Abstract
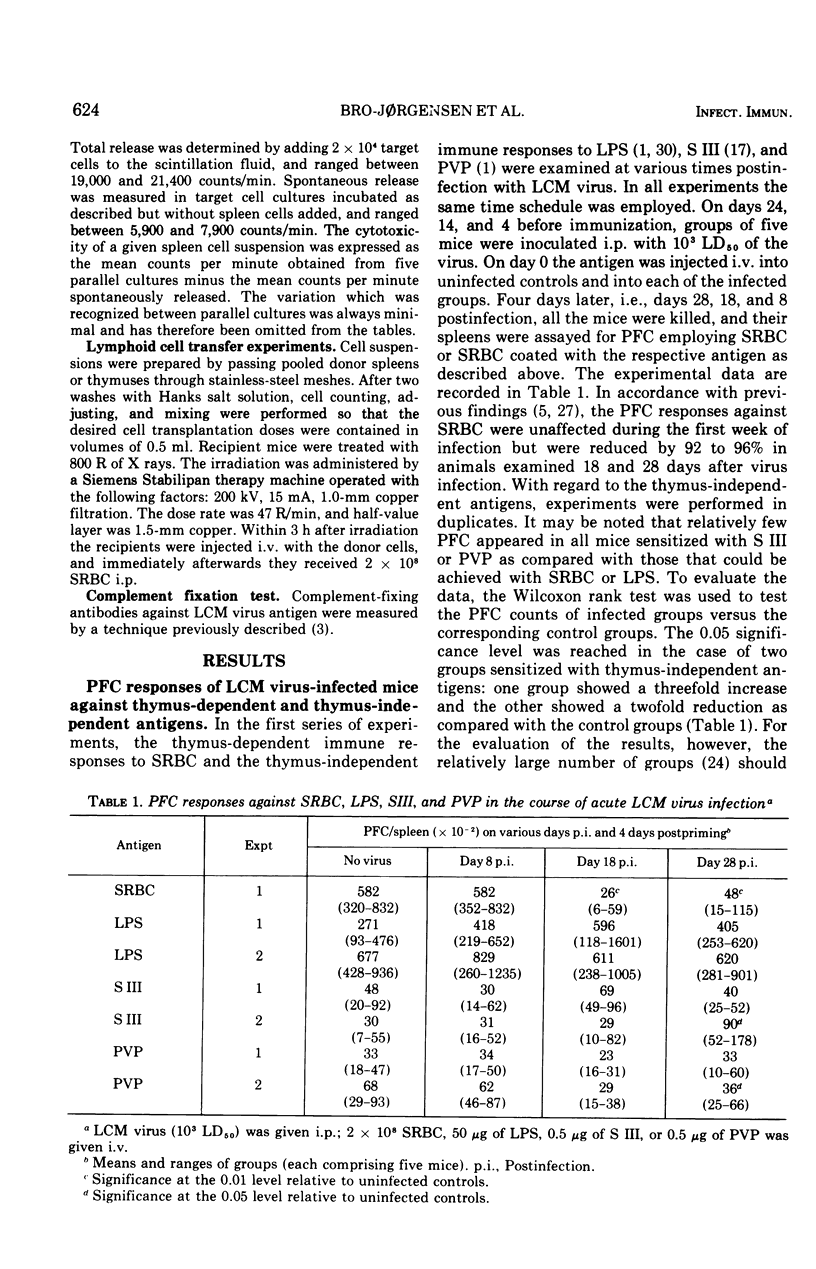
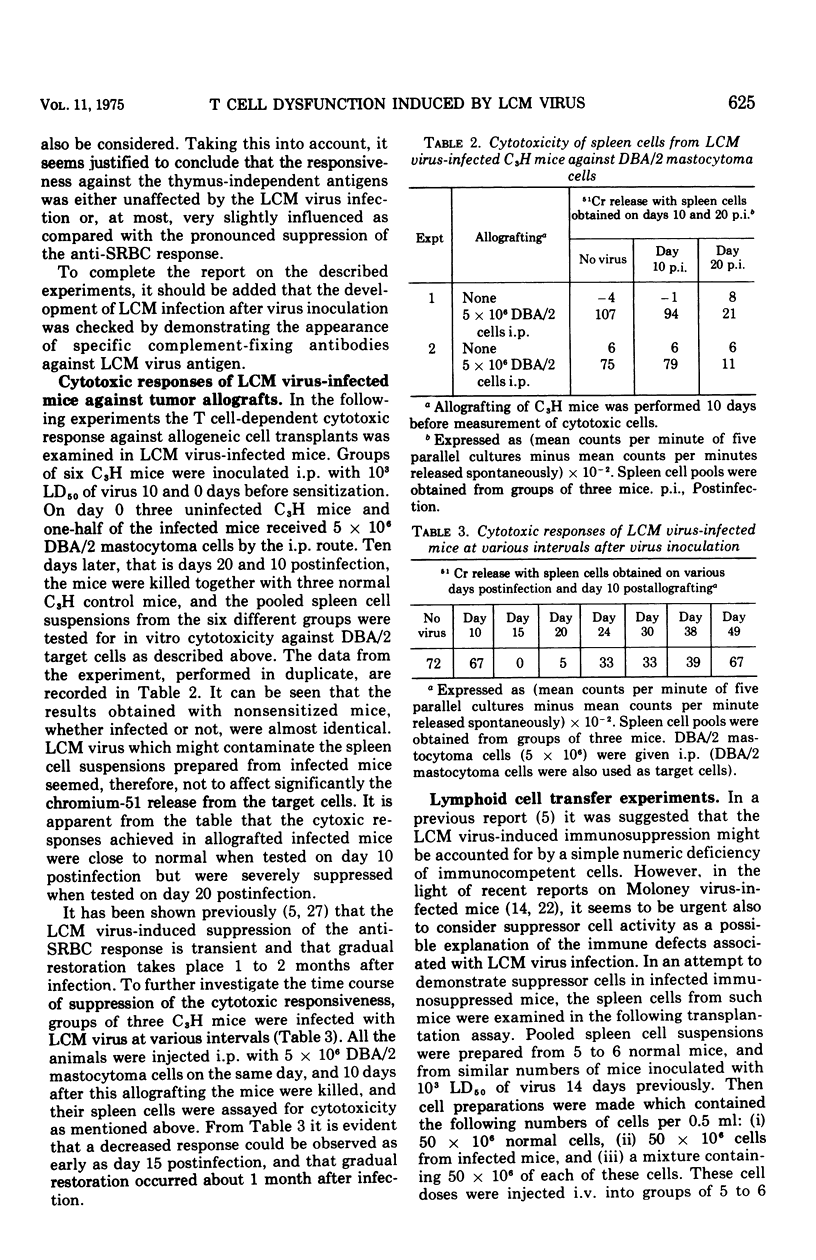
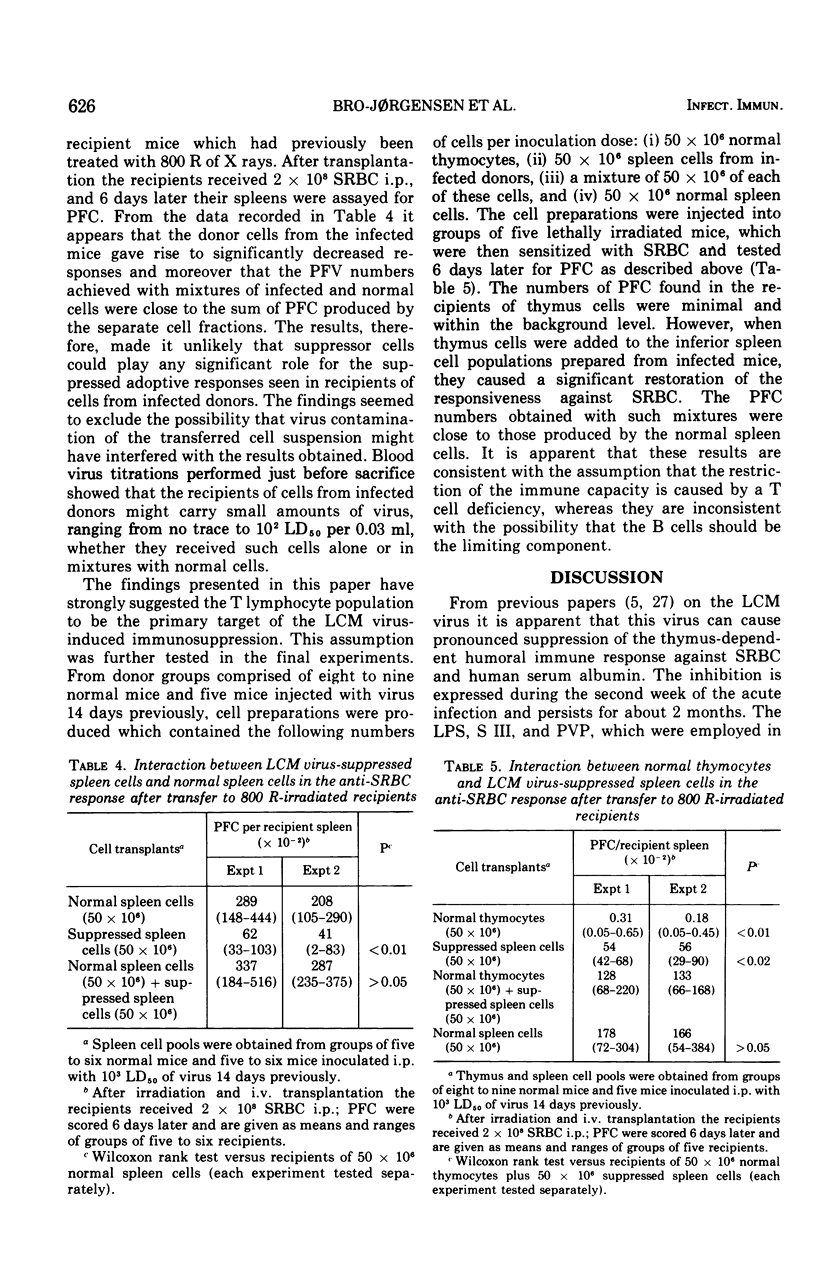
Plaque-forming cell responses against sheep erythrocytes, Escherichia coli lipopolysaccharide, pneumococcal polysaccharide, and polyvinylpyrrolidone were examined in mice infected with lymphocytic choriomeningitis virus. A 92 to 96 percent reduction of the thymus-dependent anti-sheep erythrocyte responses was observed 2 to 4 weeks after infection. However, the thymus-independent responses against the three other antigens were close to normal at all stages of the infetion. Studies on allograft immunity of infected C3H mice against DBA/2 mastocytoma cells revealed a severe suppression of the T cell-mediated cytotoxic response which was temporally related to the impaired humoral responsiveness against sheep erythrocytes. The capacity of spleen cells from infected mice to restore immune responsiveness of lethally irradiated recipients against sheep erythrocytes was significantly reduced. The adoptive responses, however, were clearly improved when normal thymus cells were added to the inferior spleen cells. Moreover, it appeared that the spleen cells from immunosuppressed donor mice could not confer suppression to normal lymphoid cells. The presented findings are consistent with the assumption that a numeric deficiency of T cells, or cells belonging to some T cell subpopulation, is the primary cause of lymphocytic choriomeningitis virus-induced immunosuppression.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Defects in the immune system of mice infected with lymphocytic choriomeningitis virus. Infect Immun. 1974 Apr;9(4):605–614. doi: 10.1128/iai.9.4.605-614.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Haemopoietic defects in mice infected with lymphocytic choriomeningitis virus. 1. The enhanced x-ray sensitivity of virus infected mice. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):845–852. doi: 10.1111/j.0365-5563.1973.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Volkert M. Haemopoietic defects in mice infected with lymphocytic choriomeningitis virus. 2. The viral effect upon the function of colony-forming stem cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):853–862. [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Cremer N. E. Selective immunoglobulin deficiencies in rats infected with Moloney virus. J Immunol. 1967 Jul;99(1):71–81. [PubMed] [Google Scholar]
- Dent P. B. Immunodepression by oncogenic viruses. Prog Med Virol. 1972;14:1–35. [PubMed] [Google Scholar]
- Gelfand M. C., Steinberg A. D. Cooperation of three lymphoid cells in allograft rejection. Cell Immunol. 1974 Mar 30;11(1-3):221–230. doi: 10.1016/0008-8749(74)90022-7. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Gery I., Waksman B. H. Suppressive effects of in vivo immunization on PHA responses in vitro. J Immunol. 1974 Jan;112(1):215–221. [PubMed] [Google Scholar]
- Golstein P., Blomgren H. Further evidence for autonomy of T cells mediating specific in vitro cytotoxicity: efficiency of very small amounts of highly purified T cells. Cell Immunol. 1973 Oct;9(1):127–141. doi: 10.1016/0008-8749(73)90174-3. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M. Immunity to murine sarcoma virus-inducted tumors. II. Suppression of T cell-mediated immunity by cells from progressor animals. J Immunol. 1974 May;112(5):1826–1838. [PubMed] [Google Scholar]
- Hanaoka M., Suzuki S., Hotchin J. Thymus-dependent lymphocytes: destruction by lymphocytic choriomeningitis virus. Science. 1969 Mar 14;163(3872):1216–1219. doi: 10.1126/science.163.3872.1216. [DOI] [PubMed] [Google Scholar]
- Hirano S., Ceglowski W. S., Allen J. L., Friedman H. Effect of Friend leukemogenic virus on antibody-forming cells to a bacterial somatic antigen. J Natl Cancer Inst. 1969 Dec;43(6):1337–1345. [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M., Leuchars E., Davies A. J. Studies on immunological paralysis. VI. Thymic-independence of tolerance and immunity to type 3 pneumococcal polysaccharide. Cell Immunol. 1971 Dec;2(6):614–626. doi: 10.1016/0008-8749(71)90009-8. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Notkins A. L., Mergenhagen S. E. Inhibition of cellular immune reactions in mice infected with lactic dehydrogenase virus. Nature. 1969 Mar 1;221(5183):873–874. doi: 10.1038/221873a0. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. N., Florentz K., Güttler F. Cytotoxicity of vivo sensitized lymphocytes on allogeneic third-party fibroblasts differing for various regions of the H-2 complex. Transplant Proc. 1974 Mar;6(1):95–99. [PubMed] [Google Scholar]
- Katz M., Papenheimer A. M., Jr Quantitative studies of the specificity of anti-pneumococcal antibodies, types 3 and 8. IV. Binding of labeled hexasaccharides derived from S3 by anti-S3 antibodies and their Fab fragments. J Immunol. 1969 Sep;103(3):491–495. [PubMed] [Google Scholar]
- Kauffman C. A., Phair J. P., Linnemann C. C., Jr, Schiff G. M. Cell-mediated immunity in humans during viral infection. I. Effect of rubella on dermal hypersensitivity, phytohemagglutinin response, and T lymphocyte numbers. Infect Immun. 1974 Jul;10(1):212–215. doi: 10.1128/iai.10.1.212-215.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Chused T. M., Herberman R. B., Holden H. T., Lavrin D. H. Evidence of suppressor cell activity in spleens of mice bearing primary tumors induced by Moloney sarcoma virus. J Exp Med. 1974 Jun 1;139(6):1473–1487. doi: 10.1084/jem.139.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers T. A., Petrich J. M., Holloway A. W., St Geme J. W., Jr Depression of tuberculin delayed hypersensitivity by live attenuated mumps virus. J Pediatr. 1970 May;76(5):716–721. doi: 10.1016/s0022-3476(70)80290-6. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Niemeyer I., Löhler J. Lymphocytic choriomeningitis of the mouse. IV. Depression of the allograft reaction. Med Microbiol Immunol. 1972;158(1):16–25. doi: 10.1007/BF02122004. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Blanden R. V. Antiviral action of immune lymphocytes in mice infected with lymphocytic choriomeningitis virus. Infect Immun. 1972 Nov;6(5):695–698. doi: 10.1128/iai.6.5.695-698.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Wainwright S. The immunodepressive action of lymphocytic choriomeningitis virus in mice. J Immunol. 1968 Oct;101(4):717–724. [PubMed] [Google Scholar]
- Moorhead J. W., Claman H. N. Subpopulations of mouse T lymphocytes. I. "Thymidine suicide" of a major proliferating, PHA-responsive cell population present in spleen but not in lymph node. J Immunol. 1974 Jan;112(1):333–338. [PubMed] [Google Scholar]
- Mortensen R. F., Ceglowski W. S., Friedman H. Leukemia virus-induced immunosuppression. X. Depression of T cell-mediated cytotoxicity after infection of mice with Friend leukemia virus. J Immunol. 1974 Jun;112(6):2077–2086. [PubMed] [Google Scholar]
- Möller G., Michael G. Frequency of antigen-sensitive cells to thymus-independent antigens. Cell Immunol. 1971 Aug;2(4):309–316. doi: 10.1016/0008-8749(71)90065-7. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Differentiation of B lymphocytes from stem cell precursors. Adv Exp Med Biol. 1973;29(0):11–18. doi: 10.1007/978-1-4615-9017-0_3. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Chiller J. M., Weigle W. O., Dixon F. J. Effect of chronic viral infection on the immune system. I. Comparison of the immune responsiveness of mice chronically infected with LCM virus with that of noninfected mice. J Immunol. 1973 May;110(5):1268–1278. [PubMed] [Google Scholar]
- Olsson L., Claësson M. H. Studies on subpopulations of theta-bearing lymphoid cells. Nat New Biol. 1973 Jul 11;244(132):50–51. doi: 10.1038/newbio244050a0. [DOI] [PubMed] [Google Scholar]
- Peled A., Haran-Ghera N. The cellular basis of immunosuppression caused by the radiation leukaemia virus. Immunology. 1974 Feb;26(2):323–329. [PMC free article] [PubMed] [Google Scholar]
- Salaman M. H. Immunodepression by mammalian viruses and plasmodia. Proc R Soc Med. 1970 Jan;63(1):11–15. doi: 10.1177/003591577006300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass M. J., Lowrey D. S., Hanna M. G., Jr Changes induced by lactic dehydrogenase virus in thymus and thymus-dependent areas of lymphatic tissue. J Immunol. 1972 Apr;108(4):877–892. [PubMed] [Google Scholar]
- Stobo J. D., Paul W. E., Henney C. S. Functional heterogeneity of murine lymphoid cells. IV. Allogeneic mixed lymphocyte reactivity and cytolytic activity as functions of distinct T cell subsets. J Immunol. 1973 Mar;110(3):652–660. [PubMed] [Google Scholar]
- Strober S., Dilley J. Maturation of B lymphocytes in the rat. I. Migration pattern, tissue distribution, and turnover rate of unprimed and primed B lymphocytes involved in the adoptive antidinitrophenyl response. J Exp Med. 1973 Dec 1;138(6):1331–1344. doi: 10.1084/jem.138.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig M. J. Antigenic competition. Curr Top Microbiol Immunol. 1973;60:125–174. doi: 10.1007/978-3-642-65502-9_4. [DOI] [PubMed] [Google Scholar]
- Taussig M. J. Demonstration of suppressor T cells in a population of 'educated' T cells. Nature. 1974 Mar 15;248(445):236–238. doi: 10.1038/248236a0. [DOI] [PubMed] [Google Scholar]
- Volkert M., Marker O., Bro-Jorgensen K. Twp populations of T lymphocytes immune to the lymphocytic choriomeningitis virus. J Exp Med. 1974 May 1;139(5):1329–1343. doi: 10.1084/jem.139.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Lymphocyte receptors for myxoviruses and paramyxoviruses. J Immunol. 1974 Jun;112(6):2176–2183. [PubMed] [Google Scholar]



