Abstract
The purpose of the present study was to determine the strength and persistence of cocaine-induced conditioned activity in young and adult rats. A one-trial protocol has proven useful for studying the ontogeny of psychostimulant-induced behavioral sensitization; therefore, a similar procedure was used to examine conditioned activity. On postnatal day (PD) 19 or PD 80, rats were injected with saline or cocaine in either a novel test chamber or the home cage. After various drug abstinence intervals (1–21 days), rats were injected with saline and returned to the test chamber, where conditioned activity was assessed. In a separate experiment, we examined whether cocaine-induced conditioned activity was a consequence of Pavlovian conditioning or a failure to habituate to the test environment. The results showed that adult rats exhibited strong one-trial conditioned activity that persisted for at least 21 days, whereas young rats did not show a conditioned locomotor response. The conditioned activity exhibited by adult rats did not result from a failure to habituate to the cocaine-paired environment. These results indicate that cocaine-paired contextual stimuli differentially affect behavior depending on age of the animal. The data provided by adult rats have potential translational relevance for humans because a single environment-drug pairing caused long-term alterations in behavior.
Keywords: conditioned activity, cocaine, ontogeny, rat
Introduction
Exposure to a variety of psychoactive compounds can induce the related phenomena of conditioned activity and behavioral sensitization (Robinson et al., 1998; Kalivas and Stewart, 1991). Conditioned activity is apparent when drug-free animals exhibit increased locomotion in an environmental context in which they previously received psychostimulant treatment (e.g., cocaine). Behavioral sensitization occurs when rats previously exposed to a psychostimulant drug exhibit an augmented behavioral response when challenged later with the same drug. The sensitized responding of adult animals can be detected for many months after final psychostimulant treatment (Leith and Kuczenski, 1982; Paulson et al., 1991). Conditioned activity may be no less resilient, because adult rats and mice given multiple environment-cocaine pairings show robust conditioned locomotion when tested up to 42 days later (Johnson et al., 2012; Tirelli et al., 2005). In the conditioned activity paradigm, both the amount of cocaine administered and the number of environment-cocaine pairings influence the amplitude of the conditioned locomotor response. More specifically, adult mice do not exhibit conditioned activity when a low dose of cocaine is given during the pretreatment phase (Michel and Tirelli, 2002a, b), whereas the conditioned locomotor response becomes progressively stronger as the number of environment-cocaine pairings increases (Michel et al., 2003). Even so, adult rats can exhibit conditioned activity after a single environment-cocaine pairing (McDougall et al., 2007, 2009), although the persistence of cocaine-induced “one-trial” conditioned activity has never been determined in adult rats.
In comparison to adult rats and mice, young rats do not typically show conditioned activity when tested in a previously drug-paired context (Wood et al., 1998; McDougall et al., 1999, 2007; Zavala et al., 2000; but see Tirelli and Ferrara, 1997). For example, young rats given from 7 to 10 environment-cocaine pairings did not exhibit a heightened locomotor response when returned to the same environmental context in a drug-free state (Wood et al., 1998; Zavala et al., 2000). An absence of conditioned activity was also apparent when young rats were given a single environment-cocaine pairing and then tested 24 h later on postnatal day (PD) 20 (McDougall et al., 2007). The latter result is consistent with findings from the behavioral sensitization literature, because environment-drug associations do not modify the one-trial behavioral sensitization of young rats (McDougall et al., 2009, 2011; Herbert et al., 2010).
Recently, Valijent et al. (2010) have highlighted the advantages of using a one-trial procedure, which they refer to as a “two-injection protocol (TIPS),” for examining the neural mechanisms mediating behavioral sensitization (see also Weiss et al., 1989). Most notably, administering only a single injection of psychostimulant largely avoids the competing processes of tolerance and dependence (Valjent et al., 2010). In addition, the one-trial procedure is particularly sensitive at detecting changes in signaling mechanisms that may underlie the development and persistence of behavioral sensitization (Stipanovich et al., 2008; Valjent et al., 2010). It is uncertain whether the one-trial procedure would confer similar benefits to the study of psychostimulant-induced conditioned activity, because the longevity of the conditioned response has not been determined using this experimental protocol.
The purpose of the present study was three-fold. First, the persistence of cocaine-induced conditioned activity was determined in young and adult rats using a one-trial procedure. Young rats do not typically exhibit a conditioned locomotor response when testing occurs soon after the final environment-cocaine pairing (e.g., Wood et al., 1998); thus conditioned activity was examined for up to 21 days after cocaine pretreatment. Second, we assessed whether conditioned activity would show an “incubation of drug craving” effect, in which a cocaine-paired cue elicits greater behavioral effects after longer periods of drug abstinence (Pickens et al., 2011). Lastly, conditioned activity could be a consequence of contextual conditioning (i.e., the novel environment serves as a conditioned stimulus) or a failure to habituate to the test chamber (i.e., exposure to a perceived novel environment triggers an elevated locomotor response). Therefore, a separate experiment was conducted in which a non-habituated control group was used to determine whether the conditioned activity exhibited by cocaine-pretreated rats was the result of Pavlovian processes or a loss of habituation.
Methods
Subjects
A total of 318 male Sprague-Dawley rats were used. Adult male rats (N = 202) were purchased from Charles River (Hollister, California, USA) and were group-housed in large polycarbonate maternity cages (56 × 34 × 22 cm) with wire lids. Young male rats (N = 114) were born and bred at California State University, San Bernardino (CSUSB) and were kept with the dam and littermates in maternity cages. Litters were culled to 10 pups on PD 3 and weaned at PD 24. Food and water were freely available. The colony room was maintained at 22–24°C and kept under a 12 L:12 D cycle. Subjects were cared for according to the “Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council, 2010) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in commercially available activity monitoring chambers (Coulbourn Instruments, Allentown, Pennsylvania, USA), housed in a testing room separate from the animal colony. The activity chambers had acrylic walls, a gray plastic floor, and an open top. Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to measure horizontal locomotor activity (distance traveled). The position of each rat was determined every 100 msec. To somewhat control for differences in body size, rats tested on PD 19 and PD 20 were placed in smaller sized chambers (26 × 26 × 41 cm) than rats tested on PD 33–100 (41 × 41 × 41 cm) (see also Campbell et al., 1969; Shalaby and Spear, 1980). In all other regards, the different-sized chambers were identical to each other.
Procedures
Experiment 1: Adult rats
On PD 79, adult male rats (n = 8 per group) were randomly assigned to one of three pretreatment conditions. In the Saline control group, rats were given saline injections both immediately prior to being placed in the test chamber and 30 min after being returned to the home cage. In the ‘Unpaired’ group, rats were injected with saline before being placed in the test chamber and then injected with cocaine (30 mg/kg, i.p.) 30 min after being returned to the home cage. In the ‘Paired’ group, rats were injected with cocaine (30 mg/kg, i.p.) before being placed in the test chamber and then injected with saline 30 min after being returned to the home cage. The pretreatment phase occurred on a single day, with distance traveled (locomotor activity) being measured in the test chamber for 60 min.
To determine the persistence of one-trial conditioned activity, adult rats were tested after an interval of 1, 3, 5, 7, 14, or 21 days (i.e., on PD 80, 82, 84, 86, 93, or 100). On the test days, rats were injected with saline and immediately placed in the test chambers, where locomotion was measured for 90 min.
Experiment 2: Young rats
In Experiment 2a, young male rats (n = 8 per group) were randomly assigned to the Saline control group, the Unpaired group, or the Paired group on PD 19. To determine the occurrence of one-trial conditioned activity, young rats were tested one day later (i.e., on PD 20) using the same procedure as described for the adult rats. Because adult rats showed strong conditioned activity after both 14- and 21-day intervals, Experiment 2b was conducted in which young rats were pretreated with cocaine and/or saline on PD 19 and tested 14 or 21 days later (i.e., on PD 33 or PD 40). Once again, procedures were the same as described previously with the exception that rats in the Unpaired and Paired groups were injected with either 15 or 30 mg/kg cocaine on the pretreatment day (i.e., a lower dose of cocaine was added to the experiment). After an interval of 14 or 21 days, young rats (n = 9 per group) were injected with saline and immediately placed in test chambers where locomotion was measured for 90 min.
Experiment 3: Non-Habituated controls
In Experiment 3, adult male rats (n = 10 per group) were randomly assigned to a Saline control group, a Non-Habituated control group, or a Paired group on PD 79. In the Saline control group, rats were given a single injection of saline immediately prior to being placed in the test chamber. In the Non-Habituated control group, rats were injected with cocaine (30 mg/kg, IP) and directly returned to the home cage (i.e., rats were not placed in the test chamber on the pretreatment day). In the Paired group, rats were injected with cocaine (30 mg/kg, IP) before being placed in the test chamber. After an interval of 3 or 14 days (i.e., on PD 82 or PD 93), adult rats were injected with saline and placed in the test chambers where locomotion was measured for 90 min.
Drugs
(–)-Cocaine hydrochloride (Sigma-Aldrich, St. Louis, Missouri, USA) was dissolved in saline and injected i.p. at a volume of 5 ml/kg for preweanling rats and 1 ml/kg for adolescent and adult rats.
Data analysis
Separate omnibus multifactor analyses of variance (ANOVAs) were used to statistically analyze the behavioral data of young and adult rats. Distance traveled scores on the pretreatment day were analyzed using Group × Time Block repeated-measures ANOVAs, whereas test day data were analyzed using Group × Interval ANOVAs. When the assumption of sphericity was violated, as determined by Mauchly’s test of sphericity, the Greenhouse-Geisser epsilon statistic was used to adjust degrees of freedom (Geisser and Greenhouse, 1958). Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a”. When required, significant higher-order interactions were further analyzed using one-way ANOVAs. Post hoc analysis of behavioral data was done using Tukey tests (P < 0.05). For the younger age groups (PD 19–40), litter effects were minimized by assigning no more than one subject per litter into a particular group. In situations where this procedure was not possible (e.g., on the pretreatment day), a single litter mean was calculated from multiple littermates assigned to the same group (Holson and Pearce, 1992; Zorrilla, 1997). For presentation purposes, test-day data from the first two experiments were collapsed across the time variable, whereas data from the third experiment were depicted across 10-min time blocks.
Results
Experiment 1: Adult rats
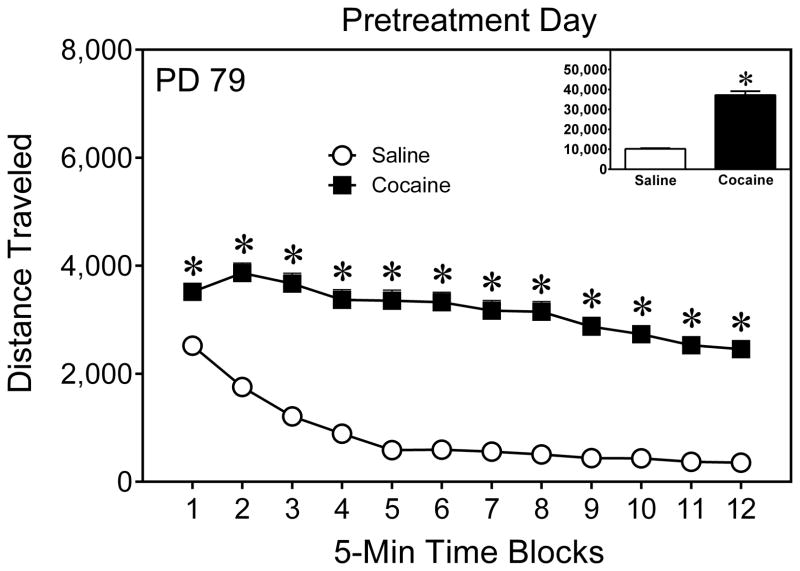
On the pretreatment day (PD 79), cocaine significantly enhanced the locomotor activity of adult rats (Fig. 1) [Drug main effect, F(1,142) = 422.91, P < 0.001]. Rats locomoted less as the testing session progressed [aTime Block main effect, F(6,898) = 120.32, P < 0.001], with cocaine-treated rats exhibiting more locomotor activity than saline-treated rats on time blocks 1–12 [aDrug × Time Block interaction, F(6,898) = 25.79, P < 0.001; and Tukey tests, P < 0.05].
Fig. 1.
Mean (+SEM) distance traveled (cm) by adult rats given a pretreatment injection of saline or cocaine (30 mg/kg, IP) on PD 79. The inset shows mean distance traveled collapsed across time blocks 1–12. * Significant difference between the cocaine and saline groups (P < 0.05).
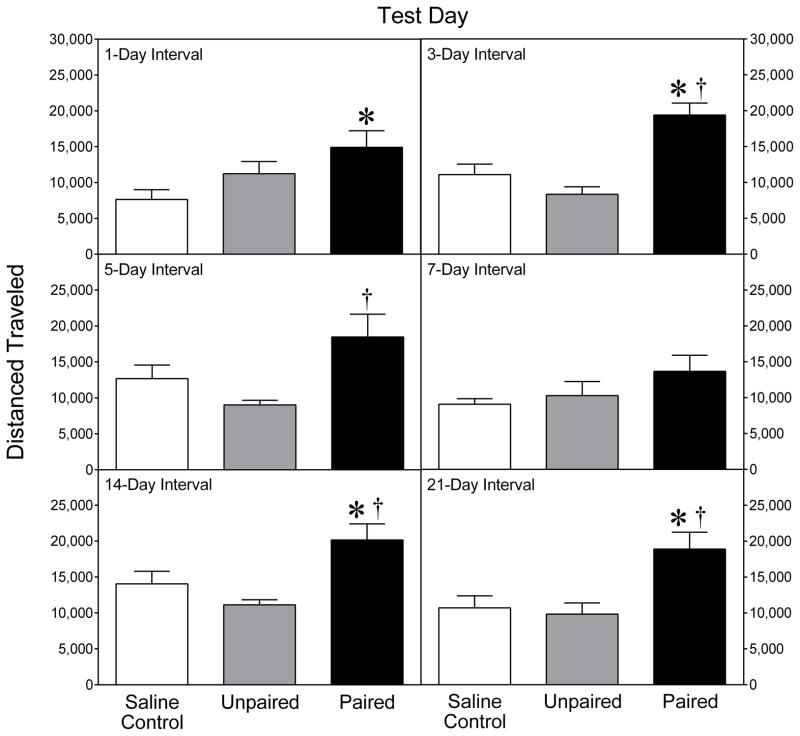
When collapsed across the six test days, a strong conditioned activity response was evident (Fig. 2), because adult rats pretreated with cocaine in the test chambers (i.e., the Paired groups) exhibited significantly more locomotor activity than rats pretreated with either saline alone (i.e., the Saline control groups) or cocaine in the home cage (i.e., the Unpaired groups) [Group main effect, F(2,126) = 31.61, P < 0.001; and Tukey tests, P < 0.05]. The lack of a significant Group × Day interaction suggests that the strength of the conditioned locomotor response did not vary across the 21-day interval.
Fig. 2.
Mean (+SEM) distance traveled (cm) by adult rats (n = 8 per group) tested after a 1-, 3-, 5-, 7-, 14-, or 21-day interval (i.e., on PD 80, PD 82, PD 84, PD 86, PD 93, or PD 100). Data shown are collapsed across the 90-min testing session. * Significantly different from the Saline control group of the same age (P < 0.05). † Significantly different from the Unpaired group of the same age (P < 0.05).
To determine more precisely whether the intensity of the conditioned response changed across the testing period, separate one-way ANOVAs were used to examine the data from each test day. Conditioned activity was evident when rats were returned to the test chamber after a 1- or 3-day interval (i.e., on PD 80 or PD 82), because the Paired groups locomoted significantly more than the saline controls (Fig. 2, upper panels) [Group main effects, F(2,21) = 3.94, P < 0.05, F(2,21) = 16.45, P < 0.001, respectively; and Tukey tests, P < 0.05]. On PD 82, rats in the Paired group also locomoted more than the Unpaired group (Tukey tests, P < 0.05). After a 5-day interval (i.e., on PD 84), the Paired group exhibited significantly more locomotion than the Unpaired group, but not the Saline control group (Fig. 2, middle left panel) [Group main effect, F(2,21) = 4.87, P < 0.05; and Tukey tests, P < 0.05]. After a 7-day interval (i.e., on PD 86), the three groups did not differ. Interestingly, after a 14- or 21-day-interval (i.e., on PD 93 or PD 100), a strong conditioned activity response was apparent (Fig. 2, lower panels,), since the Paired groups exhibited significantly more locomotor activity than both the Unpaired and Saline control groups [Group main effects, F(2,21) = 7.45, P < 0.01, F(2,21) = 6.97, P < 0.01, respectively; and Tukey tests, P < 0.05].
Experiment 2: Young rats
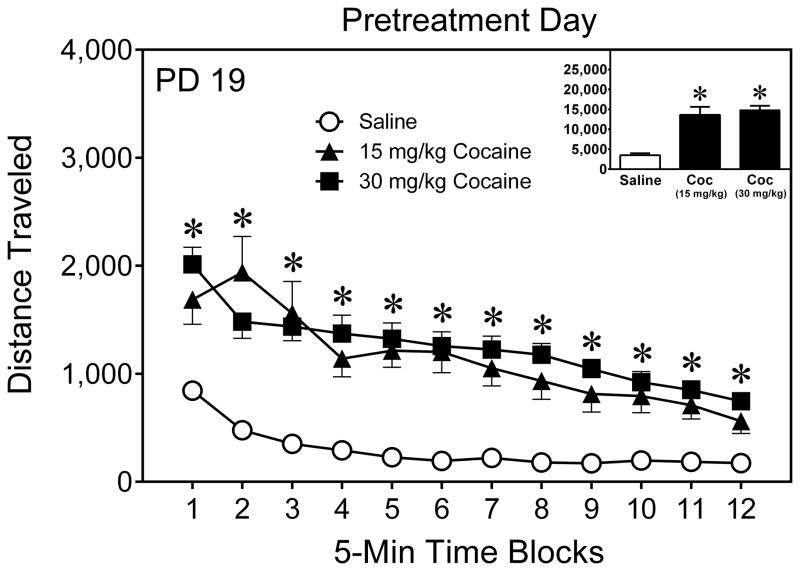
On the pretreatment day (PD 19), rats given cocaine (15 or 30 mg/kg) locomoted more than saline-treated rats on time blocks 1–12 (Fig. 3) [Drug main effect, F(2,52) = 49.55, P < 0.001; aDrug × Time Block interaction, F(10,259) = 4.05, P < 0.001]. Locomotor activity declined across the 60-min testing session [aTime Block main effect, F(5,258) = 38.31, P < 0.001].
Fig. 3.
Mean (±SEM) distance traveled (cm) by young rats given a pretreatment injection of saline or cocaine (30 mg/kg, IP) on PD 19. The inset shows mean distance traveled collapsed across time blocks 1–12. * Significant difference between the cocaine (15 and 30 mg/kg) and saline groups (P < 0.05).
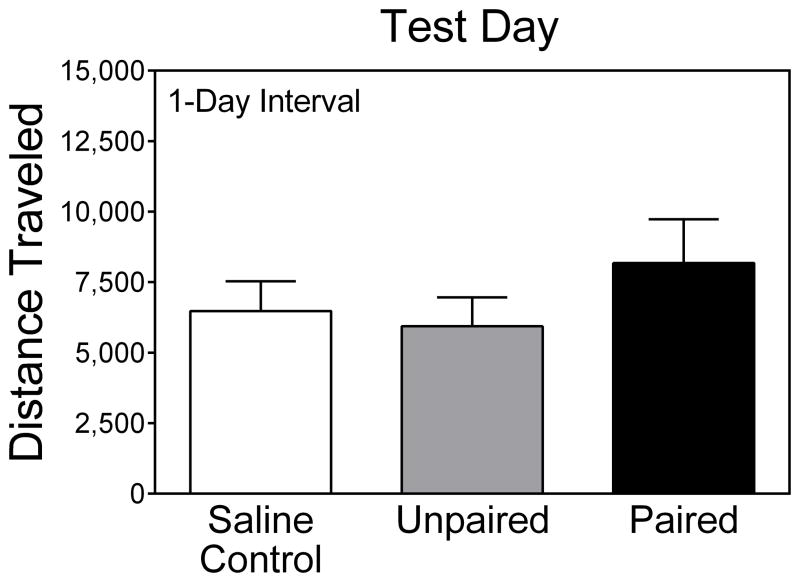
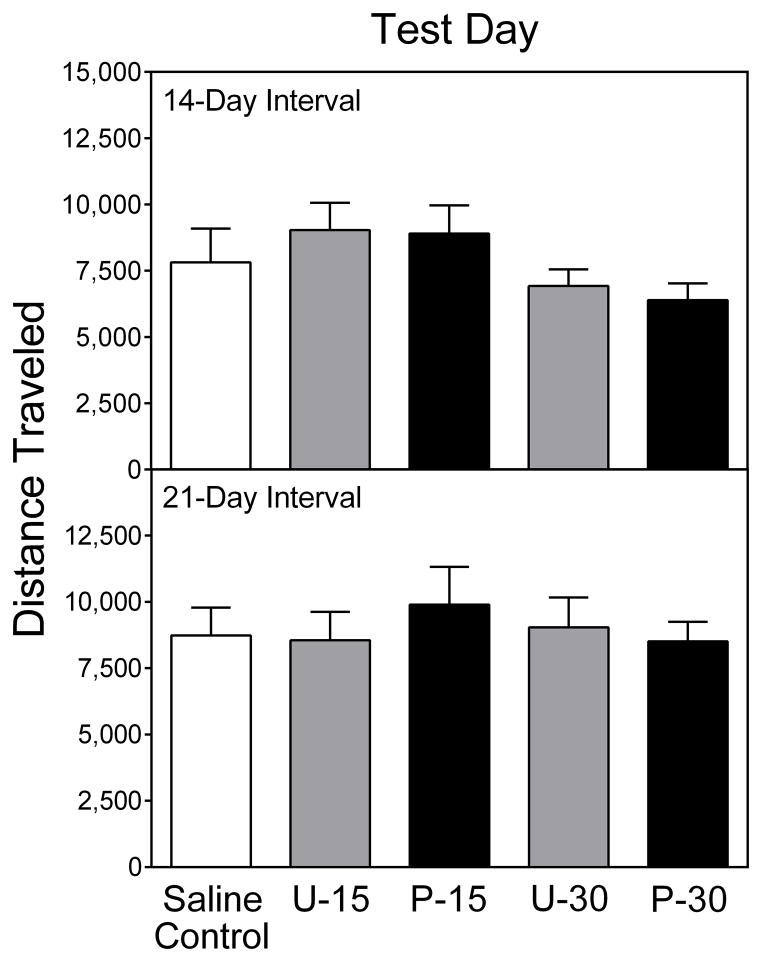
Young rats did not show evidence of conditioned activity on PD 20 (Fig. 4), since rats in the Paired group did not exhibit more locomotor activity than rats in the Unpaired or Saline control groups. Conditioned activity was also not evident when young rats were tested after a 14- or 21-day interval (i.e., on PD 33 or PD 40) (Fig. 5).
Fig. 4.
Mean (+SEM) distance traveled (cm) by young rats (n = 8 per group) tested after a 1-day interval (i.e., on PD 20). Data shown are collapsed across the 90-min testing session.
Fig. 5.
Mean (+SEM) distance traveled (cm) by young rats (n = 9 per group) tested after a 14- or 21-day interval (i.e., on PD 33 or PD 40). Data shown are collapsed across the 90-min testing session. U-15 = Unpaired group-15 mg/kg cocaine; P-15 = Paired group-15 mg/kg cocaine; U-30 = Unpaired group-30 mg/kg cocaine; P-30 = Paired group-30 mg/kg cocaine.
Experiments 1 and 2: Cross-age comparisons
On the pretreatment day (i.e., PD 19 and PD 79), saline- and cocaine-treated adult rats locomoted more than similarly treated preweanling rats (Table 1) [Age × Drug interaction, F(1,186) = 31.18, P < 0.001]; however, cocaine did not differentially affect the locomotor activity of the two age groups if the data were transformed to percent of same-aged saline controls. Among the Saline control groups (i.e., rats injected with saline on both the pretreatment and test days), the test day locomotor activity of preweanling (PD 20, x̄ = 6,475 cm, SEM = 1,057) and adolescent rats (PD 33, x̄ = 7,820 cm, SEM = 1,271; PD 40, x̄ = 8,732 cm, SEM = 1,053) was significantly less than that of adult rats (PD 80–100, x̄ = 12,816 cm, SEM = 517) (compare Figs. 2, 4, and 5) [Age main effect, F(3,166) = 5.69, P < 0.01].
Table 1.
Mean (+SEM) distance traveled scores of saline- and cocaine-treated preweanling and adult rats on the pretreatment day.
| Drug Group | Age
|
|
|---|---|---|
| PD 19 | PD 79 | |
| Saline | 3,512 (+469) | 10,223 (+357) * |
| 15 mg/kg Cocaine | 13,609 (+2018) | Not tested |
| 30 mg/kg Cocaine | 14,849 (+1023) | 37,184 (+1,946) * |
| Cocaine (relative to saline) | 4.2-fold increase | 3.6-fold increase |
Significantly different from PD 19 rats.
Experiment 3: Non-Habituated controls
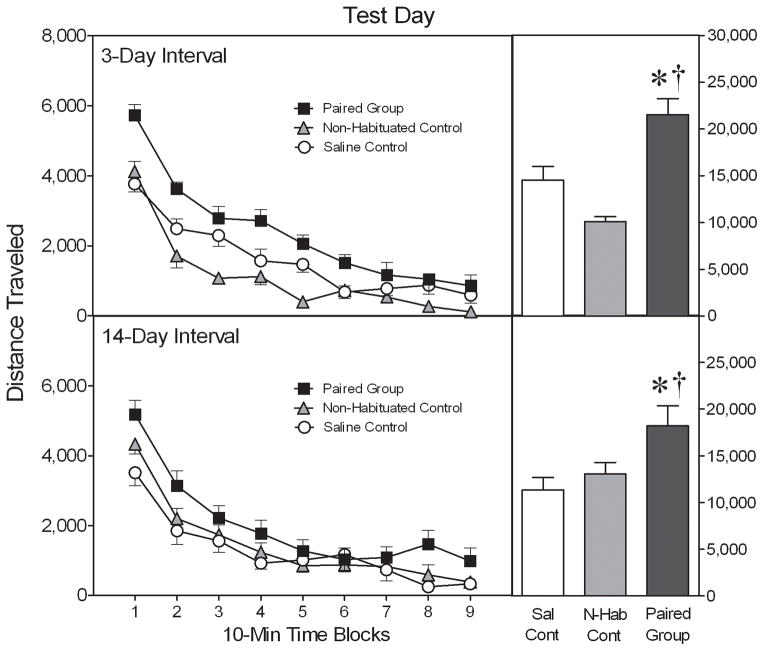
On the pretreatment day (PD 79), adult rats injected with cocaine (x̄ = 44,558 cm; SEM = 2,182) exhibited significantly more locomotor activity than rats given saline (x̄ = 13,125 cm; SEM = 934) [Drug main effect, F(1,38) = 175.45, P < 0.001]. Although not shown graphically, the saline- and cocaine-treated rats performed essentially the same as in Experiment 1.
Overall, adult rats pretreated with cocaine in the test chambers (i.e., the Paired group) exhibited significantly more test day locomotor activity than rats in the Non-Habituated or Saline control groups (Fig. 6) [Group main effect, F(2,54) = 17.81, P < 0.001; and Tukey tests, P < 0.05]. Interactions involving the day variable were not statistically significant, suggesting that the same pattern of group differences occurred after a 3- or 14-day drug abstinence interval (i.e., on PD 82 or PD 93). When data were collapsed across the day variable, rats in the Paired group exhibited more locomotor activity than the Saline control group on time blocks 1, 2, 3, 6, and 8 [aGroup × Time Block interaction, F(11,308) = 2.75, P < 0.01; and Tukey tests, P < 0.05]. The Paired group also locomoted more than the Non-Habituated control group on all except time blocks 5 and 7 [Tukey tests, P < 0.05].
Fig. 6.
Mean (±SEM) distance traveled (cm) by adult rats (n = 10 per group) tested after a 3- or 14-day interval (i.e., on PD 82 or PD 93). The inset shows mean distance traveled collapsed across time blocks 1–9. * Significantly different from the Saline control group (P < 0.05). † Significantly different from the Non-Habituated control group (P < 0.05).
Discussion
As hypothesized, adult rats showed robust one-trial cocaine-induced conditioned activity that persisted for at least 21 days. More specifically, rats given a single environment-cocaine pairing on PD 79 exhibited an elevated locomotor response when injected with saline in the same environmental context on PD 100. Such findings are not unique in the literature, because adult wild-type C57BL/6 mice show a strong conditioned locomotor response when tested 17 days after a single environment-amphetamine pairing (McDougall et al., 2005). An interesting effect often observed in drug-seeking tasks is called “incubation of drug craving,” in which cocaine-paired cues induce greater levels of instrumental responding at longer drug abstinence intervals (for reviews, see Lu et al., 2004; Pickens et al., 2011). Such an effect was not observed in the present study, because the magnitude of the conditioned locomotor response was not significantly greater on PD 100 than PD 80 (i.e., 21 days vs. 1 day after drug pretreatment). Curiously, the strength of the conditioned response declined on PD 86 (i.e., after a 7-day interval), but response magnitude fully recovered at longer drug abstinence intervals. The absence of an incubation-like effect was not overly surprising, since “incubation of drug craving” is often not observed if the conditioned activity task relies on contextual cues and Pavlovian associations (Tirelli et al., 2005; Johnson et al., 2012; Diehl et al., 2013). Therefore, while an incubation effect was not apparent, the present study did show that a single environment-drug pairing is sufficient to produce strong conditioned activity that persists for at least three weeks in adult rats.
Conditioned activity is typically explained in terms of Pavlovian learning principles. Presumably, an excitatory Pavlovian association forms between the environmental context (conditioned stimulus, CS) and psychostimulant (unconditioned stimulus, US), such that the CS can elicit enhanced locomotion (conditioned response, CR) when the animal is returned to the same environmental context (Franklin and Druhan, 2000; Michel and Tirelli, 2002a; Johnson et al., 2012). Results from conditioned activity studies are generally, but not always, consistent with the basic tenets of Pavlovian theory. For example, the magnitude of the CR (i.e., the locomotor response) is at least partially dependent on the intensity of the CS (i.e., the dose of drug administered). In other words, as the psychostimulant dose increases the strength of the conditioned locomotor response also increases (Tilson and Rech, 1973; Michel and Tirelli, 2002a, b). Moreover, the size of the CR and resistance to extinction should be a function of the number of CS-US pairings (Mackintosh, 1974). Consistent with this basic tenet, Michel et al. (2003) showed that increasing the number of environment-cocaine pairings from 3 to 12 resulted in progressively more conditioned locomotion on the test day (but see Ahmed et al., 1996). Further, the exaggerated CR evident after 12 CS-US pairings was more resistant to extinction under conditions of repeated testing (Michel et al., 2003). In the present study, adult rats showed robust one-trial conditioned activity when cocaine was administered prior to placement in the test chamber, but not when cocaine was administered in the home cage. The latter finding shows that it is the CS-US pairing, rather than some nonassociative drug effect, that is responsible for the elevated locomotor response on the test day. Although assessing conditioned activity after a single environment-drug pairing is unusual, the present results conform to established Pavlovian learning principles since there are multiple examples of “one-trial” learning in the presence of an intense US and salient CS (Mackintosh, 1974).
Alternatively, various research groups have suggested that conditioned activity might result from a failure to habituate rather than contextual conditioning (e.g., Gold et al., 1988; Damianopoulos and Carey, 1992; Ahmed et al., 1995). According to the habituation hypothesis, cocaine interferes with the normal habituation process; thus, rats in the Paired group exhibit increased locomotor activity on the test day because of a hyperactive response to the seemingly novel environment. Evidence for the habituation hypothesis is exclusively based on studies using multi-trial procedures (Gold et al., 1988; Damianopoulos and Carey, 1992; Ahmed et al., 1995), so the extent to which cocaine-induced conditioning and/or anti-habituation effects are responsible for one-trial conditioned activity is unknown. One way to assess the relative impact of conditioning and habituation is to compare the test day performance of the Paired group with a Non-Habituated control group (i.e., a group of cocaine-treated rats that is restricted to the home cage on the pretreatment day). If a loss of habituation is responsible for increased locomotor activity on the test day then the Non-Habituated control group should respond no differently than the Paired group when placed in the test chamber (Damianopoulos and Carey, 1992). This result was not obtained: the Non-Habituated control group exhibited significantly less locomotor activity than the Paired group when tested after 3 or 14 days (see Fig. 6). In addition, Damianopoulos and Carey (1994) stated that drug-induced anti-habituation effects do not persist for 21 days. Therefore, the strong conditioned activity exhibited by our PD 100 rats, which were tested after a 21-day drug abstinence interval, supports the conclusion that Pavlovian conditioning, rather than a failure to habituate, is responsible for the one-trial cocaine-induced conditioned activity of adult rats.
Unlike what was observed in adult rats, young rats did not show conditioned activity after either a short or long drug abstinence interval. More specifically, rats given a single environment-cocaine pairing on PD 19 did not exhibit an elevated locomotor response when tested in the same environmental context on PD 20, PD 33, or PD 40. This pattern of results was not due to an insufficient number of acquisition trials, because administering once daily environment-cocaine pairings on PD 11–PD 20 also does not induce conditioned activity in young rats (Zavala et al., 2000; see also Wood et al., 1998; McDougall et al., 1999). The seeming inability of environment-drug associations to increase locomotor responding is consistent with studies showing that contextual conditioning does not modify the one-trial behavioral sensitization of rats on PD 20 (McDougall et al., 2009, 2011; Herbert et al., 2010).
It is possible that associative learning or memory deficits are responsible for the absence of conditioned activity in younger animals. Consistent with this idea, Spear and colleagues have shown that young and adult rats perceive and process CSs differently (for reviews, see Spear et al., 1988; Spear and McKenzie, 1994); whereas, retention deficits are characteristic of younger animals (Campbell et al., 1968, 1974; Guanowsky et al., 1983; Anderson et al., 2004). That being said, it is doubtful whether associative learning or memory deficits are sufficient to explain the absence of conditioned activity, because even very young rats and mice (PD 12–PD 22) exhibit cocaine- and morphine-induced conditioned place preferences (CPP) (Laviola et al., 1992; Pruitt et al., 1995; Bolanos et al., 1996; Randall et al., 1998). In the CPP and conditioned activity paradigms the nature of the CS-US relationship (context-drug) is similar, albeit the CPP apparatus typically includes a combination of visual, tactile, and olfactory cues. The extra cocaine-paired cues may explain why young rats exhibit CPP but not conditioned activity; alternatively, the CR of interest frequently differs between the two tasks (i.e., locomotion vs. place preference). Therefore, it is possible that the lack of conditioned locomotion on the test day may not reflect a learning or retention deficit, but rather the CR being expressed may differ from the UR (i.e., locomotor activity). It is often found that the CR closely resembles the UR, but there are many examples, frequently involving a drug US, where the nature of the CR and UR differ substantially (Mackintosh, 1974; Siegel, 1975).
In addition to these conditioned effects, basal and cocaine-induced locomotor activity varied across ontogeny. More specifically, saline-treated adult rats (PD 79) exhibited almost 3-fold more locomotor activity than preweanling rats when tested on PD 19. Adult rats also showed more cocaine-induced locomotor activity than preweanling rats, but this drug effect more than disappeared when basal differences in locomotor activity were accounted for. Responsiveness of young and adult rats to acute psychostimulant administration is greatly affected by drug dose (Campbell et al., 1969; McDougall et al., 2013); therefore generalizations based on a single high dose of cocaine should be made with caution. In contrast, analysis of the Saline control groups allowed basal locomotor activity to be unambiguously assessed across three ontogenetic periods. In this case, the basal locomotor activity of preweanling and adolescent rats did not differ, but was substantially reduced relative to adult rats. Whether adolescent or adult rodents exhibit greater basal locomotor activity is a contentious issue, with studies frequently reporting age-dependent differences in opposing directions (adolescent > adult, Lanier and Isaacson, 1977; Spear and Brake, 1983; adolescent < adult, Adriani and Laviola, 2000; Koek et al., 2012; adolescent = adult, White and Holtzman, 2005; Zombeck et al., 2010). Koek et al. (2012) have suggested that procedural differences involving the novelty of the test environment may explain why adolescent and adult rodents are alternately reported to exhibit greater or lesser amounts of locomotor activity. Consistent with this explanation, the basal locomotor activity of adolescent rats increases under conditions of repeated daily testing, while the locomotor activity of adult rats declines or stays stable (Bronstein, 1972, 1973).
In conclusion, adult rats exhibit strong one-trial conditioned activity that persists for at least 21 days, whereas young rats do not show a conditioned locomotor response under the same conditions. These finding have potential translational relevance for human models of drug-taking, since it is apparent that a single environment-psychostimulant pairing can have long-term effects on the behavior of adult rats tested in a drug-free state. At the same time, the strength of the conditioned response did not increase across time, suggesting that the “incubation” effect does not occur when conditioning relies on Pavlovian associations involving contextual cues (see also Tirelli et al., 2005; Johnson et al., 2012; Diehl et al., 2013). Lastly, Valjent et al. (2010) have argued that the one-trial procedure provides a number of practical and theoretical advantages over multi-trial procedures for the study of behavioral sensitization (e.g., reduced impact of tolerance and dependence). It is likely that the same advantages would benefit researchers using a one-trial procedure to study psychostimulant-induced conditioned activity.
Acknowledgments
We thank Arturo Zavala and Jena Shaddox for their technical assistance. This research was supported by the National Institute on Drug Abuse (NIDA) research grant DA027985 (SAM) and the National Institute of General Medical Sciences (NIGMS) training grants GM083883 (RJL and SMC) and DA025319 (TD).
Footnotes
Disclosures: The National Institutes for Health (NIH) funded this project; the authors have no conflicts of interest or additional funding sources to disclose.
References
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Cador M, Le Moal M, Stinus L. Amphetamine-induced conditioned activity in rats: comparison with novelty-induced activity and role of the basolateral amygdala. Behav Neurosci. 1995;109:723–733. doi: 10.1037//0735-7044.109.4.723. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Oberling P, Di Scala G, Sandner G. Amphetamine-induced conditioned activity does not result from a failure of rats to habituate to novelty. Psychopharmacology (Berl) 1996;123:325–332. doi: 10.1007/BF02246642. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Barnes GW, Briggs JF, Ashton KM, Moody EW, Joynes RL, et al. Effects of ontogeny on performance of rats in a novel object-recognition task. Psychol Rep. 2004;94:437–443. doi: 10.2466/pr0.94.2.437-443. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the κ-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Bronstein PM. Open-field behavior of the rats as a function of age: cross-sectional and longitudinal investigations. J Comp Physiol Psych. 1972;80:335–341. [Google Scholar]
- Bronstein PM. Replication report: age and open-field activity of rats. Psychol Rep. 1973;32:403–406. [Google Scholar]
- Campbell BA, Jaynes J, Misanin JR. Retention of a light-dark discrimination in rats of different ages. J Comp Physiol Psych. 1968;66:467–472. doi: 10.1037/h0026360. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Lytle LD, Fibiger HC. Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat. Science. 1969;166:635–637. doi: 10.1126/science.166.3905.635. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Misanin JR, White BC, Lytle LD. Species differences in ontogeny of memory: indirect support for neural maturation as a determinant of forgetting. J Comp Physiol Psych. 1974;87:193–202. [Google Scholar]
- Damianopoulos EN, Carey RJ. Pavlovian conditioning of CNS drug effects: a critical review and new experimental design. Rev Neurosci. 1992;3:65–77. doi: 10.1515/REVNEURO.1992.3.1.65. [DOI] [PubMed] [Google Scholar]
- Damianopoulos EN, Carey RJ. A new method to assess Pavlovian conditioning of psychostimulant drug effects. J Neurosci Methods. 1994;53:7–17. doi: 10.1016/0165-0270(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Diehl GW, Wachtel JM, Paine TA. Cue-induced conditioned activity does not incubate but is mediated by the basolateral amygdala. Pharmacol Biochem Behav. 2013;104:69–79. doi: 10.1016/j.pbb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology. 2000;23:633–644. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Geisser S, Greenhouse SW. An extension of Box’s results on the use of the F distribution in multivariate analysis. Ann Math Statist. 1958;29:885–891. [Google Scholar]
- Gold LH, Swerdlow NR, Koob GF. The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine. Behav Neurosci. 1988;102:544–552. doi: 10.1037//0735-7044.102.4.544. [DOI] [PubMed] [Google Scholar]
- Guanowsky V, Misanin JR, Riccio DC. Retention of conditioned taste aversion in weanling, adult, and old-age rats. Behav Neural Biol. 1983;37:173–178. doi: 10.1016/s0163-1047(83)91187-1. [DOI] [PubMed] [Google Scholar]
- Herbert MS, Der-Ghazarian T, Palmer AG, McDougall SA. One-trial cocaine-induced behavioral sensitization in preweanling rats: role of contextual stimuli. Exp Clin Psychopharm. 2010;18:284–295. doi: 10.1037/a0019142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Sediqzadah S, Erb S. Expression and resilience of a cocaine-conditioned locomotor response after brief and extended drug-free periods. Behav Brain Res. 2012;230:69–77. doi: 10.1016/j.bbr.2012.01.049. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Koek W, France CP, Javors MA. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacology (Berl) 2012;219:1027–1037. doi: 10.1007/s00213-011-2432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LP, Isaacson RL. Early developmental changes in the locomotor response to amphetamine and their relation to hippocampal function. Brain Res. 1977;126:567–575. doi: 10.1016/0006-8993(77)90610-2. [DOI] [PubMed] [Google Scholar]
- Laviola G, Dell’Omo G, Alleva E, Bignami G. Ontogeny of cocaine hyperactivity and conditioned place preference in mice. Psychopharmacology (Berl) 1992;107:221–228. doi: 10.1007/BF02245141. [DOI] [PubMed] [Google Scholar]
- Leith NJ, Kuczenski R. Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology (Berl) 1982;76:310–315. doi: 10.1007/BF00449116. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. New York: Academic Press; 1974. [Google Scholar]
- McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharm. 1999;7:208–218. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Reichel CM, Cyr MC, Karper PE, Nazarian A, Crawford CA. Importance of D1 receptors for associative components of amphetamine-induced behavioral sensitization and conditioned activity: a study using D1 receptor knockout mice. Psychopharmacology (Berl) 2005;183:20–30. doi: 10.1007/s00213-005-0146-9. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Baella SA, Stuebner NM, Halladay LM, Crawford CA. Cocaine-induced behavioral sensitization in preweanling and adult rats: effects of a single drug-environment pairing. Psychopharmacology (Berl) 2007;193:323–332. doi: 10.1007/s00213-007-0788-x. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Cortez AM, Palmer AG, Herbert MS, Martinez CE, Charntikov S, et al. Importance of environmental context for one- and three-trial cocaine-induced behavioral sensitization. Psychopharmacology (Berl) 2009;206:377–388. doi: 10.1007/s00213-009-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Pothier AG, Der-Ghazarian T, Herbert MS, Kozanian OO, Castellanos KA, et al. Importance of associative learning processes for the one-trial behavioral sensitization of preweanling rats. Behav Pharmacol. 2011;22:693–702. doi: 10.1097/FBP.0b013e32834affb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Nuqui CM, Quiroz AT, Martinez CM. Early ontogeny of D-amphetamine-induced one-trial behavioral sensitization. Pharmacol Biochem Behav. 2013;104:154–162. doi: 10.1016/j.pbb.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A, Tirelli E. Post-sensitisation conditioned hyperlocomotion induced by cocaine is augmented as a function of dose in C57BL/6J mice. Behav Brain Res. 2002a;132:179–186. doi: 10.1016/s0166-4328(01)00400-4. [DOI] [PubMed] [Google Scholar]
- Michel A, Tirelli E. Conditioned hyperkinesia induced by cocaine in mice is dose-dependent but not correlated with the unconditioned response or the contextually-sensitized response. Behav Pharmacol. 2002b;13:59–71. doi: 10.1097/00008877-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Michel A, Tambour S, Tirelli E. The magnitude and the extinction duration of the cocaine-induced conditioned locomotion-activated response are related to the number of cocaine injections paired with the testing context in C57BL/6J mice. Behav Brain Res. 2003;145:113–123. doi: 10.1016/s0166-4328(03)00106-2. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. Washington: National Academies Press; 2010. [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt DL, Bolanos CA, McDougall SA. Effects of dopamine D1 and D2 receptor antagonists on cocaine-induced place preference conditioning in preweanling rats. Eur J Pharmacol. 1995;283:125–131. doi: 10.1016/0014-2999(95)00309-9. [DOI] [PubMed] [Google Scholar]
- Randall CK, Kraemer PJ, Bardo MT. Morphine-induced conditioned place preference in preweanling and adult rats. Pharmacol Biochem Behav. 1998;60:217–222. doi: 10.1016/s0091-3057(97)00585-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Shalaby IA, Spear LP. Psychopharmacological effects of low and high doses of apomorphine during ontogeny. Eur J Pharmacol. 1980;67:451–459. doi: 10.1016/0014-2999(80)90186-7. [DOI] [PubMed] [Google Scholar]
- Siegel S. Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psych. 1975;89:498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear NE, McKenzie DL. Intersensory integration in the infant rat. In: Lewkowicz DJ, Terrace H, editors. The development of intersensory perception: comparative perspectives. Hillsdale: Lawrence Erlbaum Associates; 1994. pp. 133–161. [Google Scholar]
- Spear NE, Kraemer PJ, Molina JC, Smoller DE. Developmental change in learning and memory: infantile disposition for unitization. In: Delacour J, Levy JCS, editors. Systems with learning and memory abilities. New York: Elsevier; 1988. pp. 27–52. [Google Scholar]
- Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn JH, Maroteaux M, et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson HA, Rech RH. Prior drug experience and effects of amphetamine on schedule controlled behavior. Pharmacol Biochem Behav. 1973;1:129–132. doi: 10.1016/0091-3057(73)90068-3. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Ferrara M. Neonatal and preweanling rats are able to express short-term behavioral sensitization to cocaine. Eur J Pharmacol. 1997;328:103–114. doi: 10.1016/s0014-2999(97)83036-1. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Michel A, Brabant C. Cocaine-conditioned activity persists for a longer time than cocaine-sensitized activity in mice: implications for the theories using Pavlovian excitatory conditioning to explain the context-specificity of sensitization. Behav Brain Res. 2005;165:18–25. doi: 10.1016/j.bbr.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault JA. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SRB, Post RM, Pert A, Woodward R, Murman D. Context-dependent cocaine sensitization: differential effect of haloperidol on development versus expression. Pharmacol Biochem Behav. 1989;34:655–661. [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Wood RD, Tirelli E, Snyder KJ, Heyser CJ, LaRocca TM, Spear LP. Evidence for behavioral sensitization to cocaine in preweanling rat pups. Psychopharmacology (Berl) 1998;138:114–123. doi: 10.1007/s002130050653. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Nazarian A, Crawford CA, McDougall SA. Cocaine-induced behavioral sensitization in the young rat. Psychopharmacology (Berl) 2000;151:291–298. doi: 10.1007/s002130000377. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Lewicki AD, Patel K, Gupta T, Rhodes JS. Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6J mice. Neuroscience. 2010;165:1087–1099. doi: 10.1016/j.neuroscience.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]