Abstract
Mutations in sarcomere genes have been found in many inheritable human diseases, including hypertrophic cardiomyopathy. Elucidating the molecular mechanisms of sarcomere assembly shall facilitate understanding of the pathogenesis of sarcomere-based cardiac disease. Recently, biochemical and genomic studies have identified many new genes encoding proteins that localize to the sarcomere. However, their precise functions in sarcomere assembly and sarcomere-based cardiac disease are unknown. Here, we review zebrafish as an emerging vertebrate model for these studies. We summarize the techniques offered by this animal model to manipulate genes of interest, annotate gene expression, and describe the resulting phenotypes. We survey the sarcomere genes that have been investigated in zebrafish and discuss the potential of applying this in vivo model for larger-scale genetic studies.
Keywords: cardiomyopathy, genetics, myofibrillogenesis, sarcomere, zebrafish
Introduction to Sarcomere Assembly
The sarcomere is the basic contractile unit of myofibrils in striated muscles, including cardiac and skeletal muscles (Clark, McElhinny et al. 2002). When viewed with transmission electron microscopy (TEM), sarcomeres appear as semicrystalline structures, with substructures of 2 filamentary systems, ie, thick and thin filaments. The electron-dense A-bands are the myosin-based thick filaments, whereas the least electron-dense I-bands are the actin-based thin filaments. M-lines are located in the middle of A-bands and anchor the bipolar thick filaments. In the middle of the I-bands, Z-discs define the border of 2 neighboring sarcomere units, anchoring the end of the thin filaments and titin filaments, the latter being the third filament system in a sarcomere (Pyle and Solaro 2004; Frank, Kuhn et al. 2006). Titin is the largest known protein, spanning half of a sarcomere as a single molecule. The N-terminus of titin anchors in the Z-disc, and its C-terminus extends into the M-line (Tskhovrebova and Trinick 2003; LeWinter, Wu et al. 2007). The energy-consuming movement between the myosin-based thick filament and actin-based thin filament is the molecular basis for active contraction, whereas the Titin filament functions as a molecular spring and contributes to passive contraction.
Mutations in sarcomeric genes have been shown to cause many human cardiac diseases. For example, inheritable hypertrophic cardiomyopathy (HCM) has been found to be associated with more than 1,400 mutations in 11 or more genes encoding cardiac sarcomere proteins (Seidman and Seidman 2001; Seidman and Seidman 2011; Maron and Maron 2013). Thus, HCM has been described as a disease of the sarcomere. Disturbance of the sarcomeric structure, including myofibrillar disarray or Z-disc streaming, is a crucial pathologic step in the development of heart failure by various mechanisms (Knoll, Hoshijima et al. 2003). To understand the molecular mechanism of this step, fundamental knowledge of sarcomere assembly is necessary. The basic mechanisms obtained from studying de novo assembly, which can be done in experimentally accessible model systems, are expected to facilitate understanding of the pathogenesis of human diseases.
As a complicated multistep process, sarcomere assembly, also termed myofibrillogenesis, has fascinated scientists for more than 100 years. Recent extensive studies suggest several models of myofibrillogenesis (Sanger, Kang et al. 2005; Boateng and Goldspink 2008). One model emphasizes the existence of a stress fiber–like structure, composed of nonmuscle proteins in the cell periphery that functions as a reusable template for the recruitment of sarcomeric proteins to form myofibrils (Dlugosz, Antin et al. 1984). A second model hypothesizes that actin filaments attached to Z-bands (I-Z-I brushes) and A-bands form independently and are subsequently stitched together by Titin to form myofibrils (Schultheiss, Lin et al. 1990; Holtzer, Hijikata et al. 1997; Ojima, Lin et al. 1999). A third model emphasizes the formation of premyofibrils along the cell membrane; these have a short mini-sarcomere structure that includes Z-bodies, attached actin filaments, and miniature A-bands (Rhee, Sanger et al. 1994). Premyofibrils are composed of sarcomeric proteins, except for mini A-bands, which are composed of nonmuscle Myosin II filaments. The addition of titin and overlapping muscle myosin II filaments to Z-bodies transforms premyofibrils to nascent myofibrils (Rhee, Sanger et al. 1994; Dabiri, Turnacioglu et al. 1997; Du, Sanger et al. 2003; Sanger, Wang et al. 2009). The addition of Myosin-binding protein C and M-band proteins, the alignment of the thick filaments into A-bands, and the loss of nonmuscle Myosin II leads to the formation of mature myofibrils (Sanger, Chowrashi et al. 2002; Sanger and Sanger 2008; Sanger, Wang et al. 2010). These models describing the different aspects of sarcomere assembly are supported by varying levels of in vivo and in vitro evidence. The first and second models are based solely on fixed and antibody-stained muscle cells. The premyofibril model is based on immunofluorescence experiments and by the assembly of mature myofibrils, as revealed by sarcomeric protein fused to fluorescent protein in transfected living muscle cells (Dabiri, Turnacioglu et al. 1997; Sanger, Chowrashi et al. 2002; Stout, Wang et al. 2008; Liu, Chakkarapani et al. 2013).
In recent years, the study of myofibrillogenesis has attracted more attention through the identification of many new sarcomeric components and because previously known proteins were found to localize to the sarcomere (Sanger, Wang et al. 2009). The identification of these proteins suggests that the sarcomere not only provides a structural basis for contraction but also acts as a docking station for signaling molecules. The statement is exemplified by recent research on the Z-disc substructure that consists of Titin, Actin, and α-Actinin (Pyle and Solaro 2004; Frank, Kuhn et al. 2006). In addition to Actin, Nebulin and Obscurin were later found to also bind to Titin (Kontrogianni-Konstantopoulos and Bloch 2005; Linke 2008). Many proteins were detected in the Z-disc that bind to α-Actinin, including FATZ/calsarcin/myozenin, Myotilin, Actinin-associated LIM Protein (ALP), ZASP/Cypher/Oracle, Muscle Lim protein (MLP), Nebulette/Lasp-2, Myospryn, Myomaxin, Myopalladin, Enigma homologue (ENH), CLP36/Elfin/CLIM1, and Zyxin (Durham, Brand et al. 2006; Frank, Kuhn et al. 2006; Huang, Brand et al. 2006; Linke 2008). These sarcomeric proteins typically interact with more than one partner to form an intricate protein network. Meanwhile, several Z-disc components bind to membrane proteins and link the Z-disc to membrane structures such as the transverse-tubule, costamere, and sarcoplasmic reticulum, whereas others bind to signaling molecules such as Calcineurin, Protein kinase C (PKC), Protein kinase D (PKD), and RhoA (Frank and Frey 2011; Knoll, Buyandelger et al. 2011; Bennett 2012). Transcription factors or cofactors such as Cardiac ankyrin repeat protein (CARP) and MLP are also found within the Z-disc and may translocate to the nuclei and regulate gene transcription (Pyle and Solaro 2004; Frank, Kuhn et al. 2006; Hoshijima 2006). In summary, the discovery of these new proteins indicates that the sarcomere assembly process is much more complicated than originally thought. To unify different models of sarcomere assembly and to decipher the relationship between sarcomere structural changes and pathogenesis in the heart, a systematic genetic study of the sarcomeric genes, especially in vivo, is needed.
Techniques to Study Sarcomere Assembly in Zebrafish
Early studies of myofibrillogenesis were conducted using an in vitro cell culture system. Despite the convenience of high-resolution imaging and easy molecular manipulation, significant limitations of this system have been recognized. For example, 3-dimensional analysis of cell-cell communication that is crucial for sarcomere assembly is difficult to be recapitulated faithfully. Instead of examining de novo sarcomere assembly, a process of reassembling sarcomere components has been studied (Gregorio and Antin 2000; Rudy, Yatskievych et al. 2001; Russell, Curtis et al. 2010). The mouse has been the major whole-animal model for genetic studies of sarcomere assembly because of the elegant physiology analysis tools available. However, its in utero development means that embryos are less accessible for phenotyping the sarcomere assembly processes. A functional circulation system is crucial for survival; therefore, secondary defects of a dysfunctional circulation system complicate the interpretation of genetic studies in mice. To address these shortcomings, other animal models have been explored for sarcomere assembly studies, including the chicken and quail ex vivo culture systems (Rudy, Yatskievych et al. 2001; Du, Sanger et al. 2003; Du, Sanger et al. 2008). However, despite the gain of embryonic accessibility, the absence of genetic tools in these avian models prevents systematic genetic studies of sarcomere assembly.
In the past 2 decades, the zebrafish (Danio rerio) has become a popular model organism for studying embryogenesis (Driever, Stemple et al. 1994; Ingham 1997; Grunwald and Eisen 2002), including cardiac development (Staudt and Stainier 2012; Tu and Chi 2012) and myofibrillogenesis in skeletal muscle (Sanger, Wang et al. 2009; Raeker, Shavit et al. 2014). The advance of novel gene manipulation techniques and the experimentally accessible embryos of zebrafish offer several advantages for studying sarcomere assembly. First, zebrafish embryos are transparent and develop ex utero. Myofibrillogenesis occurs in a single layer of cardiomyocytes, which is ideal for live observation, immunostaining, and fluorescent-tagged imaging (Schoenebeck and Yelon 2007). Second, a collection of genetic tools has been developed in zebrafish to conduct either gain-of-function or loss-of-function studies. Third, zebrafish mutant embryo can survive for several days without a functional circulation system. Because of their small size, oxygen diffuses into their bodies in quantities sufficient to support early development (Burggren and Pinder 1991). This unique feature is beneficial for studying sarcomeres because disruption of sarcomere assembly typically results in severe defects in the cardiac system. Below, we summarize major experimental techniques offered by zebrafish to study sarcomere assembly.
Techniques Used to Generate Loss-Of-Function Mutants
Mutagenesis Screening
The completion of 2 large-scale mutagenesis screens in 1996 was a milestone needed to establish zebrafish as a new vertebrate model (Haffter, Granato et al. 1996; Stainier, Fouquet et al. 1996). Using N-ethyl-N-nitrosourea (ENU) as a chemical mutagen, approximately 695 stable mutants were identified, of which 58 had cardiac phenotypes (Chen, Haffter et al. 1996; Stainier, Fouquet et al. 1996). Positional cloning identified causative genes for pickwick/pik and silent heart/sil as titin and cardiac troponin T, respectively (Sehnert, Huq et al. 2002; Xu, Meiler et al. 2002), and this opened the door for genetic studies of cardiac sarcomere assembly using the zebrafish model.
In addition to chemical mutagens, virus-based vectors have been explored as a method of conducting insertional mutagenesis screens. Because of the exogenous DNA of viral vectors, the cloning of the insertion loci is efficiently conducted by using polymerase chain reaction (PCR)–based techniques. Indeed, among 525 mutants with recessive embryonic lethal mutations identified from a large-scale virus-based screen, the majority have been cloned (Amsterdam, Burgess et al. 1999; Amsterdam, Nissen et al. 2004).
The other insertional mutagen that has been successfully developed is based on transposon vectors (Kawakami, Takeda et al. 2004). The higher cargo capacity of transposon-based vectors allows transgene of multiple DNA fragments, including promoters, splicing donors, and genes encoding fluorescent proteins. The pGBT-RP2.1 (RP2) vector is highly effective, with a typical knock-down efficiency of more than 97% (Clark, Balciunas et al. 2011; Ding, Liu et al. 2013). More than 350 mutant fish lines have been generated and cloned (Clark, Argue et al. 2012). In addition to RP2, other transposon-based systems have been reported, including the Flip-trap (Trinh le, Hochgreb et al. 2011) and FlEx-based conditional gene-trap mutagenesis systems (Ni, Lu et al. 2012), which generate conditionally mutated alleles that allow temporal- and spatial-specific manipulation of the gene. Most of these mutants and those generated via ENU and the virus are deposited in the Zebrafish International Resource Center (http://zebrafish.org/zirc/home/guide.php) or distributed via their own web-based platform (www.zFishbook.org; http://kawakami.lab.nig.ac.jp/ztrap/; http://www.fliptrap.org/static/index_new.html) (Varshney, Huang et al. 2013; Varshney, Lu et al. 2013) to enable sharing of these genetic resources with the research community.
Morpholino Technology
Morpholinos are modified antisense oligonucleotides that effectively knock down targeted gene expression by either blocking translation or splicing pre-mRNA. Zebrafish embryos develop ex utero from a single fertilized cell; therefore, morpholinos can be injected at the single-cell stage, which allows effective distribution to every cell to achieve whole-body knock down (Nasevicius and Ekker 2000). Morpholino technology has been widely used in loss-of-function studies, including many sarcomeric genes. However, we note that morpholinos are a transient gene knock-down technology. The dilution of morpholinos after multiple rounds of cell division causes them to lose their knock-down capacity after approximately 5 days postfertilization (dpf). Similar to other antisense technologies, the injection of morpholinos, especially at higher doses, leads to nonspecific adverse effects (eg, activation of p53 and induction of apoptosis) that complicate interpretation of the phenotype. Therefore, the specificity of morpholinos needs to be carefully determined, usually by injecting a second morpholino to confirm the phenotype, by conducting rescue experiments with co-injection of mRNA, or by partially eliminating secondary defects with co-injection of a morpholino against p53 (Robu, Larson et al. 2007).
TILLING Technology
Targeting induced local lesions in genomes (TILLING) technology enables the identification of stable gene-specific point mutants in zebrafish using high-throughput screening (Wienholds, van Eeden et al. 2003; Moens, Donn et al. 2008; Kettleborough, Bruijn et al. 2011). Building on its success in zebrafish screening, ENU mutagenesis can be used to generate a randomly mutagenized zebrafish library that will be sufficient to cover most genes in the genome. Gene-specific primer pairs are used to screen mutations in the targeted gene via PCR using DNA templates generated from either sperm or fin. This technology has been successfully implemented in large research centers, including the Sanger Institute in the UK and the Fred Hutchinson Cancer Research Center. More than 11,000 mutants covering 45% of the genome have been generated and are available to the research community (Kettleborough, Busch-Nentwich et al. 2013).
TALENs and CRISP-Cas9 Technology
The lack of genetic tools to generate targeted gene knock-outs in a typical laboratory had been a bottleneck in the zebrafish model for many years. The recent development of zinc-finger nucleases (ZFN) (Meng, Noyes et al. 2008), transcription activator–like effectors nucleases (TALENs), and clustered, regularly interspaced, short palindromic repeats (CRISPR)–associated (Cas) systems effectively addressed this challenge. These 3 systems all contain endonucleases that incorporate a sequence-specific DNA-binding domain to recognize and cleave a precise genomic locus. To generate mutations in a targeted gene using TALENs technology, 2 TALEN constructs that recognize sequences surrounding the targeted locus are generated and co-injected, allowing the FokI nuclease from the 2 constructs to form an activated dimer and induce DNA double-strand breaks in the targeted locus (Christian, Cermak et al. 2010; Campbell, Hartjes et al. 2013). In most cases, the double-strand breaks are repaired via the nonhomologous end-joining mechanism, an intrinsically error-prone process that often results in small insertions or deletions (or both) at the target site that disrupt gene function. The high efficiency of TALEN in producing loss-of-function mutants has been demonstrated in zebrafish (Huang, Xiao et al. 2011; Sander, Cade et al. 2011; Dahlem, Hoshijima et al. 2012). Moreover, double-strand breaks can also be repaired by a homologous recombination–based mechanism. Using either short single-strand oligonucleotides or long double-strand DNA molecules as templates, sequence tags and gene replacements can be introduced into a specific genomic locus (Bedell, Wang et al. 2012; Wefers, Meyer et al. 2013). Further development of these genome-editing tools is being actively researched and will significantly affect zebrafish as a model for genetic studies of sarcomere assembly.
Techniques for Studying Gene Overexpression
Transient Injection
Similar to Xenopus, ectopic expression of a gene can be achieved by the injection of either DNA or mRNA into zebrafish embryos at the single-cell stage. These transient injection assays can be used to conduct rescue experiments, to prove the specificity of morpholinos, to reveal subcellular expression of the protein of interest (if tagged with a fluorescent protein), and to determine the consequences of overexpressing a mutated sarcomeric gene. DNA with a circular or linear format can be injected. Cardiac-specific expression can be achieved by cloning a cardiac-specific promoter before the gene of interest. Several heart-specific promoters have been identified, including a promoter from cmlc2 that drives cardiomyocyte-specific expression (Huang, Tu et al. 2003), a promoter from titin that drives both cardiomyocyte and somite expression (Yang and Xu 2012), and a promoter from vmhc or amhc that drives chamber-specific expression (Zhang and Xu 2009; Zhang, Han et al. 2013). Among these promoters, the titin promoter drives earliest-onset gene expression in cardiomyocytes, which is important for studying earlier stages of sarcomere assembly. Of note, DNA plasmid injections typically lead to mosaic expression. This unique feature can be leveraged to conduct rescue experiments because the surrounding cells without ectopic gene expression serve as ideal controls (Huang, Zhang et al. 2009). In contrast to DNA injection, mRNA injection leads to ubiquitous expression in the whole zebrafish embryo.
Transgenic Technology
Transgenic technology has been widely used in mice for functional studies of sarcomeric genes, especially genes with disease-causing mutations (Robbins 2000). In zebrafish, the transgenic rate was significantly improved after the introduction of transposon-based vectors such as Sleeping Beauty (SB) (Davidson, Gratsch et al. 2009), Tc3 (Raz, van Luenen et al. 1998), and Tol-2 (Kawakami, Takeda et al. 2004). When zebrafish embryos are co-injected with a Tol2-based vector and transposase-encoding mRNA, more than 50% of the F0 offspring are founders, making zebrafish possibly the easiest vertebrate model for generating a stable transgenic line.
To facilitate the cloning process, a recombination-based cloning system such as the Tol2Kit can be modified (Kwan, Fujimoto et al. 2007). Various promoters can be cloned into the 5′ entry vector, and fragments encoding various fluorescent proteins or other sequence tags can be cloned into a 3′ entry vector. After the gene of interest is cloned into the middle entry vector, 4-fragment recombination can be achieved by incubating a 5′-entry clone, a middle clone, a 3′-entry clone, and a Tol2-containing destination vector. A single cloning step with a recombinase can produce the final construct that is ready for injection. This versatile, recombination-based system can be used to swap different promoters and different tags to study sarcomeric genes of interest.
Techniques for Annotating Gene and Protein Expression
Define the mRNA Expression Pattern via In Situ Hybridization
Whole-mount in situ technology can be used to reveal the tissue-specific expression pattern of a gene of interest and to show the onset of gene transcription during cardiogenesis. Although the former is crucial to determine homologs or isoforms that function in the heart vs somites, the latter aids interpretation when different sarcomeric proteins appear in cardiomyocytes and participate in the sarcomere assembly process (Thisse and Thisse 2008). Because of their high fecundity and ex utero development, zebrafish embryos are readily available in large quantities for whole-mount in situ experiments.
Define Protein Expression via Immunostaining
Immunostaining is one of the most important experimental tools available to assess the sarcomere assembly process. Whole-mount embryos can be stained to label the heart, but poor penetration of antibodies prevents generation of high-quality images. To address this technical challenge, we developed a protocol in which the heart is dissected from the body and then stained on the surface of a microscope slide (Yang and Xu 2012). The improvement of antibody penetration allowed us to define different stages of sarcomere assembly in a developing zebrafish heart (Huang, Zhang et al. 2009).
Define Protein Subcellular Expression via Fluorescence-Tagged Imaging Technology
As detailed later in this article, a panel of antibodies against mammalian sarcomeric proteins has been confirmed to work in zebrafish. However, antibodies for many other sarcomere proteins are not yet available in zebrafish. An alternative approach is to generate fluorescent protein–tagged fusion proteins that reflect the assembly behavior of the endogenous proteins. This approach has been shown to work effectively in cell culture systems (Dabiri, Turnacioglu et al. 1997). In zebrafish, green fluorescent protein (GFP)–tagged Actn2 or Actn3 have been reported to faithfully show Z-disc localization of actinin proteins (Lin, Swinburne et al. 2012; Yang and Xu 2012). Moreover, a Tg(cmlc2:Cypher-EGFP) transgenic line was generated to assess the Z-disc assembly of Cypher.
Techniques for Annotating Mutant Phenotypes
Annotation of Sarcomere Assembly Defects via TEM and Immunostaining
TEM was the first technique used extensively to assess sarcomeric phenotypes in a zebrafish heart (Wanga, Eckberg et al. 2001; Sehnert, Huq et al. 2002; Xu, Meiler et al. 2002). We recommend treating hearts with relaxation buffer to ensure that the samples are imaged at the same contraction stage. Compared with TEM, immunostaining has been less frequently used in the phenotypic analysis of sarcomeric mutants, partially because of the penetration problems. In fact, immunostaining using antibodies against different sarcomeric substructures can yield more information on sarcomeric defects compared with TEM alone (Huang, Zhang et al. 2009; Yang and Xu 2012).
Annotation of Cellular Changes via Immunostaining and Transgenic Fish Lines
To access the morphometric changes of cardiomyocytes during cardiogenesis, the cell border of individual cardiomyocytes can be revealed by immunostaining using β-catenin or Zn5 antibodies. Transgenic fish lines with membrane-tagged fluorescent proteins have also been used, including Tg(titin:mGFP) and Tg(myl7:mkate-caax) with membrane-tagged GFP to define cell membranes (Lin, Swinburne et al. 2012; Yang and Xu 2012). The nuclei of cardiomyocytes are revealed either by immunostaining using Mef2 antibody or transgenic fish lines using nuclei-tagged fluorescent protein, including Tg(cmlc2:nDsRed) (Rottbauer, Saurin et al. 2002). The proliferation of cardiomyocytes can be measured by immunostaining using antibodies against proliferating cell nuclear antigen (PCNA) or Phosphorylation of histone H3 (pH3) or transgenic fish lines such as Tg(ccnb1:EGFP) (Kassen, Thummel et al. 2008). Apoptosis can be assessed by either TUNEL staining or transgenic fish lines (UAS-secA5-YFP) (van Ham, Mapes et al. 2010).
Annotation of Cardiac Functions
As an in vivo vertebrate animal model, zebrafish has a major advantage because of its ability to quantify cardiac function changes that accompany the sarcomeric defects. Because of the transparency of fish embryos, the heartbeat of a living animal can be documented directly under a dissecting microscope. Cardiac function indices, including heart rate, ejection fraction, and myocardial wall velocity, can be calculated by analyzing video clips (Denvir, Tucker et al. 2008; Hoage, Ding et al. 2012).
Summary
Zebrafish are a mature vertebrate model with a collection of convenient genetic tools that enable the study of sarcomere genes via loss-of-function and gain-of-function manipulations. Mutants for most genes are already available for experimental investigation. The resultant phenotypes at the organ and cellular levels can be uncovered by using this whole-animal model for genetic studies of sarcomere assembly.
Zebrafish With Sarcomere Gene Mutations
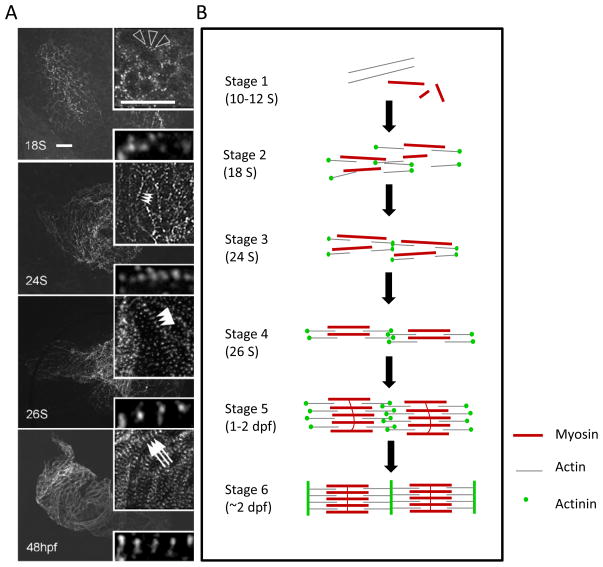
The improved antibody-staining technique enables identification of the 6 stages of sarcomere assembly in a zebrafish heart (Figure) (Huang, Zhang et al. 2009; Yang and Xu 2012; Yang and Xu 2012). Of note, the timeline reflects the process in cardiac muscle, not skeletal muscle. During the first stage at approximately 10–12 somites (S), thin filaments appear as a perimembrane network, while thick filaments assemble independently into rodlets of various lengths. At the second stage (18 S), these thin and thick filaments assemble to form a rudimentary sarcomere structure, in which α-actinin integrates into Z-bodies, appearing as irregular spaced dots. The sarcomere at this stage resembles premyofibrils, as previously described (Dabiri, Turnacioglu et al. 1997). At the third stage (24 S), Z bodies form regular spaced dots, with shorter distance between neighboring dots (1.2 μm). The fourth stage corresponds to the initiation of heart contraction at 26 S. The distance between Z-bodies increases (1.9 μm) and becomes consistent, resembling nascent myofibrils. In this fourth stage, the Z-bodies fuse laterally to form longer Z-bands (1 to 2 dpf) at the fifth stage and then align with each other to form well-registered sarcomeres at the sixth stage at approximately 2 dpf. Genetic studies of actn2 suggest the fifth stage as an intermediate step because depletion of this major Z-disc protein is required for lateral alignment but not lateral fusion of the Z-discs (Yang and Xu 2012). This 6-stage sarcomere assembly process provides the structural basis for the initiation of peristaltic contraction at 26 S and the switch to a much stronger and more coordinated contraction at 2 dpf. Accompanied with the sarcomere assembly process, the zebrafish heart transforms from a tube-like structure at 26 S into a 2-chambered heart with a ventricle and atrium. This baseline characterization of sarcomere assembly sets the stage for the interpretation of phenotypes in sarcomere mutants. Below, we review known sarcomeric genes with cardiac expression that have been studied in zebrafish.
Figure. Sarcomere Assembly in a Zebrafish Heart.
A, Immunostaining of a zebrafish heart using anti-Actinin antibody on embryos at the 18 somite (S), 24 S, 26 S, and 48 hours postfertilization (hpf) stages, respectively. Scale bar indicates 20 μm. Arrows and arrowheads indicate Z-bodies and Z-discs at different developmental stages. Images adapted from (Huang, Zhang et al. 2009). B, Schematic of the 6 stages of sarcomere assembly in the zebrafish heart. Stage 1, around 10–12 S, the actin filament is assembled along the cell membrane while the myosin filaments independently form rodlet structures. Stage 2, around 18 S, actin filaments and thick filaments are integrated where Actinin is randomly located as irregular spaced dots. Stage 3, around 24 S, the sarcomere becomes more organized, with consistent shorter length. Stage 4, around 26 S, the striated sarcomere becomes elongated. Stage 5, 1–2 days postfertilization (dpf), myofibrils are bundled through lateral fusion of adjacent myofibrils. Stage 6, around 2 dpf, mature sarcomeres appear after a lateral registration process, as reflected by the formation of well-aligned Z-discs.
Thin Filament
The thin filaments consist of polarized Actin polymers, Tropomyosin, and Troponin. The assembly process of thin filaments in a zebrafish heart is revealed by either immunostaining with an antibody against Tropomyosin (CH1) or by phalloidin staining of actin filaments (Huang, Zhang et al. 2009). The mutants and morphants described below have been generated and studied.
Actin
Zebrafish have at least 4 sarcomeric actin genes: acta1a, acta1b, acta2, and actc1a. Two actin mutants have been identified, one is the cardiofunk (cfk−/−) mutant affecting acta1b, generated via ENU mutagenesis screening (Bartman, Walsh et al. 2004); the other is s434, which affects actc1a (Glenn, McKane et al. 2012). The R177H mutation in cfk−/− and the Y169S mutation in s434 both affect polymerization of actin (Wen and Rubenstein 2003; Bartman, Walsh et al. 2004), but the sarcomere structure is differentially affected. The cfk mutant has no visible sarcomere defects, whereas the s434 mutant has severe sarcomere defects, including loss and shortening of myofibrils. A further experiment indicated that acta1b and actc1a have partially redundant functions in the heart (Glenn, McKane et al. 2012). Both mutants show cardiac dilation, blood regurgitation, and cardiac cushion defects.
Troponin T
Calcium wave regulation of heart contractions is conferred by the troponin complex that consists of Troponin C, Troponin I, and Troponin T. Of the 3 troponin T genes in zebrafish (tnnt1, tnnt2, and tnnt3), tnnt2a is most likely the only homolog that is expressed in the heart. The mutant alleles of silent heart (sih) were identified from an ENU mutagenesis screen; these alleles affect either a splicing acceptor in intron 2 or the translation start site of the tnnt2a gene (Sehnert, Huq et al. 2002). In addition, 2 morpholinos against the splicing donor of either exon 12 (Huang, Zhang et al. 2009) or exon 13 (Becker, Deo et al. 2011) were reported to mimic the C-terminus truncation mutation in humans with cardiomyopathy.
In contrast to the well-aligned and laterally fused thin and thick filaments in wild-type fish, only a few scattered myofibrils were noted in sih fish by ultrastructural analysis (Sehnert, Huq et al. 2002). More information regarding the sarcomere assembly defects has been revealed by immunostaining studies. Without Troponin T, both Tropomyosin and Troponin I are detached from the thin filaments. The striation of thin filaments, thick filaments, and Z-discs are all absent (Huang, Zhang et al. 2009). These data revealed an essential function of Troponin T in actin filament assembly in the first stage of sarcomere assembly, and the loss of Troponin T prevented the sarcomere assembly from progressing to the second stage, ie, the formation of premyofibrils. The C-terminus of Troponin T is indispensable for this function because both thin and thick filaments appear striated in embryos injected with the morpholino against the exon 12 splice donor (e12 morphants). The regular spaced periodic Z bodies can be detected, suggesting that the N-terminus of Troponin T is capable of driving sarcomere assembly to form premyofibril structures (Huang, Zhang et al. 2009).
The distinct sarcomere defects in sih and e12 morphants explain the different phenotypes in cardiac function and morphologic changes. Although heart beating and blood circulation were not noted in sih embryos, weak contraction was still detectable in e12 morphants. In contrast to normal ventricular chamber size in sih fish, e12 morphant fish had significantly smaller ventricles. This suggests that Troponin T truncated at the C-terminus might exert dominant functions via activating unique signaling pathways in e12 morphant fish (Huang, Zhang et al. 2009). Supporting this possibility, microarray studies of embryos injected with the morpholino against the exon 13 splice donor showed a phenotype that resembled HCM in human patients (Becker, Deo et al. 2011).
Troponin C
Among the 3 known troponin C homologs in zebrafish, only troponin C1a (tnnc1a) exhibits cardiac ventricle–specific expression; tnnc1b and tnnc2 are expressed in skeletal muscles. Two mutations in the tnnc1a gene were reported in silent partner (sil) mutant fish that were identified from an ENU mutagenesis screen (Sogah, Serluca et al. 2010). The sil m656 allele has a point mutation in the splicing donor in the second intron, and the sil sk25 allele contains a deletion in this locus. Both mutations significantly attenuate troponin C expression and sequentially affect ventricle myosin heavy chain (vmhc) expression, resulting in consistent sarcomere defects. The sarcomere manifests as sparse and poorly organized structures under electron microscopy. Consistent with the ventricle-specific expression pattern of tnnc1a, depletion of Tnnc1a results in a silent ventricle but a beating atrium. Consequently, the ventricle becomes compacted and the atrium appears enlarged. Despite undetectable cardiac expression of tnnc1b in situ, co-injection of a tnnc1b morpholino and tnnc1a morpholino results in complete silence of both chambers, suggesting that tnnc1b has residual expression in the atrium and is responsible for sarcomere assembly in this chamber. Together, these genetic studies underscore a crucial function of troponin C in sarcomere assembly, presumably via supporting thin filament assembly. Of note, immunostaining studies that could reveal the precise function of Troponin C in sarcomere assembly have yet to be conducted.
Thick Filament
The assembly process of thick filaments in the zebrafish heart can be revealed by immunostaining using myosin-specific antibodies, including MF20 for the ventricle, S46 for the atrium, and F59 for both cardiac chambers (Berdougo, Coleman et al. 2003; Auman, Coleman et al. 2007; Huang, Zhang et al. 2009). The mutants and morphants described below have been generated and studied.
Myosin Heavy Chain
Several myosin heavy-chain homologs have been reported in the zebrafish genome Zv9. Mutants have been generated for myh7 and myh6, also named ventricle-specific myosin heavy chain (vmhc) and atrium-specific myosin heavy chain (amhc), respectively. A nonsense mutation in myh7 was found in the half-hearted (haf) mutant that results in truncated Vmhc (Auman, Coleman et al. 2007), and a nonsense mutation in myh6 was found in the weak atrium (wea) mutant that results in the deletion of C-terminal amino acids of Amhc (Berdougo, Coleman et al. 2003). In both mutants, a significantly reduced myofibril number is noted, supporting the essential function of myosin in sarcomere assembly. Detailed studies of sarcomeric defects have not been conducted to date.
Consistent with sarcomeric defects in different chambers, contractility is completely lost in the ventricle of haf mutants and in the atrium of wea mutants. Interestingly, the cell size of ventricular cardiomyocytes is increased in haf mutants but reduced in wea mutants. Further studies have uncovered an important function of heart contraction in modulating heart morphogenesis (Auman, Coleman et al. 2007).
Essential Myosin Light Chain
By screening the expression of a panel of essential myosin light chain (ELC) homologs in zebrafish, cardiac myosin light chain1 (cmlc1) was found to be specifically expressed in the heart. Genetic studies of cmlc1 were conducted using morpholinos (Chen, Huang et al. 2008). The mutant lazy susan (laz), identified from an ENU mutagenesis screen, contains a nonsense mutation resulting in the truncation of 11 amino acids in the C terminus of Cmlc1 (Meder, Laufer et al. 2009).
In cmlc1 morphants, myosin still assembles into rodlet structures, which integrate with thin filaments. Striated thin filaments and Z-discs can be observed by electron microscopy. However, the myofibrils appear narrow because lateral fusion does not occur. The length of individual sarcomeres becomes longer and irregular. Together, these observations suggest that ELC is not essential for the initial assembly of thick or thin filaments or for their initial interaction. Instead, ELC has an important role in the later lateral fusion step (stage 4). The 11 amino acids in the C-terminus are indispensable for this ELC function because the sarcomere structure appears normal in laz fish.
In both laz mutants and cmlc1 morphants, cardiac contractility is severely reduced, suggesting an essential function of Cmlc1 and its C terminus in regulating heart beating. An enlarged ventricular chamber was noted in cmlc1 morphants; however, a small and severely collapsed ventricular chamber was noted in laz mutant fish.
Regulatory Myosin Light Chain
Among several regulatory myosin light chain homologs, cardiac myosin light chain 2 (cmlc2) is specifically expressed in the heart. In addition to a morpholino study, the function of cmlc2 has also been studied in the tell tale (tel) mutant, which was identified in an ENU mutagenesis screen that affects a splice donor site and leads to early truncation of the Cmlc2 protein (Rottbauer, Wessels et al. 2006; Chen, Huang et al. 2008).
As revealed by TEM studies, only Z-bodies and immaturely assembled thin filaments are noted in tel mutants. In a cross-sectional view, the hexagonal lattice of thin and thick filaments is disrupted, and only scattered thin filaments are noted (Rottbauer, Wessels et al. 2006). An immunostaining study of both the tel mutant and cmlc2 morphants showed that the early step of sarcomere assembly is not disturbed (Chen, Huang et al. 2008). Myosin forms irregular spaced dots or continuous lines along myofibril; however, Actin still forms striated and periodic dots. Consequently, the sarcomere length in cmlc2 morphants appears shorter, unlike the longer sarcomere in cmlc1 morphants. Moreover, morpholino studies of myosin light chain kinase (Cardiac-MLCK) suggest that phosphorylation of cmlc2 is crucial for its function in sarcomere assembly (Seguchi, Takashima et al. 2007).
Heart contraction is compromised in cmlc2 morphants and tel mutants. In the tel mutant, cardiomyocytes in the ventricle and atrium are completely silent, and only the cardiomyocytes in the atrioventricular junction continue to contract rhythmically (Rottbauer, Wessels et al. 2006). Small ventricle chamber size was reported in cmlc2 morphant fish, unlike the enlarged size in the cmlc1 morphant (Chen, Huang et al. 2008).
Myosin-Binding Protein C3
Only one gene for myosin-binding protein c3 (mybpc3) is present in zebrafish, and it is evolutionarily conserved with the human homolog. However, 3 different transcripts of mybpc3 are dynamically expressed during embryogenesis, and all are expressed in the heart and slow muscle. Morpholinos have been used to knock down mybpc3 expression (translational blockage). A specific cardiac phenotype was noted, but a detailed analysis of the sarcomere structure was not conducted. Both cardiac chambers, but especially the atrium, were enlarged. The ventricle wall thickness was reduced, and the ventricular diastolic relaxation velocity was reduced. The electrophysiologic phenotype in morphants, including ventricular action potential duration, resembles the phenotype in human patients with heart failure (Chen, Pai et al. 2013).
Titin Filament
In contrast to the single titin gene in mammals, the zebrafish genome contains 2 titin homologs that are localized in tandem on chromosome 9 (Seeley, Huang et al. 2007). Most of the functional domains are encoded by ttna and ttnb, except the Novex3 domain that is encoded only by ttna. Morpholinos against either ttna or ttnb cause specific embryonic lethal phenotypes, indicating that these genes are not functionally redundant (Seeley, Huang et al. 2007). The injection of a morpholino against the cardiac-specific N2B exon of ttna results in cardiac defects, whereas injection of a morpholino against the N2A exon of ttna results in somite phenotypes (Xu, Meiler et al. 2002). A group of mutants named pickwick (pik), herzschlag, and runzel have been identified from ENU mutagenesis screens (Xu, Meiler et al. 2002; Steffen, Guyon et al. 2007; Myhre, Hills et al. 2013). A nonsense mutation was identified in the N2B domain of ttna from one of the pik alleles (Xu, Meiler et al. 2002). Due to the extremely large size of the ttna gene, mutations in herzschlag, runzel and other pik alleles have not been identified. From epitope mapping, the mutation in the herzschlag was suggested to be between exons 27 and 103, which presumably truncates the A-band rod domain of Ttna (Myhre, Hills et al. 2013).
Both ttna and ttnb are expressed in the heart; ttna is by far the earliest sarcomere gene that is transcribed in the heart, with a transcript detectable as early as the 5 S stage (using whole-mount in situ staining). This is earlier than the onset of the vmhc transcript at 10 S, the cmlc1/cmlc2 transcripts at 15 S, and the ttnb transcript at 30 hpf (Seeley, Huang et al. 2007; Chen, Huang et al. 2008). Therefore, ttna likely has a more important role than ttnb in the heart. Indeed, significantly reduced myofibril number and disrupted sarcomere structure were noted under TEM in pikwick mutants that affect ttna, but this was not observed in morphants affecting ttnb. Although Z-bodies and myosin filaments still form, they fail to laterally fuse and fail to form Z-discs and A-bands in pikwick fish (Seeley, Huang et al. 2007). These observations support the hypothesis that titin is required for sarcomere assembly, including the lateral fusion of Z-discs and A-bands. Of note, less severe sarcomeric phenotypes were noted in somites of the herzschlag mutant (Myhre, Hills et al. 2013). Stable null mutations are needed for both ttna and ttnb for more comprehensive genetic studies of titin.
As a consequence of the primary sarcomere defects, the cardiac contractility of pickwick fish is significantly reduced. Individual cardiomyocytes appear thin and stretched, resembling dilated cardiomyopathy (Xu, Meiler et al. 2002).
Z-Disc
The Z-disc defines the border of a sarcomere, where the thin filaments and Titin filaments are anchored. The Actinin antibody is widely used to mark the Z-disc, which conveniently defines different stages of sarcomere assembly in a zebrafish heart. The transformation of irregular spaced and shorter Actinin-immunopositive dots to periodically spaced dots at approximately 22 S reflects the advance of sarcomere assembly from premyofibrils (stage 2) to nascent myofibrils (stage 3) (Huang, Zhang et al. 2009). Later, the lateral expansion of Actinin-immunopositive Z-bodies to form longer Z-discs represents the advance of the sarcomere assembly from stage 3 to stage 5 (Figure). A large number of Z-disc genes have been studied genetically in zebrafish, as summarized below.
α-Actinin
As a major component of the Z-disc, α-Actinin binds a large number of proteins in the Z-disc, including Actin via its N-terminal binding domain and Titin via its central 4 spectrin repeats (Young, Ferguson et al. 1998; Clark, McElhinny et al. 2002). Together with its early appearance within the Z-disc, Actinin is postulated to function as a cross-linker, with an essential function in Z-disc assembly. Among the 4 α-actinin homologs in zebrafish (actn1, actn2, actn3a, and actn3b), only actn2 is expressed in the heart (Holterhoff, Saunders et al. 2009; Gupta, Discenza et al. 2012; Yang and Xu 2012). Actn2 protein can be detected as irregular spaced dots at approximately 18 S in a developing zebrafish heart, before detection of 2 other Z-disc proteins, Tcap and Mlp (Zhang, Yang et al. 2009). Genetic studies of actn2 have been conducted with several morpholinos (Holterhoff, Saunders et al. 2009; Yang and Xu 2012).
In actn2 morphants, sarcomere assembly proceeds to stage 4. Well-defined thin filaments, thick filaments, and Z-discs can still be detected. However, Z-discs manifest as zigzagged lines or noncontinuous pieces. Therefore, genetic studies in zebrafish do not support the idea that Actn2 is required for Z-disc assembly. Rather than functioning in the early steps of sarcomere assembly, actn2 appears to be specifically needed for the lateral alignment of Z discs.
Regarding cardiac function, Actn2 deficiency attenuates the shortening fraction without disturbing the heart rate. The ventricle chamber size is significantly reduced, but this phenotype can be rescued by cessation of contraction, supporting the importance of mechanical force in regulating heart morphogenesis (Yang and Xu 2012).
Cypher
Cypher has been shown to form a protein complex with Actinin and Titin and to function as a linker-strut supporting the Z-disc structure (Sheikh, Bang et al. 2007). Genetic studies in the cypher knock-out mouse uncovered severe Z-disc defects, including a wide and zigzagged Z line, suggesting the function of cypher in Z-disc maintenance (Zhou, Chu et al. 2001). A recent study in a Drosophila mutant confirmed the important role of cypher in adult Z-disc stability and pupal myofibril assembly (Katzemich, Liao et al. 2013). A cypher ortholog was identified in zebrafish (van der Meer, Marques et al. 2006). In total, 13 isoforms from this gene locus have been detected as a consequence of alternative splicing, and isoforms 2α, 3α, 3β, and 3γ are expressed in the heart. Morpholino studies were designed to knock down these isoforms of cypher. Consistent with sarcomere defects in mouse cypher knock outs, deformed somites and pericardiac edema were found in cypher morphant fish (van der Meer, Marques et al. 2006). In addition to reduced cardiac contraction, a reduced ventricle chamber size with a thinner ventricular wall was noted.
Obscurin
The obscurin gene encodes a giant sarcomere protein that is detected in both Z-discs and M-lines. Based on its interaction with Titin in sarcomeres, as well as an interaction with Small Ankyrin-1 in sarcolemma, Obscurin is hypothesized to have a role supporting sarcomere structure and aligning sarcomeres with the sarcoplasmic reticulum (Kontrogianni-Konstantopoulos and Bloch 2005). There is a single obscurin ortholog in zebrafish, and 2 morpholinos have been designed to knock down zebrafish obscurin. An ATG morpholino blocks the translation of full-length obscurin, whereas the other splicing-donor morpholino specifically deletes the RhoGEF domain at the C-terminus (Raeker, Su et al. 2006; Raeker, Bieniek et al. 2010). Full-length Obscurin is required for lateral alignment between adjacent myofibrils but not for the early steps of sarcomere assembly, as indicated by impaired lateral alignment of the adjacent myofibrils and disorganized sarcoplasmic reticulum but properly striated thin filaments, thick filaments, and Z-discs. Moreover, a normal and well-aligned sarcomere structure was detected in embryos injected with the splicing morpholino, suggesting that the structural property but not the RhoGEF activity of Obscurin is important for sarcomere assembly. Detailed analysis of the splicing donor morphant suggests a function of the RhoGEF domain in the formation of the intercalated disc. Despite different sarcomere structural defects, both morphants have similar cardiac morphologic and functional defects, including cardiac edema, reduced heart rate, and reduced shortening fraction.
Nexilin
Nexilin is a novel Z-disc protein that was discovered and genetically characterized in zebrafish. The injection of a translation-blocking morpholino results in sarcomere defects (Hassel, Dahme et al. 2009). Both thin and thick filaments still form but have a disrupted structure that frequently detaches from irregular spaced and blurred Z discs. Ruptured sarcomeres were increasingly noted when the fish developed from 2 dpf to 3 dpf, suggesting an essential role of Nexilin in the maintenance (rather than in the initial assembly) of the sarcomere. Further studies revealed a severe sarcomere disruption in stretched skeletal muscle, suggesting a function of nexilin in protecting the sarcomere from mechanical trauma.
As a consequence of the sarcomere defects, nexilin morphants have dilated atriums and ventricles, as well as reduced shortening fractions. These phenotypes prompted the search of mutations in NEXILIN from human patients lacking mutations in known cardiomyopathy-causing genes. Indeed, mutations in NEXILIN have been found in patients with dilated cardiomyopathy; as such, NEXILIN was then defined as a novel causative gene (Hassel, Dahme et al. 2009). Z-disc defects similar to those observed in nexilin morphant fish were also noted in these human patients. Notably, overexpression of zebrafish nexilin with a human mutation also leads to Z-disc damage and heart failure in zebrafish embryos.
Cytoskeletal Heart-Enriched Actin-Associated Protein
Cytoskeletal heart-enriched actin-associated protein (Chap) is a newly identified Z-disc protein that can translocate to the nucleus (Beqqali, Monshouwer-Kloots et al. 2010). Two chap orthologs, chap1 and chap2, are found in zebrafish and expressed in the heart. The chap1 transcript is expressed at an earlier stage and has a higher expression level than the chap2 transcript. A translation-blocking morpholino against chap1 leads to severely disrupted Z-discs, as revealed by electron microscopy. Heart looping consequently is impaired at 32 hpf, and both the atrium and ventricle broaden at 48 hpf.
Core-Binding Factor β
Z-disc localization of the core-binding factor β (Cbfβ) has been determined in both skeletal and cardiac myocytes. Depletion of full-length cbfβ in morphant fish causes thinner myofibrils, misaligned Z-discs, and misaligned intercalated discs, indicating an essential role of cbfβ in sarcomere lateral alignment and lateral fusion (Meder, Just et al. 2010).
M-Line
The M-line is located in the middle of a sarcomere and anchors the bipolar myosin bundles to form thick filaments. In zebrafish, immunostaining with a Myomesin-specific antibody shows that the M-line is assembled at approximately 18 to 22 S, when thick filaments start to interact and assemble with thin filaments (Huang, Zhang et al. 2009). The initial irregular spaced dots of Myomesin along the myofibril become periodic at 26 S, when the heart begins beating. Zebrafish have 4 myomesin genes: myom1a, myom1b, myom2, and myom3. Knocking down myom3 in slow muscle has little effect on sarcomere assembly (Xu, Gao et al. 2012). The specific myomesins expressed in the heart and their function in sarcomere assembly during zebrafish heart development remain unclear.
Zebrafish have 2 SET and MYND domain–containing protein 1 genes (smyd1a and smyd1b). The smyd1b gene encodes an M-line protein that is expressed in the heart and in skeletal muscle (Just, Meder et al. 2011). Smyd1a has similar function as Smyd1b in slow muscle but not in cardiomyocytes (Li, Zhong et al. 2013). In addition to interacting with myosin in the M-line, Smyd1b also translocates into the nucleus to exert its function as a transcriptional suppressor. A nonsense mutation in zebrafish flat line (fla) leads to a truncated and unstable Symd1b protein. Sarcomeric defects, including premature sarcomere I-Z-I structure and loss of thick filaments, were noted in fla mutants by using electron microscopy for ultrastructural analysis (Just, Meder et al. 2011). This sarcomere defect was also confirmed in fish embryos injected with a smyd1b ATG morpholino (Li, Zhong et al. 2013). As a consequence of the sarcomere defects, the heart stops beating in fla mutant fish.
Conclusion
A panel of sarcomeric genes has been studied in zebrafish, generating a wealth of information on sarcomere assembly. Although studies of genes such as cypher supported conclusions reached in previous genetic studies of mouse and Drosophila models, studies of other genes such as tnnt2, cmlc1, cmlc2, and actn2 were able to generate new knowledge on sarcomere assembly. The zebrafish is becoming an increasingly popular model to elucidate functions of novel sarcomere genes, as exemplified by studies of nexilin, chap, and cbfβ. Previously, TEM was the major and, in many cases, only technique to be used to assess sarcomeric defects, but immunostaining now is able to elicit greater detail regarding sarcomere-related phenotypes and will be an indispensable assay for future genetic studies. As an in vivo vertebrate model, the zebrafish offers a wealth of convenient genetic and phenotyping tools to elucidate gene function beyond sarcomere assembly, including cardiomyocyte morphology and cardiac function. Indeed, genetic studies of cardiofunk, haf, wea, and actn2 mutants promote use of fish embryos as an in vivo model to interrogate the functional mechanism of mechanotransduction, ie, how contraction affects cardiomyocyte remodeling (Bartman, Walsh et al. 2004; Auman, Coleman et al. 2007; Yang and Xu 2012).
One challenge in generating null mutants in zebrafish for a particular sarcomere component is the coexistence of multiple homologs of several sarcomeric genes, including those encoding actin, myosin, and titin. To elucidate the functions of these sarcomeric components, compound mutants are needed, as was done for the 3 tnnt2 homologs in zebrafish somites (Ferrante, Kiff et al. 2011). Of note, compound mutants that completely remove 1 of the 3 main filament systems (ie, thin, thick, and Titin filaments) are yet to be generated, but these will be pursued to further understand the de novo assembly of each filament and their interactions. The convenient morpholino technology will continue to provide additional insights into sarcomere assembly. However, because of its adverse effects at higher doses, the specificity of the null phenotypes of a morpholino is difficult to determine. Therefore, we anticipate that future genetic studies in zebrafish will be conducted using stable knock-out mutants.
In addition to analyzing the phenotypes of null mutations, zebrafish embryos can be a fruitful platform for investigating disease-causing mutations. As exemplified by genetic studies of tnnt2, cmlc1, titin, and obscurin, the functions of particular domains in a sarcomeric protein can be uncovered by comparing the phenotypes among mutants and morphants containing different truncation alleles (Xu, Meiler et al. 2002; Huang, Zhang et al. 2009; Meder, Laufer et al. 2009; Raeker, Bieniek et al. 2010). The feasibility of using fish embryos to study point mutations identified from humans has been proven (Hassel, Dahme et al. 2009; Sarparanta, Jonson et al. 2012). To study stable mutants, genetic tools such as TALENs and the Cas9 system are being developed and can be used to generate knock-in mutants that mimic point mutations (Bedell, Wang et al. 2012; Campbell, Hartjes et al. 2013; Hruscha, Krawitz et al. 2013).
The completed zebrafish genome opens the door for a comprehensive analysis of genes involved in sarcomere assembly. Efforts are being coordinated to generate a near-complete collection of loss-of-function mutants for every zebrafish gene. Thousands of mutants have already been deposited in the Zebrafish International Resource Center, including mutants generated by ENU and virus-based mutagenesis screens and by transposon-based insertional screens. Moreover, mutants for approximately half the genome have been generated via TILLING technology at the Sanger Institute (Kettleborough, Busch-Nentwich et al. 2013). Mutants for most genes are expected to be available to the research community within a decade. Together with the maturing phenotyping tools, the zebrafish is emerging as a premier model for genetic studies of sarcomere assembly and will facilitate understanding of the relationship among gene mutations, sarcomere structural changes, and sarcomere-based cardiac disease.
Acknowledgments
We apologize to those colleagues whose work was overlooked.
Grant sponsor: NIH/NHLBI
Grant number: HL107304, HL081753
References
- Amsterdam A, Burgess S, et al. A large-scale insertional mutagenesis screen in zebrafish. Genes & development. 1999;13(20):2713–2724. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Nissen RM, et al. Identification of 315 genes essential for early zebrafish development. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(35):12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auman HJ, Coleman H, et al. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS biology. 2007;5(3):e53. doi: 10.1371/journal.pbio.0050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartman T, Walsh EC, et al. Early myocardial function affects endocardial cushion development in zebrafish. PLoS biology. 2004;2(5):E129. doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JR, Deo RC, et al. Human cardiomyopathy mutations induce myocyte hyperplasia and activate hypertrophic pathways during cardiogenesis in zebrafish. Disease models & mechanisms. 2011;4(3):400–410. doi: 10.1242/dmm.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491(7422):114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett PM. From myofibril to membrane; the transitional junction at the intercalated disc. Frontiers in bioscience. 2012;17:1035–1050. doi: 10.2741/3972. [DOI] [PubMed] [Google Scholar]
- Beqqali A, Monshouwer-Kloots J, et al. CHAP is a newly identified Z-disc protein essential for heart and skeletal muscle function. Journal of cell science. 2010;123(Pt 7):1141–1150. doi: 10.1242/jcs.063859. [DOI] [PubMed] [Google Scholar]
- Berdougo E, Coleman H, et al. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130(24):6121–6129. doi: 10.1242/dev.00838. [DOI] [PubMed] [Google Scholar]
- Boateng SY, Goldspink PH. Assembly and maintenance of the sarcomere night and day. Cardiovasc Res. 2008;77(4):667–675. doi: 10.1093/cvr/cvm048. [DOI] [PubMed] [Google Scholar]
- Burggren WW, Pinder AW. Ontogeny of cardiovascular and respiratory physiology in lower vertebrates. Annual review of physiology. 1991;53:107–135. doi: 10.1146/annurev.ph.53.030191.000543. [DOI] [PubMed] [Google Scholar]
- Campbell JM, Hartjes KA, et al. New and TALENted genome engineering toolbox. Circulation research. 2013;113(5):571–587. doi: 10.1161/CIRCRESAHA.113.301765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JN, Haffter P, et al. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- Chen YH, Pai CW, et al. Inactivation of Myosin binding protein C homolog in zebrafish as a model for human cardiac hypertrophy and diastolic dysfunction. Journal of the American Heart Association. 2013;2(5):e000231. doi: 10.1161/JAHA.113.000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Huang W, et al. Depletion of zebrafish essential and regulatory myosin light chains reduces cardiac function through distinct mechanisms. Cardiovascular research. 2008;79(1):97–108. doi: 10.1093/cvr/cvn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Cermak T, et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186(2):757–761. doi: 10.1534/genetics.110.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KA, McElhinny AS, et al. Striated muscle cytoarchitecture: an intricate web of form and function. Annual review of cell and developmental biology. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Clark KJ, Argue DP, et al. zfishbook: connecting you to a world of zebrafish revertible mutants. Nucleic acids research. 2012;40(Database issue):D907–911. doi: 10.1093/nar/gkr957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KJ, Balciunas D, et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nature methods. 2011;8(6):506–515. doi: 10.1038/nmeth.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabiri GA, Turnacioglu KK, et al. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(17):9493–9498. doi: 10.1073/pnas.94.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem TJ, Hoshijima K, et al. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS genetics. 2012;8(8):e1002861. doi: 10.1371/journal.pgen.1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AE, Gratsch TE, et al. Use of the Sleeping Beauty transposon system for stable gene expression in mouse embryonic stem cells. Cold Spring Harbor protocols. 2009;2009(8) doi: 10.1101/pdb.prot5270. pdb prot5270. [DOI] [PubMed] [Google Scholar]
- Denvir MA, Tucker CS, et al. Systolic and diastolic ventricular function in zebrafish embryos: influence of norepenephrine, MS-222 and temperature. BMC biotechnology. 2008;8:21. doi: 10.1186/1472-6750-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Liu W, et al. Trapping cardiac recessive mutants via expression-based insertional mutagenesis screening. Circulation research. 2013;112(4):606–617. doi: 10.1161/CIRCRESAHA.112.300603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz AA, Antin PB, et al. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984;99(6):2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Stemple D, et al. Zebrafish: genetic tools for studying vertebrate development. Trends in genetics : TIG. 1994;10(5):152–159. doi: 10.1016/0168-9525(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Du A, Sanger JM, et al. Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev Biol. 2003;257(2):382–394. doi: 10.1016/s0012-1606(03)00104-0. [DOI] [PubMed] [Google Scholar]
- Du A, Sanger JM, et al. Cardiac myofibrillogenesis inside intact embryonic hearts. Developmental biology. 2008;318(2):236–246. doi: 10.1016/j.ydbio.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham JT, Brand OM, et al. Myospryn is a direct transcriptional target for MEF2A that encodes a striated muscle, alpha-actinin-interacting, costamere-localized protein. J Biol Chem. 2006;281(10):6841–6849. doi: 10.1074/jbc.M510499200. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, Kiff RM, et al. Troponin T is essential for sarcomere assembly in zebrafish skeletal muscle. Journal of cell science. 2011;124(Pt 4):565–577. doi: 10.1242/jcs.071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Frey N. Cardiac Z-disc signaling network. The Journal of biological chemistry. 2011;286(12):9897–9904. doi: 10.1074/jbc.R110.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Kuhn C, et al. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med. 2006;84(6):446–468. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Glenn NO, McKane M, et al. The W-loop of alpha-cardiac actin is critical for heart function and endocardial cushion morphogenesis in zebrafish. Molecular and cellular biology. 2012;32(17):3527–3540. doi: 10.1128/MCB.00486-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio CC, Antin PB. To the heart of myofibril assembly. Trends in cell biology. 2000;10(9):355–362. doi: 10.1016/s0962-8924(00)01793-1. [DOI] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish -- emergence of a new model vertebrate. Nature reviews Genetics. 2002;3(9):717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Gupta V, Discenza M, et al. alpha-Actinin-2 deficiency results in sarcomeric defects in zebrafish that cannot be rescued by alpha-actinin-3 revealing functional differences between sarcomeric isoforms. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(5):1892–1908. doi: 10.1096/fj.11-194548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter P, Granato M, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Hassel D, Dahme T, et al. Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nature medicine. 2009;15(11):1281–1288. doi: 10.1038/nm.2037. [DOI] [PubMed] [Google Scholar]
- Hoage T, Ding Y, et al. Quantifying cardiac functions in embryonic and adult zebrafish. Methods in molecular biology. 2012;843:11–20. doi: 10.1007/978-1-61779-523-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holterhoff CK, Saunders RH, et al. Sequence and expression of the zebrafish alpha-actinin gene family reveals conservation and diversification among vertebrates. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238(11):2936–2947. doi: 10.1002/dvdy.22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H, Hijikata T, et al. Independent assembly of 1.6 microns long bipolar MHC filaments and I-Z-I bodies. Cell Struct Funct. 1997;22(1):83–93. doi: 10.1247/csf.22.83. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290(4):H1313–1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruscha A, Krawitz P, et al. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development. 2013;140(24):4982–4987. doi: 10.1242/dev.099085. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Tu CT, et al. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 2003;228(1):30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- Huang HT, Brand OM, et al. Myomaxin is a novel transcriptional target of MEF2A that encodes a Xin-related alpha-actinin-interacting protein. J Biol Chem. 2006;281(51):39370–39379. doi: 10.1074/jbc.M603244200. [DOI] [PubMed] [Google Scholar]
- Huang P, Xiao A, et al. Heritable gene targeting in zebrafish using customized TALENs. Nature biotechnology. 2011;29(8):699–700. doi: 10.1038/nbt.1939. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang R, et al. Myofibrillogenesis in the developing zebrafish heart: A functional study of tnnt2. Developmental biology. 2009;331(2):237–249. doi: 10.1016/j.ydbio.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW. Zebrafish genetics and its implications for understanding vertebrate development. Human molecular genetics. 1997;6(10):1755–1760. doi: 10.1093/hmg/6.10.1755. [DOI] [PubMed] [Google Scholar]
- Just S, Meder B, et al. The myosin-interacting protein SMYD1 is essential for sarcomere organization. Journal of cell science. 2011;124(Pt 18):3127–3136. doi: 10.1242/jcs.084772. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Thummel R, et al. The Tg(ccnb1:EGFP) transgenic zebrafish line labels proliferating cells during retinal development and regeneration. Molecular vision. 2008;14:951–963. [PMC free article] [PubMed] [Google Scholar]
- Katzemich A, Liao KA, et al. Alp/Enigma family proteins cooperate in Z-disc formation and myofibril assembly. PLoS genetics. 2013;9(3):e1003342. doi: 10.1371/journal.pgen.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Takeda H, et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Developmental cell. 2004;7(1):133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kettleborough RN, Bruijn E, et al. High-throughput target-selected gene inactivation in zebrafish. Methods in cell biology. 2011;104:121–127. doi: 10.1016/B978-0-12-374814-0.00006-9. [DOI] [PubMed] [Google Scholar]
- Kettleborough RN, Busch-Nentwich EM, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496(7446):494–497. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll R, Buyandelger B, et al. The sarcomeric Z-disc and Z-discopathies. Journal of biomedicine & biotechnology. 2011;2011:569628. doi: 10.1155/2011/569628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll R, Hoshijima M, et al. Cardiac mechanotransduction and implications for heart disease. Journal of molecular medicine. 2003;81(12):750–756. doi: 10.1007/s00109-003-0488-x. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A, Bloch RJ. Obscurin: a multitasking muscle giant. Journal of muscle research and cell motility. 2005;26(6–8):419–426. doi: 10.1007/s10974-005-9024-7. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, et al. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236(11):3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- LeWinter MM, Wu Y, et al. Cardiac titin: structure, functions and role in disease. Clin Chim Acta. 2007;375(1–2):1–9. doi: 10.1016/j.cca.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Li H, Zhong Y, et al. Smyd1b is required for skeletal and cardiac muscle function in zebrafish. Molecular biology of the cell. 2013;24(22):3511–3521. doi: 10.1091/mbc.E13-06-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YF, Swinburne I, et al. Multiple influences of blood flow on cardiomyocyte hypertrophy in the embryonic zebrafish heart. Developmental biology. 2012;362(2):242–253. doi: 10.1016/j.ydbio.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77(4):637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Liu X, Chakkarapani E, et al. Effect of cardiac compressions and hypothermia treatment on cardiac troponin I in newborns with perinatal asphyxia. Resuscitation. 2013;84(11):1562–1567. doi: 10.1016/j.resuscitation.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381(9862):242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- Meder B, Just S, et al. JunB-CBFbeta signaling is essential to maintain sarcomeric Z-disc structure and when defective leads to heart failure. Journal of cell science. 2010;123(Pt 15):2613–2620. doi: 10.1242/jcs.067967. [DOI] [PubMed] [Google Scholar]
- Meder B, Laufer C, et al. A single serine in the carboxyl terminus of cardiac essential myosin light chain-1 controls cardiomyocyte contractility in vivo. Circulation research. 2009;104(5):650–659. doi: 10.1161/CIRCRESAHA.108.186676. [DOI] [PubMed] [Google Scholar]
- Meng X, Noyes MB, et al. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nature biotechnology. 2008;26(6):695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens CB, Donn TM, et al. Reverse genetics in zebrafish by TILLING. Briefings in functional genomics & proteomics. 2008;7(6):454–459. doi: 10.1093/bfgp/eln046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre JL, Hills JA, et al. The titin A-band rod domain is dispensable for initial thick filament assembly in zebrafish. Developmental biology. 2013 doi: 10.1016/j.ydbio.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nature genetics. 2000;26(2):216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Ni TT, Lu J, et al. Conditional control of gene function by an invertible gene trap in zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(38):15389–15394. doi: 10.1073/pnas.1206131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima K, Lin ZX, et al. Initiation and maturation of I-Z-I bodies in the growth tips of transfected myotubes. J Cell Sci. 1999;112(Pt 22):4101–4112. doi: 10.1242/jcs.112.22.4101. [DOI] [PubMed] [Google Scholar]
- Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res. 2004;94(3):296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- Raeker MO, Bieniek AN, et al. Targeted deletion of the zebrafish obscurin A RhoGEF domain affects heart, skeletal muscle and brain development. Developmental biology. 2010;337(2):432–443. doi: 10.1016/j.ydbio.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeker MO, Shavit JA, et al. Membrane-myofibril cross-talk in myofibrillogenesis and in muscular dystrophy pathogenesis: lessons from the zebrafish. Frontiers in physiology. 2014;5:14. doi: 10.3389/fphys.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeker MO, Su F, et al. Obscurin is required for the lateral alignment of striated myofibrils in zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235(8):2018–2029. doi: 10.1002/dvdy.20812. [DOI] [PubMed] [Google Scholar]
- Raz E, van Luenen HG, et al. Transposition of the nematode Caenorhabditis elegans Tc3 element in the zebrafish Danio rerio. Current biology : CB. 1998;8(2):82–88. doi: 10.1016/s0960-9822(98)70038-7. [DOI] [PubMed] [Google Scholar]
- Rhee D, Sanger JM, et al. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton. 1994;28(1):1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Robbins J. Remodeling the cardiac sarcomere using transgenesis. Annual review of physiology. 2000;62:261–287. doi: 10.1146/annurev.physiol.62.1.261. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, et al. p53 activation by knockdown technologies. PLoS genetics. 2007;3(5):e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottbauer W, Saurin AJ, et al. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell. 2002;111(5):661–672. doi: 10.1016/s0092-8674(02)01112-1. [DOI] [PubMed] [Google Scholar]
- Rottbauer W, Wessels G, et al. Cardiac myosin light chain-2: a novel essential component of thick-myofilament assembly and contractility of the heart. Circulation research. 2006;99(3):323–331. doi: 10.1161/01.RES.0000234807.16034.fe. [DOI] [PubMed] [Google Scholar]
- Rudy DE, Yatskievych TA, et al. Assembly of thick, thin, and titin filaments in chick precardiac explants. Developmental dynamics : an official publication of the American Association of Anatomists. 2001;221(1):61–71. doi: 10.1002/dvdy.1125. [DOI] [PubMed] [Google Scholar]
- Russell B, Curtis MW, et al. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. Journal of molecular and cellular cardiology. 2010;48(5):817–823. doi: 10.1016/j.yjmcc.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Cade L, et al. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nature biotechnology. 2011;29(8):697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Sanger JW. The dynamic Z bands of striated muscle cells. Science signaling. 2008;1(32):pe37. doi: 10.1126/scisignal.132pe37. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Chowrashi P, et al. Myofibrillogenesis in skeletal muscle cells. Clinical orthopaedics and related research. 2002;403(Suppl):S153–162. doi: 10.1097/00003086-200210001-00018. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Kang S, et al. How to build a myofibril. J Muscle Res Cell Motil. 2005;26(6–8):343–354. doi: 10.1007/s10974-005-9016-7. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Wang J, et al. Assembly and dynamics of myofibrils. Journal of biomedicine & biotechnology. 2010;2010:858606. doi: 10.1155/2010/858606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Wang J, et al. Myofibrillogenesis in skeletal muscle cells in zebrafish. Cell motility and the cytoskeleton. 2009;66(8):556–566. doi: 10.1002/cm.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarparanta J, Jonson PH, et al. Mutations affecting the cytoplasmic functions of the co-chaperone DNAJB6 cause limb-girdle muscular dystrophy. Nature genetics. 2012;44(4):450–455. S451–452. doi: 10.1038/ng.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenebeck JJ, Yelon D. Illuminating cardiac development: Advances in imaging add new dimensions to the utility of zebrafish genetics. Seminars in cell & developmental biology. 2007;18(1):27–35. doi: 10.1016/j.semcdb.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss T, Lin ZX, et al. Differential distribution of subsets of myofibrillar proteins in cardiac nonstriated and striated myofibrils. J Cell Biol. 1990;110(4):1159–1172. doi: 10.1083/jcb.110.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley M, Huang W, et al. Depletion of zebrafish titin reduces cardiac contractility by disrupting the assembly of Z-discs and A-bands. Circulation research. 2007;100(2):238–245. doi: 10.1161/01.RES.0000255758.69821.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguchi O, Takashima S, et al. A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart. The Journal of clinical investigation. 2007;117(10):2812–2824. doi: 10.1172/JCI30804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, et al. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nature genetics. 2002;31(1):106–110. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- Seidman CE, Seidman JG. Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circulation research. 2011;108(6):743–750. doi: 10.1161/CIRCRESAHA.110.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104(4):557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- Sheikh F, Bang ML, et al. ”Z”eroing in on the role of Cypher in striated muscle function, signaling, and human disease. Trends in cardiovascular medicine. 2007;17(8):258–262. doi: 10.1016/j.tcm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogah VM, Serluca FC, et al. Distinct troponin C isoform requirements in cardiac and skeletal muscle. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239(11):3115–3123. doi: 10.1002/dvdy.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY, Fouquet B, et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- Staudt D, Stainier D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annual review of genetics. 2012;46:397–418. doi: 10.1146/annurev-genet-110711-155646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen LS, Guyon JR, et al. The zebrafish runzel muscular dystrophy is linked to the titin gene. Developmental biology. 2007;309(2):180–192. doi: 10.1016/j.ydbio.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AL, Wang J, et al. Tracking changes in Z-band organization during myofibrillogenesis with FRET imaging. Cell motility and the cytoskeleton. 2008;65(5):353–367. doi: 10.1002/cm.20265. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nature protocols. 2008;3(1):59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Trinh le A, Hochgreb T, et al. A versatile gene trap to visualize and interrogate the function of the vertebrate proteome. Genes & development. 2011;25(21):2306–2320. doi: 10.1101/gad.174037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J. Titin: properties and family relationships. Nat Rev Mol Cell Biol. 2003;4(9):679–689. doi: 10.1038/nrm1198. [DOI] [PubMed] [Google Scholar]
- Tu S, Chi NC. Zebrafish models in cardiac development and congenital heart birth defects. Differentiation; research in biological diversity. 2012;84(1):4–16. doi: 10.1016/j.diff.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer DL, I, Marques J, et al. Zebrafish cypher is important for somite formation and heart development. Developmental biology. 2006;299(2):356–372. doi: 10.1016/j.ydbio.2006.07.032. [DOI] [PubMed] [Google Scholar]
- van Ham TJ, Mapes J, et al. Live imaging of apoptotic cells in zebrafish. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(11):4336–4342. doi: 10.1096/fj.10-161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney GK, Huang H, et al. The Zebrafish Insertion Collection (ZInC): a web based, searchable collection of zebrafish mutations generated by DNA insertion. Nucleic acids research. 2013;41(Database issue):D861–864. doi: 10.1093/nar/gks946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney GK, Lu J, et al. A large-scale zebrafish gene knockout resource for the genome-wide study of gene function. Genome research. 2013;23(4):727–735. doi: 10.1101/gr.151464.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanga J, Eckberg WR, et al. Ultrastructural differentiation of cardiomyocytes of the zebrafish during the 8–26-somite stages. Journal of submicroscopic cytology and pathology. 2001;33(3):275–287. [PubMed] [Google Scholar]
- Wefers B, Meyer M, et al. Direct production of mouse disease models by embryo microinjection of TALENs and oligodeoxynucleotides. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):3782–3787. doi: 10.1073/pnas.1218721110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen KK, Rubenstein PA. Biochemical consequences of the cardiofunk (R177H) mutation in yeast actin. The Journal of biological chemistry. 2003;278(48):48386–48394. doi: 10.1074/jbc.M308980200. [DOI] [PubMed] [Google Scholar]
- Wienholds E, van Eeden F, et al. Efficient target-selected mutagenesis in zebrafish. Genome research. 2003;13(12):2700–2707. doi: 10.1101/gr.1725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Gao J, et al. Functional analysis of slow myosin heavy chain 1 and myomesin-3 in sarcomere organization in zebrafish embryonic slow muscles. Journal of genetics and genomics = Yi chuan xue bao. 2012;39(2):69–80. doi: 10.1016/j.jgg.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Meiler SE, et al. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nature genetics. 2002;30(2):205–209. doi: 10.1038/ng816. [DOI] [PubMed] [Google Scholar]
- Yang J, Xu X. alpha-Actinin2 is required for the lateral alignment of Z discs and ventricular chamber enlargement during zebrafish cardiogenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(10):4230–4242. doi: 10.1096/fj.12-207969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Xu X. Immunostaining of dissected zebrafish embryonic heart. Journal of visualized experiments JoVE. 2012;(59):e3510. doi: 10.3791/3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Ferguson C, et al. Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin. The EMBO journal. 1998;17(6):1614–1624. doi: 10.1093/emboj/17.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Han P, et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature. 2013;498(7455):497–501. doi: 10.1038/nature12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Xu X. Transient and transgenic analysis of the zebrafish ventricular myosin heavy chain (vmhc) promoter: an inhibitory mechanism of ventricle-specific gene expression. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238(6):1564–1573. doi: 10.1002/dvdy.21929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Yang J, et al. Depletion of zebrafish Tcap leads to muscular dystrophy via disrupting sarcomere-membrane interaction, not sarcomere assembly. Human molecular genetics. 2009;18(21):4130–4140. doi: 10.1093/hmg/ddp362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Chu PH, et al. Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. The Journal of cell biology. 2001;155(4):605–612. doi: 10.1083/jcb.200107092. [DOI] [PMC free article] [PubMed] [Google Scholar]