Abstract
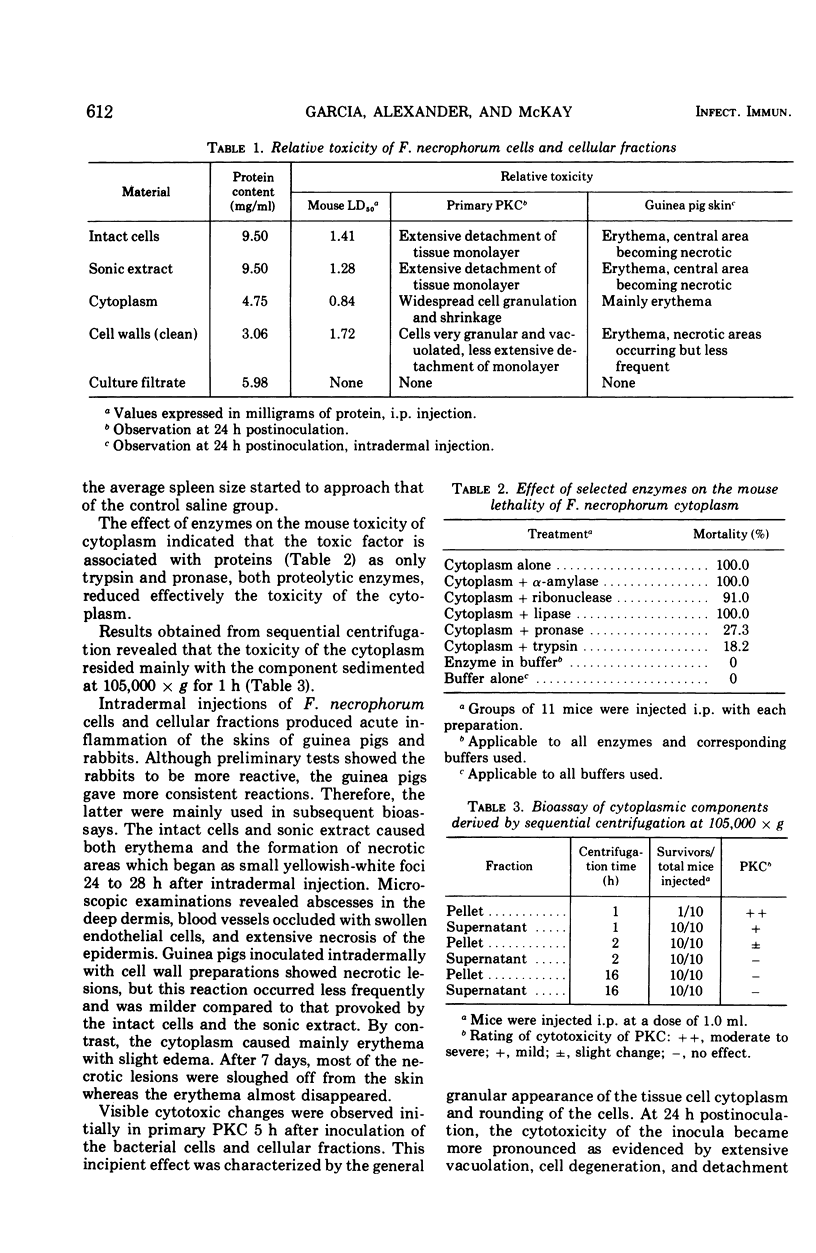
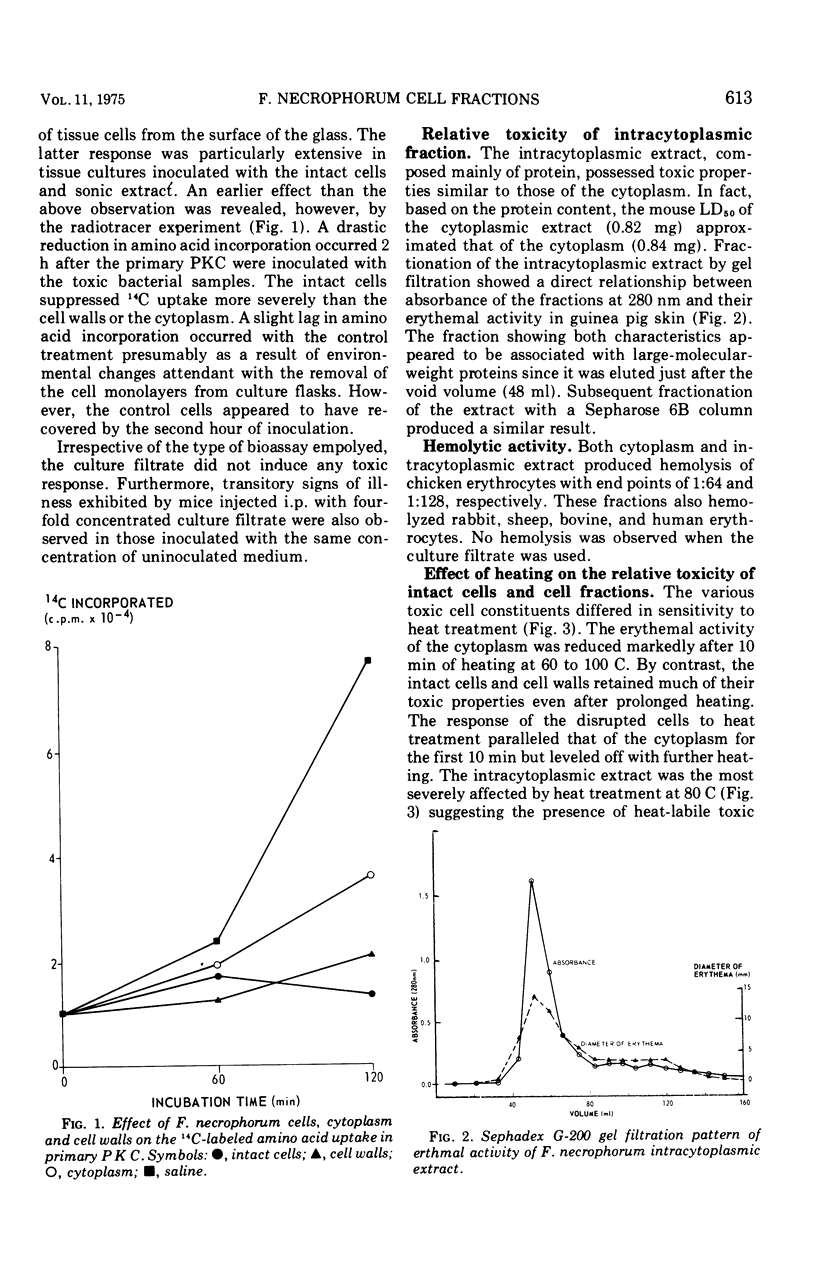
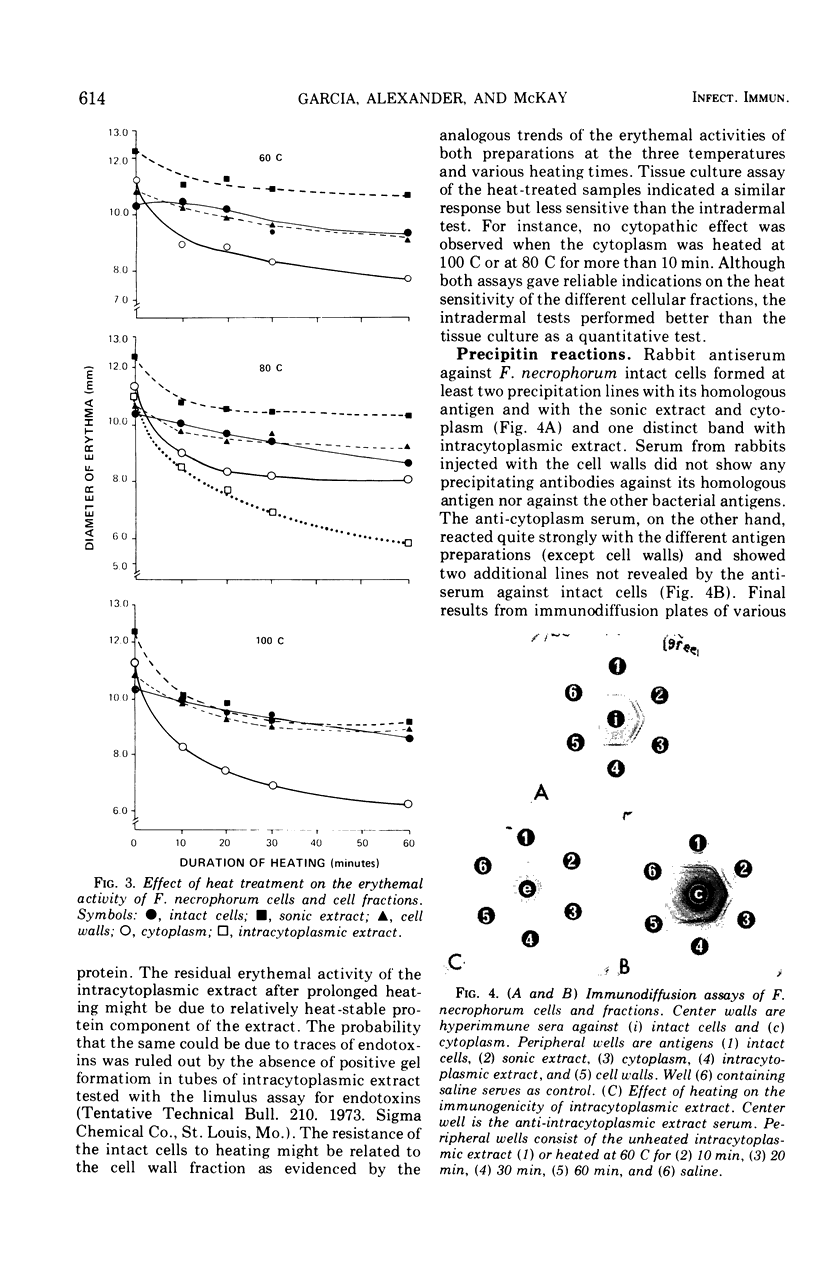
Fusobacterium necrophorum isolated from bovine liver abscesses was grown in bulk at 37 C for 24 h under a strict anaerobic atmosphere. Harvested washed cells were disrupted ultrasonically and fractionated by differential centrifugation into the intracellular (cytoplasm) and cell wall fractions. Both intact cells and cell fractions induced generalized cytopathic effect on primary pig kidney cultures and caused a variety of signs of illness and/or death of intraperitoneally injected mice. The intact cells, disrupted cells, and cell walls produced necrotic lesions and erythema on intradermally injected guinea pigs and rabbits, whereas the cytoplasm mainly erythema. By contrast, the used culture medium (culture filtrate) of F. necrophorum did not show any detectable toxicity. The toxic component of the cytoplasm appears to be associated with nondialyzable, hemolytic, high-molecular-weight proteins and its toxicity is reduced by trypsin and pronase. Heating at 60 C for 10 min decreased markedly its erythemal and cytotoxic ability, wheras the toxicity of the cell walls appeared to be only slightly affected even when heated at 100 C for 1 h. These results suggest that at leasttwo distinct cell-bound toxic factors are present in F. necrophorum cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke K., Gray G. W., Reaveley D. A. The extraction of cell walls of Pseudomonas aeruginosa with aqueous phenol. Material from the phenol layer. Biochem J. 1967 Nov;105(2):755–758. doi: 10.1042/bj1050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Ileal loop fluid accumulation and production of diarrhea in rabbits by cell-free products of Clostridium perfringens. J Bacteriol. 1969 Oct;100(1):86–94. doi: 10.1128/jb.100.1.86-94.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. M., Charlton K. M., McKay K. A. Characterization of endotoxin from Fusobacterium necrophorun. Infect Immun. 1975 Feb;11(2):371–379. doi: 10.1128/iai.11.2.371-379.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. M., Dorward W. J., Alexander D. C., Magwood S. E., McKay K. A. Results of a preliminary trial with Sphaerophorus necrophorus toxoids to control liver abscesses in feedlot cattle. Can J Comp Med. 1974 Jul;38(3):222–226. [PMC free article] [PubMed] [Google Scholar]
- Garcia M. M., McKay K. A. A satisfactory medium and technique for the mass cultivation of Sphaerophorus necrophorus. Can J Microbiol. 1973 Feb;19(2):296–298. doi: 10.1139/m73-046. [DOI] [PubMed] [Google Scholar]
- Garcia M. M., Neil D. H., McKay K. A. Application of immunofluorescence to studies on the ecology of Sphaerophorus necrophorus. Appl Microbiol. 1971 May;21(5):809–814. doi: 10.1128/am.21.5.809-814.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstad T., Kristoffersen T. Preparation and chemical characteristics of endotoxic lipopolysaccharide from three strains of Sphaerophorus necrophorus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):385–390. doi: 10.1111/j.1699-0463.1971.tb00077.x. [DOI] [PubMed] [Google Scholar]
- JENSEN R., FLINT J. C., GRINER L. A. Experimental hepatic necrobacillosis in beef cattle. Am J Vet Res. 1954 Jan;15(54):5–14. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Orcutt M. L. A STUDY OF BACILLUS NECROPHORUS OBTAINED FROM COWS. J Bacteriol. 1930 Nov;20(5):343–360. doi: 10.1128/jb.20.5.343-360.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. S. Toxic, allergenic and immunogenic factors of Fusiformis necrophorus. J Comp Pathol. 1970 Apr;80(2):247–257. doi: 10.1016/0021-9975(70)90092-7. [DOI] [PubMed] [Google Scholar]