tissue transcriptomes. Nat Genet 41, 424–429 (2009). [DOI] [PubMed] [Google Scholar]
- Dallosso A. R. et al. Frequent long-range epigenetic silencing of protocadherin gene clusters on chromosome 5q31 in Wilms' tumor. PLoS Genet 5, e1000745 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko R., Kawaguchi M., Toyama T., Taguchi Y. & Yagi T. Expression levels of Protocadherin-alpha transcripts are decreased by nonsense-mediated mRNA decay with frameshift mutations and by high DNA methylation in their promoter regions. Gene 430, 86–94 (2009). [DOI] [PubMed] [Google Scholar]
- Kawaguchi M. et al. Relationship between DNA methylation states and transcription of individual isoforms encoded by the protocadherin-alpha gene cluster. J Biol Chem 283, 12064–12075 (2008). [DOI] [PubMed] [Google Scholar]
- Tasic B. et al. Promoter choice determines splice site selection in protocadherin alpha and gamma pre-mRNA splicing. Mol Cell 10, 21–33 (2002). [DOI] [PubMed] [Google Scholar]
- Toyoda S. et al. Developmental epigenetic modification regulates stochastic expression of clustered protocadherin genes, generating single neuron diversity. Neuron 82, 94–108 (2014). [DOI] [PubMed] [Google Scholar]
- Ribich S., Tasic B. & Maniatis T. Identification of long-range regulatory elements in the protocadherin-alpha gene cluster. Proc Natl Acad Sci U S A 103, 19719–19724 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehayova P., Monahan K., Chen W. & Maniatis T. Regulatory elements required for the activation and repression of the protocadherin-alpha gene cluster. Proc Natl Acad Sci U S A 108, 17195–17200 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S. et al. Identification of the cluster control region for the Protocadherin-{beta} genes located beyond the Protocadherin-{gamma} cluster. J Biol Chem (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss S. H., Omura M. & Mombaerts P. Local and cis effects of the H element on expression of odorant receptor genes in mouse. Cell 130, 373–384 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Mutually exclusive expression of human red and green visual pigment-reporter transgenes occurs at high frequency in murine cone photoreceptors. Proc Natl Acad Sci U S A 96, 5251–5256 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montavon T., Le Garrec J. F., Kerszberg M. & Duboule D. Modeling Hox gene regulation in digits: reverse collinearity and the molecular origin of thumbness. Genes Dev 22, 346–359 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura T., Hosoya T. & Kawamura S. A single enhancer regulating the differential expression of duplicated red-sensitive opsin genes in zebrafish. PLoS Genet 6, e1001245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T., Tarusawa E., Yoshimura Y., Galjart N. & Yagi T. CTCF is required for neural development and stochastic expression of clustered Pcdh genes in neurons. Cell Rep 2, 345–357 (2012). [DOI] [PubMed] [Google Scholar]
- Lachman H. M. et al. Analysis of protocadherin alpha gene deletion variant in bipolar disorder and schizophrenia. Psychiatr Genet 18, 110–115 (2008). [DOI] [PubMed] [Google Scholar]
- Iossifov I. et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha A. et al. Protocadherin alpha (PCDHA) as a novel susceptibility gene for autism. Journal of psychiatry & neuroscience: JPN 38, 192–198 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa E. et al. Analysis of protocadherin alpha gene enhancer polymorphism in bipolar disorder and schizophrenia. Schizophr Res 102, 210–219 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukkola-Vuoti L. et al. Genome-wide copy number variation analysis in extended families and unrelated individuals characterized for musical aptitude and creativity in music. PLoS One 8, e56356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanciulli M., Petretto E. & Aitman T. J. Gene copy number variation and common human disease. Clin Genet 77, 201–213 (2010). [DOI] [PubMed] [Google Scholar]
- Vacic V. et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature 471, 499–503 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crayton M. E. 3rd, Powell B. C., Vision T. J. & Giddings M. C. Tracking the evolution of alternatively spliced exons within the Dscam family. BMC evolutionary biology 6, 16 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi S., Kaneko R., Kawamura Y. & Yagi T. Split single-cell RT-PCR analysis of Purkinje cells. Nat Protoc 1, 2143–2151 (2006). [DOI] [PubMed] [Google Scholar]
- Li L. C. & Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics 18, 1427–1431 (2002). [DOI] [PubMed] [Google Scholar]
- Kumaki Y., Oda M. & Okano M. QUMA: quantification tool for methylation analysis. Nucleic Acids Res 36, W170–175 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information