Abstract
Background
Effective perioperative analgesia is lacking for children owing to interindividual variations and underdosing of opioids caused by fear of adverse effects. We investigated the role of COMT SNPs on postoperative pain management in children.
Methods
One hundred and forty nine children undergoing adenotonsillectomy were enrolled. The associations of four COMT SNPs (rs6269, rs4633, rs4818 and rs4680) with postoperative pain were analyzed and outcome measures included maximum pain scores, need for postoperative opioid interventions and postoperative morphine requirements.
Results
We detected an association of postoperative opioid intervention need with all four COMT SNPs. Minor allele carriers of COMT SNPs were approximately three-times more likely to require analgesic interventions than homozygotes of major alleles (p-value range: 0.0031–0.0127; odds ratio range: 2.6–3.1). In addition, significant association was detected between maximum Face, Leg, Activity, Consolability, Cry (FLACC) pain scores and three COMT SNPs (rs6269, rs4633 and rs4680). Haplotype 1 (ATCA: 51.3%) and Haplotype 2 (GCGG: 36.2%) are more frequent. Haplotype 2 was associated with higher odds of intravenous analgesic intervention need in postanesthesia recovery unit with an odds ratio of 2.6 (95% CI: 1.2–5.4; p-value = 0.022).
Conclusion
COMT SNPs may play a significant role in interindividual variation in postoperative pain perception and postoperative morphine requirements in children.
Keywords: COMT, genetics, pain, pediatric pain, postoperative pain
Perioperative pain management in children is often inadequate with up to 50% experiencing inadequate pain control and serious side effects from narcotics [1–4]. There is a wide range of responses to analgesics administered to treat postoperative pain secondary to variability in patient age [2], gender, race (ancestry), anxiety [5], type of surgery, previous experience [2] and genetic factors. It is currently believed that up to two-thirds of the variability in morphine response and adverse effects results from genetic variation in adults and children [6,7].
Several adult pain studies have shown an association between COMT genetic variations and interindividual variability in pain perception [8–11]. COMT is the enzyme mainly responsible for the metabolism of noradrenaline, adrenaline and dopamine. COMT is a key regulator of pain perception, cognitive function and mood, and is a pivotal regulator of catecholamine concentrations in the pain perception pathway [12,13]. Four common COMT SNPs (rs6269, rs4633, rs4818 and rs4680 – Val158Met) define haplotypes predictive of lower COMT enzyme activity [10]. Lower COMT activity increases catecholamine levels, causes hyperalgesia through stimulation of β2-adrenergic receptors [14,15] and increases dopaminergic transmission leading to depletion of encephalin in the brain and upregulation of opioid receptors [16]. Catecholamines and their metabolism may play a significant role in pediatric surgical pain. Therefore, a better understanding of the COMT gene in children may allow for individual tailoring and optimization of analgesic therapy, including opioids.
Little literature exists on the effect of COMT variations on postoperative pain perception and morphine analgesia in children [17,18], and large pediatric studies with homogenous pain pheno-type are lacking. We studied COMT candidate polymorphisms, which have been shown in adults to be significantly associated with pain perceptions and analgesia [8–11,19–24], in a homogenous sample of children undergoing tonsillectomy, to identify genetic profiles associated with increased postoperative pain and inadequate postoperative analgesia with morphine.
Methods
We performed a prospective, genotype-blinded, observational study in a cohort of children undergoing adenotonsillectomy. This study is part of a larger ongoing clinical study, Personalizing Perioperative Morphine Analgesia in Children, which is registered with clinicaltrials, gov (NCT01140724). The study was approved by the Cincinnati Children’s Hospital Medical Center’s institutional review board and written informed consent was obtained from parents and assent was obtained from children before enrollment.
Children scheduled for elective, outpatient adenotonsillectomy were enrolled. Children between 6 and 15 years of age and American Society of Anesthesiologists (ASA) status 1 or 2, including children with obstructive sleep apnea (OSA) and recurrent adenotonsillitis were recruited. Self-reported race by parents was used to determine ancestry. Children from European (Caucasian) and African (African–American) and other ancestries were included. Children were excluded if they were non-English speaking, had an allergy to morphine, were developmentally delayed, had liver or renal disease, or had preoperative opioid use. All children received standard and uniform perioperative care with an anesthetic consisting of an intra-operative dose of 0.2 mg/kg of morphine (children with OSA received 0.1 mg/kg of morphine). Intraoperatively, all children received prophylactic antiemetics dexamethasone (0.1 mg/kg, maximum 4 mg) and ondansetron (0.1 mg/kg, maximum 4 mg). Tonsillectomy was performed in all children using an electrocautery technique. Additional use of intra- and post-operative morphine (0.05 mg/kg/dose) was administered per discretion of the anesthesia team based on clinical need.
Analgesic outcome measures were post-operative pain scores and postoperative intra-venous analgesic interventions needed to achieve comfort, postoperative morphine use, prolonged postanesthesia recovery unit (PACU) stay due to inadequate pain control and duration to achieve PACU discharge readiness. Postoperative pain scores in PACU were simultaneously measured using the Face, Leg, Activity, Cry and Consolability (FLACC) scale by nursing staff and a subjective patient report of Numerical Rating Scale (NRS) upon arrival and every 5 min for 15 min, and then every 15 min until discharge. After intravenous opioid interventions, pain scores were measured every 5 min for 15 min until pain scores were below 4 out of 10. For pain scores >4 in PACU, intravenous opioids were administered as analgesic interventions. Intravenous morphine in 0.05 mg/kg doses were administered for significant (pain scores >4 out of 10) postoperative pain. We defined prolonged PACU stay as greater than 90 min following arrival and prior to discharge. The criteria to be met for PACU discharge were: level of consciousness arousable or awake, airway patent with adequate air exchange, core temperature ≥36.3°C, acceptable pain level, hemodynamically stable and surgical site without any bleeding or complications.
Blood was drawn in the operating room to obtain DNA for genotyping. COMT SNPs were analyzed by TaqMan® genotyping assays (Life Technologies, Applied Biosystems, CA, USA).
To test the hypothesis that COMT SNPs were associated with postoperative analgesia outcomes in children, statistical analyses were conducted using SAS 9.3 and JMP Genomics 5.1 (SAS Institute, NC, USA). Prior to analysis, distributional qualities of all outcomes were examined. For the COMT variants, allelic and genotype frequencies were examined, and Hardy–Weinberg equilibrium (HWE) was tested [25]. HWE states that both allele and genotype frequencies in a population remain constant, and thus provides an ideal baseline against which changes in a population can be analyzed [25]. To analyze dichotomous outcomes (intervention need and prolonged PACU), logistic regression was performed. For postoperative morphine use and duration for PACU discharge readiness, general linear models were used because these outcomes followed a normal distribution. The maximum NRS pain scores were not normally distributed thus were analyzed using Wilcoxon rank sum test. FLACC exhibited a zero-inflated negative binomial distribution with 27.5% of the patients experiencing no pain (score = 0). Thus, FLACC were analyzed using a zero-inflated negative binomial model to appropriately capture the distribution of this variable. Prior to evaluation of COMT variants, the effects of covariates (age, sex, ancestry, weight, OSA, intraoperative morphine and total morphine) were evaluated. To select the best fitting model, log likelihood, Akaike and Bayesian Information Criterion were compared, and residuals were examined. Covariates that significantly improved model fitting (p ≤ 0.05) were retained for genetic analyses. For dichotomous outcomes, adjusted odds ratio and 95% CIs were reported; for the rest, least square means and 95% CIs were reported. To confirm stability of the estimated odds ratios, random subsampling cross-validation was applied. Haplotypes were estimated and assigned by JMP Genomics.
In this study, since the clinical outcomes correlated with each other (mean Rho: 0.43, range: 0.16–0.74), we did not perform a very conservative Bonferroni correction for multiple testing. Instead, a correlation adjusted Bonferroni correction [101], was applied (α = 0.02). As linkage disequilibrium was extremely high between the COMT SNPs (D’ = 0.99), no additional multiple testing adjustment was performed. We also report associations reaching α = 0.05 as nominally associated.
Results
Participants were primarily non-Hispanic Caucasian (of white or European ancestry) children with slightly more girls than boys. The majority of participants were between 7 and 11 years of age. All four COMT SNPs were common with minor allele frequencies ranging from 0.37 to 0.48, and all were in HWE. Demographic data and intraoperative and total morphine requirement (mean ± standard deviation) are summarized in Table 1. Allelic and genotype frequencies of the four COMT SNPs are shown in Table 2.
Table 1.
Demographic characteristics.
| Characteristic | Value (total n = 149) |
|---|---|
| Age (years), median (IQR) | 8.4(71−11.2) |
| Weight (kg), median (IQR) | 36.1 (26.5−51.4) |
| Sex, n (%) | |
| Female | 80 (54) |
| Male | 69 (46) |
| Race, n (%) | |
| White | 121 (81) |
| Non-white | 28(19) |
| OSA | |
| Yes, n (%) | 68 (46) |
| No, n (%) | 81 (54) |
| Intraoperative morphine requirement (mg/kg), mean ± SD | 0.19 ±0.06 |
| Total morphine requirement (mg/kg), mean ± SD | 0.25 ±0.08 |
IQR: Interquartile range; OSA: Obstructive sleep apnea; SD: Standard deviation.
Table 2.
COMT alleles and genotypes.
| SNP | Allele |
Genotype |
HWE test (p-value) |
||||
|---|---|---|---|---|---|---|---|
| Allele | Count | Frequency | Genotype | Count | Frequency | ||
| rs6269 | A | 175 | 0.59 | A/A | 49 | 0.33 | 0.42 |
| G | 123 | 0.41 | A/G | 77 | 0.52 | ||
| G/G | 23 | 0.15 | |||||
| rs4633 | C | 142 | 0.48 | C/C | 30 | 0.20 | 0.21 |
| T | 156 | 0.52 | C/T | 82 | 0.55 | ||
| T/T | 37 | 0.25 | |||||
| rs4818 | C | 189 | 0.63 | C/C | 58 | 0.39 | 0.49 |
| G | 109 | 0.37 | C/G | 73 | 0.49 | ||
| G/G | 18 | 0.12 | |||||
| rs4680 | A | 154 | 0.52 | A/A | 38 | 0.26 | 0.56 |
| G | 144 | 0.48 | A/G | 78 | 0.52 | ||
| G/G | 33 | 0.22 | |||||
Four COMT polymorphisms were tested, allelic frequency and HWE from the study cohort are reported.
HWE: Hardy–Weinberg equilibrium.
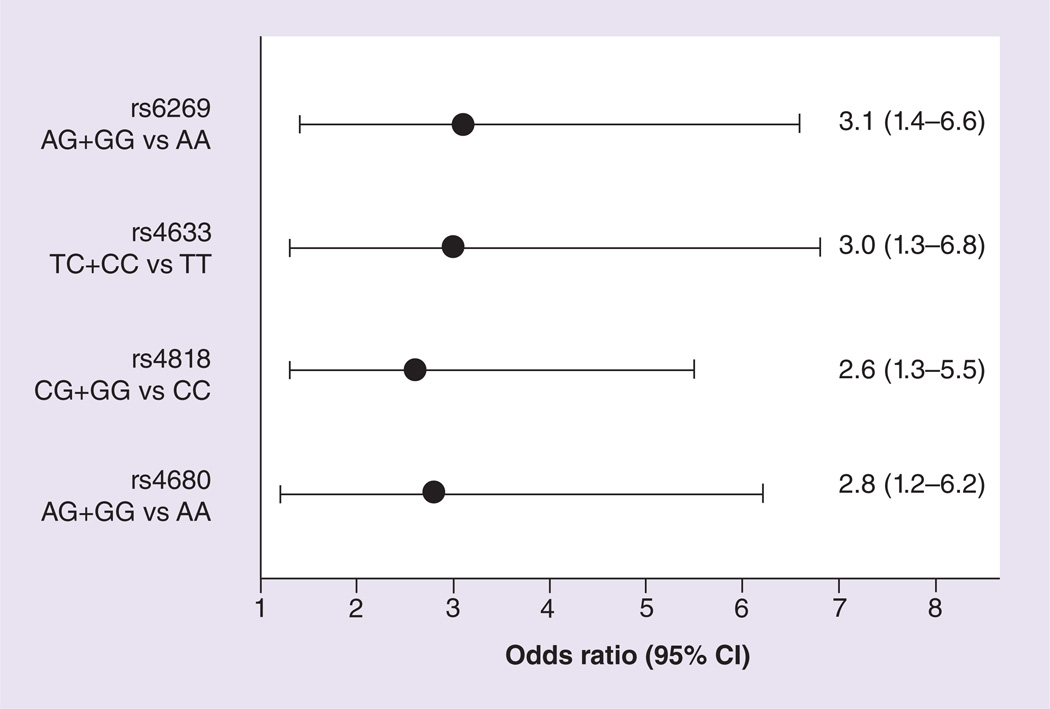
A relationship was observed between postoperative rescue analgesic intervention need and COMT genotypes, with the minor allele carriers having a larger proportion of individuals requiring postoperative rescue analgesic intervention (Table 3). After adjusting for ancestry and intraoperative morphine, significant association of intervention need was detected with all four COMT SNPs (p-value range: 0.0031–0.0127; odds ratio range: 2.6–3.1, Figure 1). Cross-validation supported the validity and stability of these findings. Children with the CC genotype of the COMT SNP rs4818 compared to minor allele carriers demonstrated a trend towards increased postoperative morphine requirement (p = 0.07; Table 3).
Table 3.
Morphine need, pain outcomes and time in postanesthesia recovery unit stratified by COMT genotypes.
| SNP |
COMT genotype |
n | Postoperative analgesic intervention need (% yes) |
Prolonged PACU stay due to pain (% yes) |
Postoperative morphine requirement, mg/kg (mean ± SD) |
Maximum FLACC score, median (IQR) |
Maximum NRS score, median (IQR) |
Time to PACU discharge readiness, min (mean ± SD) |
|---|---|---|---|---|---|---|---|---|
| rs6269 | AA | 49 | 45 | 24 | 0.04 ±0.05 | 2 (0–4) | 6 (2–8) | 104.7 ±41.3 |
| GA† | 77 | 70 | 38 | 0.07 ±0.06 | 4(1–6) | 6 (3–8) | 104.6 ±50.6 | |
| GG† | 23 | 74 | 35 | 0.07 ±0.06 | 4(1–6) | 6 (2–8) | 92.6 ±30.8 | |
| rs4633 | CC† | 30 | 70 | 33 | 0.07 ±0.06 | 4(1–6) | 6 (2–8) | 93.2 ±28.7 |
| CT† | 82 | 68 | 38 | 0.07 ±0.07 | 4 (0–6) | 6 (3–8) | 107.3 ±52.6 | |
| TT | 37 | 43 | 22 | 0.03 ±0.04 | 2 (0–3) | 6 (2–8) | 100.4 ±36.0 | |
| rs4818 | CC | 58 | 50 | 29 | 0.05 ±0.07 | 2 (0–5) | 6 (3–8) | 104.9 ±42.2 |
| CG† | 73 | 71 | 37 | 0.06 ±0.05 | 3(1–5) | 6 (4–8) | 104.7 ±49.9 | |
| GG† | 18 | 67 | 28 | 0.06 ±0.05 | 3 (1–6) | 6(2–7) | 87.9 ±28.8 | |
| rs4680 | AA | 38 | 45 | 24 | 0.04 ±0.05 | 2 (0–4) | 6 (2–8) | 100.1 ±35.5 |
| AG† | 78 | 68 | 37 | 0.07 ±0.07 | 4(0–6) | 5 (3–8) | 107.9 ±53.9 | |
| GG† | 33 | 70 | 33 | 0.06 ±0.06 | 3 (1–6) | 6 (3–9) | 93.6 ±27.7 | |
Genotypes carrying minor allele.
FLACC: Face, Leg, Activity, Cry and Consolability; IQR: Interquartile range; NRS: Numerical Rating Scale; PACU: Postanaesthesia recovery unit; SD: Standard deviation.
Figure 1. Relationship between postoperative intravenous rescue analgesic intervention need and COMT genotypes (grouped as minor and major alleles).
The minor allele carriers had a larger proportion of children requiring postoperative rescue analgesic intervention (~threefold increase). After adjusting for ancestry and intraoperative morphine, significant associations of higher intervention need (odds ratio range: 2.6–3.1) were detected with all four COMT SNPs (p-value range: 0.0031–0.0127).
Another analgesic outcome exhibiting significant association was maximum FLACC score. One SNP (rs4633) exhibited significant association and two others (rs6269 and rs4680) exhibited nominal association (Table 4). NRS, duration to PACU discharge readiness and prolonged stay in PACU due to pain were not associated with the COMT SNPs (p > 0.05).
Table 4.
Postoperative pain, morphine analgesic efficacy and genetic associations with COMT polymorphisms.
| SNP | Genotype | Postoperative† rescue analgesic intervention need |
Prolonged stay in PACU due to inadequate analgesia‡ |
Maximum FLACC pain score§ |
Postoperative morphine requirement (mg/kg)¶ |
Duration to PACU discharge readiness (min)# |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Adjusted OR (95% CI) |
p-value |
Adjusted OR (95% CI) |
p-value |
LS mean (95% CI) |
p-value |
LS mean (95% CI) |
p-value |
LS mean (95% CI) |
p-value | ||
| rs6269 | AA | 3.1 (1.4–6.6) | 0.0031 | 1.7(0.8–3.9) | 0.16 | 3.9(3.1–4.9) | 0.04 | 0.09(0.07–0.12) | 0.58 | 107.1 (94.9–119.4) | 0.39 |
| AG+GG | 5.1 (4.4–5.8) | 0.10(0.09–0.11) | 100.6(92.1–109.1) | ||||||||
| rs4633 | TT | 3.0(1.3–6.8) | 0.0074 | 1.9(0.8–4.8) | 0.14 | 3.5 (2.6–4.6) | 0.01 | 0.08(0.05–0.11) | 0.18 | 104.6(90.4–118.9) | 0.77 |
| TC+CC | 5.0(4.4–5.7) | 0.10(0.09–0.11) | 102.1 (94.0–110.2) | ||||||||
| rs4818 | CC | 2.6(1.3–5.5) | 0.0082 | 1.3(0.6–2.8) | 0.45 | 4.4(3.6–5.4) | 0.32 | 0.11 (0.09–0.13) | 0.07 | 105.3(94.2–116.5) | 0.56 |
| CG+GG | 5.0(4.3–5.7) | 0.09(0.08–0.11) | 101.1 (92.2–110.0) | ||||||||
| rs4680 | AA | 2.8(1.2–6.2) | 0.0127 | 1.7(0.7–4.0) | 0.24 | 3.8(2.9–5.0) | 0.05 | 0.09(0.06–0.12) | 0.45 | 103.7(89.7–117.7) | 0.88 |
| AG+GG | 5.0(4.4–5.7) | 0.10(0.09–0.11) | 102.4(94.3–110.6) | ||||||||
ORs are of minor allele carriers vs homozygous major allele carriers.
Adjusted for race and intraoperative morphine.
Adjusted for race, obstructive sleep apnea, and race × obstructive sleep apnea.
Adjusted for age, race, weight and intraoperative morphine.
In patients who need postoperative morphine; adjusted for race.
Adjusted for morphine by weight.
FLACC: Face, Leg, Activity, Cry and Consolability; LS: Least square; OR: Odds ratio; PACU: Postanaesthesia recovery unit.
Haplotype analyses showed that Haplotype 1 (ATCA: 51.3%) and Haplotype 2 (GCGG: 36.2%) are more frequent (Table 5). Single haplo-type association tests were carried out on continuous pain outcomes (Table 5). Nominal association was detected between Haplotype 1 and Haplotype 2 with intravenous analgesic intervention need in PACU, with opposite effects.
Table 5.
Postoperative pain, morphine analgesic efficacy and genetic associations with common COMT haplotypes.
| Haplotype (frequency) |
Postoperative† rescue analgesic intervention need |
Prolonged stay in PACU due to inadequate analgesia‡ |
Maximum FLACC pain score§ |
Postoperative morphine requirement (mg/kg)¶ |
Duration to PACU discharge readiness (min)# |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Adjusted OR (95% CI) |
p-value |
Adjusted OR (95% CI) |
p-value |
Effect size (95% CI) |
p-value |
Effect size (95% CI) |
p-value |
Effect size (95% CI) |
p-value | |
| A–T–C–A (51.3%) |
0.6(0.3–1.0) | 0.0395 | 0.8(0.5–1.4) | 0.53 | 0.88(0.74–1.04) | 0.12 | − 0.00 (− 0.02–0.01) | 0.63 | 5.4 (–4.8–15.7) | 0.30 |
| G–C–G–C (36.2%) |
1.8(1.0–3.1) | 0.0354 | 1.1 (0.6–1.8) | 0.86 | 1.08(0.92–1.28) | 0.34 | − 0.01 (− 0.03–0.01) | 0.19 | –6.9 (–17.3–3.6) | 0.20 |
| A–C–C–G (6.0%) |
0.6(0.2–1.6) | 0.30 | 0.7 (0.2–2.0) | 0.49 | 0.97(0.70–1.34) | 0.86 | 0.01 (− 0.02–0.04) | 0.63 | 2.9 (–17.3–23.0) | 0.78 |
| G–C–C–G (4.7%) |
3.0(0.5–17.2) | 0.19 | 3.5(0.8–15.7) | 0.09 | 1.12(0.80–1.56) | 0.50 | 0.06(0.03–0.09) | 0.0003 | 0.4 (–24.4–25.2) | 0.97 |
| A–T–C–G (1 %) | 0.4(0.0–5.1) | 0.47 | 0.5(0.0–6.5) | 0.56 | 1.03(0.50–2.13) | 0.94 | − 0.08 (− 0.15 to–0.01) | 0.0303 | 5.0 (–45.0–55.1) | 0.84 |
| Rare(<1%) | 3.0(0.1–124.7) | 0.18 | 7.1 (0.2–339.9) | 0.32 | 1.61 (0.84–3.08) | 0.17 | 0.05 (− 0.02–0.13) | 0.13 | 3.7 (–57.9–65.3) | 0.91 |
OR is for each additional copy of the haplotype.
Effect size: mean changes for one copy change of haplotype.
Adjusted for race and intraoperative morphine.
Adjusted for race, obstructive sleep apnea, and race × obstructive sleep apnea.
Adjusted for age, race, weight and intraoperative morphine.
In patients who need postoperative morphine; adjusted for race.
Adjusted for morphine by weight.
FLACC: Face, Leg, Activity, Cry and Consolability; OR: Odds ratio; PACU: Post-anaesthesia recovery unit.
This is consistent with our single SNP associations. Haplotypes 4 and 5 are associated with postoperative morphine requirement. In the single SNP association test, postoperative morphine requirement was associated with rs4818. The haplotype association could be due to segregation of rs4818 genotypes. Haplotypes 4, 5 and 6 are too rare to develop stable statistical models. Children that are homozygous for Haplotype 1 had lower incidence of intravenous analgesic intervention need in PACU. Heterozygous Haplotypes 1 and 2 and homozygous Haplotype 2 were comparable and they had higher intravenous analgesic intervention need in PACU than homozygous Haplotype 1. This is consistent with the single SNP association tests. Haplotype 2 was associated with odds of higher intravenous analgesic intervention need in PACU with an odds ratio of 2.6 (95%CL: 1.2–5.4; p-value = 0.022).
Discussion
Pain is a complex experience with sensory, emotional and cognitive components [4]. Morphine, one of the most commonly used opioids, has a narrow therapeutic index and large interpatient variability in both analgesic responses. Genetic variations in the gene coding for COMT have been suggested to affect clinical and experimental pain-related phenotypes [4]. Our findings indicate that COMT genetic variation is associated with the need for postoperative analgesic intervention and postoperative pain in children. Although the role of COMT genotype with postoperative analgesia and morphine requirements has been studied in adults, this is the first study to address this issue in a population of children having homogenous surgical pain.
In this homogenous group of tonsillectomy patients, the need for postoperative morphine was significantly (p < 0.013) associated with four SNPs in COMT (rs6269, rs4633, rs4818 and rs4680). The strongest association (rs6269) yielded increased odds (3.1-fold) of postoperative intravenous opioid interventions in individuals with the copy of the G allele compared with the AA genotype. Additionally, there was significant association with the FLACC pain score and rs4633 (p = 0.01). Children with the CC genotype of rs4818 demonstrated a trend towards increased postoperative morphine requirement compared with other genotypes (p = 0.07, Table 3).
Our study results are consistent with a post-operative study in adults with postlumbar disc surgery [26]. Specifically, the haplotype with the protective allele from the four COMT SNPs in our pediatric study was associated with less analgesic interventions, which is consistent with the association between the TT genotype of the COMT SNP rs4633 and improved Oswestry Disability Index (ODI) was demonstrated 1 year after surgery in adult patients [26]. In a recent study of 109 Italian adults undergoing surgery, when 12 different polymorphisms of COMT, OPRM1 and UGT2B7 genes were investigated for association with postoperative morphine requirement, only COMT polymorphisms were predictive of morphine requirement [27]. Another recent study of postoperative cardiac patients showed that the COMT polymorphism Val158Met contributed to pain sensitivity [28]. These consistent postoperative pain and functional outcomes in our study children and adults suggest that patients with certain COMT gcnotypes are at higher risk for increased immediate postoperative pain.
Our pediatric study is one of the early studies looking at associations between genetic make up and postoperative pain in a homogeneous group of healthy children undergoing one type of surgery, tonsillectomy with electrocautery surgical technique with standard and uniform intraoperative and postoperative care to minimize confounding. In another recently published small study of 168 children undergoing heterogeneous surgical procedures, including abdominal and orthopedic surgery, postoperative pain scores during mobilization were lower for GG genotype than AA and GA genotypes of the COMT polymorphism Val158Met [18]. Another recent trial comparing 68 Latino and non-Latino children showed a statistically significant difference in morphine’s response; however, none of the 28 genetic polymorphisms examined in this study, including COMT gene polymorphisms, could explain the ethnicity-related differences in morphine’s response, this is probably because of the limited sample size and the heterogeneous ethnicity of the study population [17]. Recently, the 158A>G variant in the COMT gene (rs4680), specifically the AG/GG genotype was associated with shortened length of stay and less treatment with two or more medications than the AA genotype among infants with neonatal abstinence syndrome caused by in utero opioid exposure, providing early insights into the mechanisms underlying neonatal opioid withdrawal [7]. This COMT variant has a minor allele frequency of approximately 50% in Caucasians (in our study population it was 48%) and has been associated with responses to pain and morphine dosing requirements in adults [29].
In our study, although both the pain measures of NRS and FLACC are validated measures, only FLACC exhibited an association with COMT genotyping. The failure to identify associations with NRS could be because the NRS is a subjective measure of pain versus FLACC, which is a relatively objective measure of pain. Clearly, additional studies should examine the relationship of NRS with FLACC to better understand the factors that may influence differential reporting of postoperative pain in children.
Conclusion & future perspective
By demonstrating an association between morphine need after surgery and COMT genetic variation, this study contributes to a greater understanding of the underlying genetic basis of why children vary in their sensitivity to pain and postoperative analgesia. It provides essential preliminary supportive results toward establishing associations between COMT genotypes and postoperative pain and opioid use related outcomes in children. It is important to note, however, that, besides COMT, many genes may influence morphine’s effects, and morphine’s pharmacokinetics. While variations at the COMT gene have been shown to influence pain sensitivity and postoperative morphine need in children, postoperative pain and opioid responses could be influenced by many different genes [15,17,18,30]. Large clinical trials having adequate power for population stratification to study simultaneously multiple genes potentially affecting pain and opioid responses along with gene–gene interactions over longer postoperative course in future will contribute to the essential foundation for genotype-directed analgesic selection and dosing to maximize pain relief while improving patient safety by minimizing serious side effects.
Executive summary.
Interindividual differences in postoperative pain & opioid requirement are associated with common polymorphisms of COMT gene
-
▪
Genetic variants of COMT play a role in interindividual variations in postoperative pain perception and postoperative morphine requirements in children undergoing adenotonsillectomy.
-
▪
Minor allele carriers of COMT polymorphisms (rs6269, rs4633, rs4818 and rs4680) were approximately three-times more likely to require analgesic interventions than homozygotes of major alleles (odds ratio range: 2.6–3.1; p-value range: 0.0031–0.0127).
-
▪
Haplotype 2 (GCGG: 36.2%), which was the second most common haplotype of these four COMT SNPs was associated with higher odds of intravenous analgesic intervention need in PACU with an odds ratio of 2.6 (95% CI: 1.2–5.4; p = 0.022).
Interindividual difference in postoperative pain & postoperative analgesic requirement is caused by multiple factors
-
▪
Besides COMT genetic variations, other genetic and nongenetic factors, and gene–gene interactions might contribute to variations in surgical pain perception and analgesic requirement in the perioperative period.
-
▪
Large clinical studies to simultaneously study COMT and multiple other genes affecting pain and opioid responses will contribute to the essential foundation for genotype-directed analgesic selection and dosing to maximize pain relief while improving patient safety by minimizing serious side effects.
Acknowledgments
This work was supported in part by USPHS Grant #UL1 RR026314 from the National Center for Research Resources, NIH and with the Place Outcomes Research Award and Translational Research Award, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA. Additional research funding support was provided by the Department of Anesthesia, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA. No financial support was received except for departmental salary support for the authors. This pharmacogenetic study was designed and undertaken by the authors. The sponsor of this study, the Cincinnati Children’s Hospital Medical Center (CCHMC) provided funding support for the genetic analyses and supported the salary of the research team. The authors directed and had access to all the analyses and the full clinical and genetic database, wrote all drafts of the report, decided to publish the results, and attest for the accuracy and completeness of the data.
Footnotes
Trial registration
This clinical trial reflects a portion of a larger study, Personalizing Perioperative Morphine Analgesia in Children, NCT01140724, registered at clinicaltrials.gov.
Authors’ contribution
S Sadhasivam conceptualized and designed the study, designed the data collection instruments, recruited subjects, and coordinated and supervised data collection, drafted the initial manuscript, and approved the final manuscript as submitted. V Olbrecht helped with the initial draft, reviewed and revised the manuscript, and approved the final manuscript as submitted. V Chidambaran helped with recruitment, reviewed and revised the manuscript, and approved the final manuscript as submitted. HR Esslinger helped with patient recruitment, data collection and approved the final manuscript. K Zhang helped with genetic study design and analysis. X Zhang helped with data analyses, draft statistical section, tables and revision of the manuscript. LJ Martin helped with data analyses, statistical methods, revision of the manuscript and approved the final manuscript as submitted.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Walco GA, Cassidy RC, Schechter NL. Pain, hurt, and harm. The ethics of pain control in infants and children. N. Engl. J. Med. 1994;331(8):541–544. doi: 10.1056/NEJM199408253310812. [DOI] [PubMed] [Google Scholar]
- 2.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N. Engl. J. Med. 2002;347(14):1094–1103. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu P, Cyrenne L, Mathews S, Villeneuve E, Vischoff D. Patient-controlled analgesia after spinal fusion for idiopathic scoliosis. Int. Orthop. 1996;20(5):295–299. doi: 10.1007/s002640050081. [DOI] [PubMed] [Google Scholar]
- 4.Duedahl TH, Hansen EH. A qualitative systematic review of morphine treatment in children with postoperative pain. Paediatr. Anaesth. 2007;17(8):756–774. doi: 10.1111/j.1460-9592.2007.02213.x. [DOI] [PubMed] [Google Scholar]
- 5.Schechter NL, Blankson V, Pachter LM, Sullivan CM, Costa L. The ouchless place: no pain, children’s gain. Pediatrics. 1997;99(6):890–894. doi: 10.1542/peds.99.6.890. [DOI] [PubMed] [Google Scholar]
- 6.Ross JR, Rutter D, Welsh K, et al. Clinical response to morphine in cancer patients and genetic variation in candidate genes. Pharmacogenomics J. 2005;5(5):324–336. doi: 10.1038/sj.tpj.6500327. [DOI] [PubMed] [Google Scholar]
- 7.Wachman EM, Hayes MJ, Brown MS, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA. 2013;309(17):1821–1827. doi: 10.1001/jama.2013.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both p2- and p3-adrenergic receptors. Pain. 2007;128(3):199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diatchenko L, Nackley AG, Slade GD, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125(3):216–224. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 2005;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 11.Reyes-Gibby CC, Shete S, Rakvag T, et al. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130(1–2):25–30. doi: 10.1016/j.pain.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 13.Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects muopioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 14.Khasar SG, McCarter G, Levine JD. Epinephrine produces a β-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81(3):1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 15.Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics. 2012;13(15):1719–1740. doi: 10.2217/pgs.12.152. [DOI] [PubMed] [Google Scholar]
- 16.Berthele A, Platzer S, Jochim B, et al. COMT Vall08/158Met genotype affects the muopioid receptor system in the human brain: evidence from ligand-binding, G-protein activation and preproenkephalin mRNA expression. Neuroimage. 2005;28(1):185–193. doi: 10.1016/j.neuroimage.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez N, Anderson GD, Shen DD, et al. Is ethnicity associated with morphine’s side effects in children? Morphine pharmacokinetics, analgesic response, and side effects in children having tonsillectomy. Paediatr. Anaesth. 2012;22(7):669–675. doi: 10.1111/j.1460-9592.2012.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamie C, Rebsamen MC, Morris MA, Morabia A. First evidence of a polygenic susceptibility to pain in a pediatric cohort. Anesth. Analg. 2013;116(1):170–177. doi: 10.1213/ANE.0b013e31826f0637. [DOI] [PubMed] [Google Scholar]
- 19.Oertel BG, Schmidt R, Schneider A, Geisslinger G, Lotsch J. The muopioid receptor gene polymorphism 118A>G depletes alfentanil-induced analgesia and protects against respiratory depression in homozygous carriers. Pharmacogenet. Genomics. 2006;16(9):625–636. doi: 10.1097/01.fpc.0000220566.90466.a2. [DOI] [PubMed] [Google Scholar]
- 20.Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006;105(2):334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Chou WY, Yang LC, Lu HF, et al. Association of muopioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol. Scand. 2006;50(7):787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- 22.Klepstad P, Rakvag TT, Kaasa S, et al. The 118 A>G polymorphism in the human muopioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol. Scand. 2004;48(10):1232–1239. doi: 10.1111/j.1399-6576.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 23.Coulbault L, Beaussier M, Verstuyft C, et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin. Pharmacol. Ther. 2006;79(4):316–324. doi: 10.1016/j.clpt.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Coller JK, Barratt DT, Dahlen K, Loennechen MH, Somogyi AA. ABCB1 genetic variability and methadone dosage requirements in opioid-dependent individuals. Clin. Pharmacol. Ther. 2006;80(6):682–690. doi: 10.1016/j.clpt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Stern C. The Hardy-Weinberg law. Science. 1943;97(2510):137–138. doi: 10.1126/science.97.2510.137. [DOI] [PubMed] [Google Scholar]
- 26.Dai F, Belfer I, Schwartz CE, et al. Association of catechol- O-methyltransferase genetic variants with outcome in patients undergoing surgical treatment for lumbar degenerative disc disease. Spine J. 2010;10(11):949–957. doi: 10.1016/j.spinee.2010.07.387. [DOI] [PubMed] [Google Scholar]
- 27.De Gregori M, Garbin G, De Gregori S, et al. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. Eur. J. Clin. Pharmacol. 2013;69(9):1651–1658. doi: 10.1007/s00228-013-1523-7. [DOI] [PubMed] [Google Scholar]
- 28.Ahlers SJ, Elens LL, van Gulik L, et al. The Val158Met polymorphism of the COMT gene is associated with increased pain sensitivity in morphine-treated patients undergoing a painful procedure after cardiac surgery. Br. J. Clin. Pharmacol. 2013;75(6):1506–1515. doi: 10.1111/bcp.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakvag TT, Klepstad P, Baar C, et al. The Val158Met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain. 2005;116(1–2):73–78. doi: 10.1016/j.pain.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Sadhasivam S, Krekels EH, Chidambaran V, et al. Morphine clearance in children: does race or genetics matter? J Opioid Manag. 2012;8(4):217–226. doi: 10.5055/jom.2012.0119. [DOI] [PubMed] [Google Scholar]
Website
- 101.SISA. www.quantitativeskills.com/sisa/