Abstract
Aim
Variations in the expression of cytokines during the progression of periodontitis remain ill-defined. We evaluated the expression of 19 cytokine genes related to T cell phenotype/function during initiation, progression, and resolution of periodontitis, and related these to the expression of soft and bone tissue destruction genes (TDGs).
Materials and Methods
A ligature-induced periodontitis model was used in rhesus monkeys (M. mulatta) (n=18). Gingival tissues were taken at baseline pre-ligation, 2 weeks and 1 month (Initiation), and 3 months (Progression) post-ligation. Ligatures were removed and samples taken 2 months later (Resolution). Total RNA was isolated and the Rhesus Gene 1.0 ST (Affymetrix) used for gene expression analysis. Significant expression changes were validated by qRT-PCR.
Results
Disease Initiation/Progression was characterized by over-expression of Th17/Treg cytokine genes (IL-1β, IL-6, TGFβ, and IL-21) and down-regulation of Th1/Th2 cytokine genes (IL-18, and IL-25). Increased IL-2 and decreased IL-10 levels were seen during disease resolution. Several Th17/Treg cytokine genes positively correlated with TDGs, whereas most Th1/Th2 genes exhibited a negative correlation.
Conclusion
Initiation, progression and resolution of periodontitis involve over- and under-expression of cytokine genes related to various T-helper subsets. In addition, variations in individual T-helper response subset/genes during disease progression correlated to protective/destructive outcomes.
Keywords: Periodontitis, T helper cytokines, gingival tissue, adaptive immunity, Matrix metalloproteinase, RANKL
INTRODUCTION
A balanced gingival immune response to bacterial biofilms is critical to maintain a healthy periodontium without causing tissue damage. Otherwise, a persistent inflammatory response triggered by pathogenic biofilms, leads to destruction of the soft and bone tissues of the periodontium, with the consequent loss of teeth (Van Dyke and Serhan, 2003). Cytokines are central regulators of the immunoinflammatory response that are produced by various cell types including epithelial cells, fibroblasts, dendritic cells, macrophages, and T helper (Th) cells in response to microbes. Accordingly, the net result expression of cytokines within the tissues will generate a specific local milieu that orchestrates the cellular and humoral immune responses necessary to manage oral commensals and pathogens.
Cytokines can be functionally grouped as Th1, Th2, Th17, and T regulatory (Treg), based on their expression pattern and effects on target cells or tissues. IFNγ and IL-12 are Th1 cytokines important for the activation of cell-mediated immunity to control intracellular pathogens and autoimmunity. IL-4, IL-5, IL-13 and IL-25 are Th2 cytokines which drive humoral immunity to control extracellular parasites and also have been involved in allergic-type responses mediated by IgE and mast cell activation. IL-1β, IL-6, IL-21, IL-22, IL-23 and IL-17A are Th17 cytokines that enhance an immune response against extracellular bacteria and fungi. TGFβ, IL-2 and IL-10 are considered Treg cytokines that are important to maintain a balanced immune response through negative regulation of other T-helper responses (Mucida and Cheroutre, 2010, Agnello et al., 2003). Recent evidence indicates that some fully committed Th cell subsets have the ability to produce cytokines that are normally linked to a given Th cell subset under specific conditions (e.g., Th17 cells producing IFNγ, and Tregs producing IL-17A), which suggest that Th subsets appear to exhibit cell plasticity in terms of cytokine production (Bluestone et al., 2009, Hirahara et al., 2011). This versatility associated with Th responses increases the challenge of associating unique Th response subsets/cytokines to a given disease or phase of the disease. Adding another level of complexity, the new Th9, Th22 and follicular T-helper (Tfh) cell subsets have been described, although their potential role in health and disease remains to be determined (Schmitt et al., 1994, Trifari et al., 2009, Crotty, 2011).
Variations in Th cytokine levels associated with periodontal disease have been extensively studied attempting to identify a particular Th cytokine profile/response related to early/stable or progressive periodontal lesions. Initial studies reported inconclusive results with the role of Th1 and Th2 responses, where both Th responses were associated with either protective or destructive periodontal lesions (Gaffen and Hajishengallis, 2008, Gemmell et al., 2007). Emerging evidence indicates that the recently described Th17 cytokine response appears to play a critical role in chronic periodontitis (Adibrad et al., 2012, Cardoso et al., 2009). Consistently, Th17 cytokines have shown the ability to enhance osteoclast differentiation and activation of metalloproteinases (MMPs), critical mediators of soft and hard tissue destruction in periodontitis (Okamoto and Takayanagi, 2011, van Hamburg et al., 2011). The role of Treg in periodontitis is less understood; however it has been reported that tissues from patients with chronic periodontitis presented an increased frequency of forkhead box protein 3 (Foxp3+) Tregs compared with healthy tissues, which suggests that this Th subset could be involved in the modulation of the local immune response in chronic periodontitis (Cardoso et al., 2008).
Although clinical and animal studies (i.e., mice) support that cytokines are involved in the pathogenesis of periodontal disease (Gaffen and Hajishengallis, 2008, Preshaw and Taylor, 2011), it remains unclear, what type of Th response(s) are related to initiation, progression and resolution of periodontitis.
Using the nonhuman primate model of ligature-induced periodontitis that reflects characteristics of human periodontitis we evaluated the expression of 19 Th cytokine genes related to Th1/Th2/Th17/Treg responses in gingival tissues during initiation, progression and resolution of periodontitis. Also, this model could help to delineate what type(s) of T-cell responses most closely relate to soft and bone tissue destruction and host protection.
MATERIAL AND METHODS
Animals and diet
Rhesus monkeys (Macaca mulatta) (n=18; 10 females and 8 males) housed at the Caribbean Primate Research Center (CPRC) at Sabana Seca, Puerto Rico, were used in these studies. The range of age was between 12 and 23 years old (Mean=16.1±3.7). The nonhuman primates were typically fed a 20% protein, 5% fat, and 10% fiber commercial monkey diet (diet 8773, Teklad NIB primate diet modified: Harlan Teklad). The diet was supplemented with fruits and vegetables, and water was provided ad libitum in an enclosed corral setting.
Ligature-induced periodontal disease and gingival tissue sample collection
Following a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico, anesthetized animals were examined by a single investigator using a Maryland probe on the facial aspect of the teeth, 2 proximal sites per tooth (mesio- and disto-buccal), excluding the canines and 3rd molars. The clinical examination included probing pocket depth (PD), and bleeding on probing (BOP; 0–5 scale) (Ebersole et al., 2008). At the initiation of the study, all animals were evaluated clinically for periodontal disease, and only animals with mean bleeding on probing (BOP) of ≤1 and mean pocket depths (PD) <3 mm were entered into the study. After taking the baseline gingival samples, ligatures were placed on the second premolar and first and second molars of both maxillary quadrants. Further, clinical evaluation for ligated sites was obtained and a buccal gingival papilla from each animal was taken using a standard gingivectomy technique at 2 weeks and 1 month (initiation of disease), and 3 months (progression of disease), time in which soft and alveolar bone destruction has been shown to occur in this model (Smith et al., 1993, Branch-Mays et al., 2008, Moritz et al., 1998). Then, ligatures were removed after sampling at 3 months and samples taken 2 months later (resolution). Samples were maintained frozen at −80°C in RNAlater solution until RNA preparation for microarray and real time RT-PCR analysis.
Microarray and qRT-PCR
Total RNA was isolated from each gingival tissue using Trizol reagent (Invitrogen, CA), and further cleaned up with the Qiagen RNeasy mini kit (Qiagen, Valencia, CA). All microarray RNA expression analyses were done at the University of Kentucky Microarray facility. Tissue RNA samples were submitted to the microarray core and RNA quality was assessed with an Agilent 2100 Bioanalyzer. Reverse transcription of equal amounts of RNA from each sample was performed, followed by hybridization to the GeneChip® Rhesus Gene 1.0 ST Array (Affymetrix) similar to methods we have described previously (Meka et al., Gonzalez et al., 2011). Briefly, 250ng of total RNA was amplified and labeled using Ambion WT Expression and Terminal labeling kits respectively (Affymetrix), from which 3.5μg of labeled cDNA was hybridized to the GeneChip Rhesus Macaque Gene 1.0 ST Array following the Affymetrix protocol. Post-hybridization, washing and staining of arrays were performed in an Affymetrix GeneChip Fluidics FS450 station followed by scanning using an Affymetrix GeneChip 3000 7G Scanner and Affymetrix Command Console Software version 4.0. Individual samples were used for gene expression analyses.
For qRT-PCR analysis, mRNA samples (n=18) for each time point were pooled and cytokine gene expression analyzed in triplicates. The cDNA synthesis was carried out starting with 1μg of pooled mRNA and expression of specific transcripts for cytokines/chemokines analyzed using the LightCycler 480 (Roche, IN). Primers for each cytokine gene were designed using the software Primer Quest from Integrated DNA Technologies (IDT), and synthesized by the same company (www.idtdna.com, Coralville, IA) (Table 1). Concentration ratios for the target genes were calculated by normalizing to the housekeeping gene GADPH.
Table 1.
Sequences of the primers used for qRT-PCR analysis.
| Gene | Primer sequences (5′ -3′) | Tm | Product Size (bp) | |
|---|---|---|---|---|
|
| ||||
| IL1B | Forward | TCCTCGACACACGCAATAAC | 62.2 | 111 |
| Reverse | CATATGGACCAGACATCACCAA | 62.0 | ||
|
| ||||
| IL-2 | Forward | CACTGATGTGTGAATATGCTGATG | 61.9 | 94 |
| Reverse | GTCAGTGTTGAGATGATGCTTTG | 61.9 | ||
|
| ||||
| IL-6 | Forward | CCTGAACCAACCACAAATGC | 61.9 | 114 |
| Reverse | GGACTGCAGGAACTCCTTAAA | 61.9 | ||
|
| ||||
| IL-10 | Forward | GGCGCTGTCATCGATTTCTT | 62.9 | 103 |
| Reverse | ATGGCTTTGTAGACGCCTTTC | 62.9 | ||
|
| ||||
| IL-18 | Forward | CTCTCTCCTGTGAGAACAGAATTA | 61.5 | 106 |
| Reverse | CCTGGGACACTTCTCTGAAA | 61.2 | ||
|
| ||||
| IL-21 | Forward | ACTGTGAGTGGTCAGCTATTTC | 62.1 | 116 |
| Reverse | AGGTGATTTCCTCTTCAGCTTT | 62.2 | ||
|
| ||||
| TGFB | Forward | GGCTACCATGCCAACTTCT | 61.9 | 100 |
| Reverse | CCGGGTTATGCTGGTTGT | 61.9 | ||
|
| ||||
| IL-25 | Forward | ATGGCAAGAAGGGCAACC | 62.7 | 75 |
| Reverse | CACACACACAAGCCAAGGA | 62.6 | ||
|
| ||||
| GADPH | Forward | GGTGTGAACCATGAGAAGTATGA | 62.2 | 123 |
| Reverse | GAGTCCTTCCACGATACCAAAG | 62.2 | ||
Data Analysis
The expression intensities for all genes across the samples were estimated using the Robust Multi-array Average (RMA) algorithm with probe-level quintile normalization, as implemented in the Partek Genomics Suite software version 6.6. The different time points were initially compared using one way ANOVA. For genes that had significant difference in the time point means, two sample t-tests were used to investigate differences when comparing baseline to each time point (i.e., 2 weeks, 1 month, 3 months and 5 months). We also determined correlations between the expression of T-helper cytokine genes and soft and hard tissue destruction genes during the course of the disease using a Spearman Rank correlation analysis. Statistical significance was considered by a p value ≤ 0.05.
RESULTS
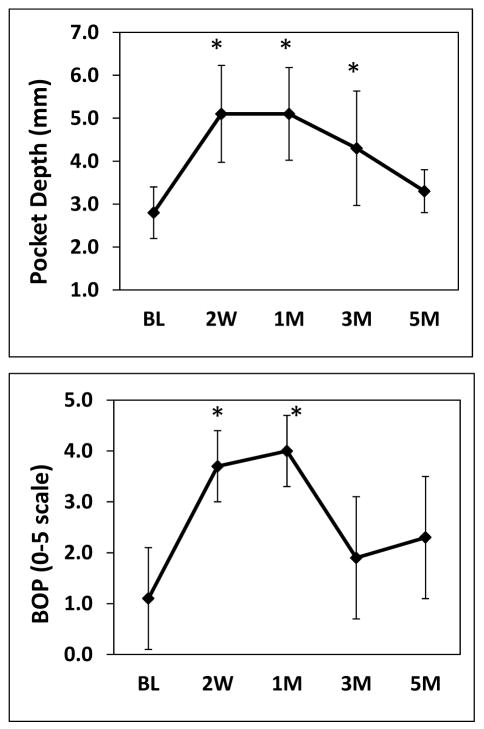
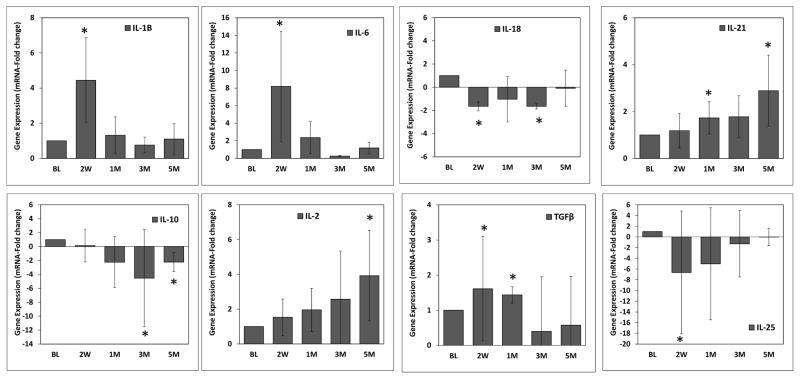
Significant increases in pocket depths were observed during initiation and progression of the disease through the third month, and these returned to basal levels at 5 months after removing the ligatures (Figure 1). Bleeding on probing was significantly elevated during the initiation of disease through the first month, and then decreased to bleeding scores comparable to basal levels at the third month even in the presence of ligatures, and 5 months (Figure 1). Expression of T helper-related cytokine genes during initiation, progression and resolution of periodontal disease analyzed by microarray are shown in Table 2. The expression of cytokines IL-1β, and IL-6 was significantly increased at 2 weeks after ligature-induced periodontitis. In contrast, IL-18 expression was reduced. The variation in the expression of IL-1β, IL-6 and IL-18 returned to basal levels at 1 month. A significant up-regulation of IL-21 was observed at 3 months. Finally, a significant decrease in IL-10 expression and increased levels of IL-2 were observed at 5 months. These cytokine expression variations observed by microarray analyses were validated by qRT-PCR demonstrating similar trends of expression during the progression of periodontitis (Figure 2), and plotted in the context of each phase of the disease using arbitrary units (Figure 3).
Figure 1.
Periodontal clinical measures in ligature-induced periodontitis. Pocket Depth (PD) and bleeding on Probing (BOP) from one site per animal (n=18) were taken at baseline and different time points after inducing disease. Data are expressed as means ± standard deviations. *p≤0.05 when baseline values where compared to the different time points, as determined by Student’s t test.
Table 2.
Expression analysis of cytokines related to T-helper responses in gingival tissues during initiation (BL and 2W), progression (3M) and resolution (5M) of periodontitis by microarray.
| Th1 | BL | 2W | 1M | 3M | 5M |
|---|---|---|---|---|---|
| IFNgamma | 19.1 (3.8) | 20.3 (4.5) | 19.2 (5.4) | 18.4 (3.9) | 19.8 (2.9) |
| IL-12A | 17.9 (2.0) | 17.0 (2.1) | 18.1 (5.1) | 17.5 (2.1) | 19.1 (3.5) |
| IL-12B | 42.0 (7.2) | 38.3 (5.8) | 41.7 (9.0) | 38.1 (4.9) | 40.2 (5.9) |
| IL-18 | 432.7 (109.5) | 336.3 (63.4) | 365.1 (60.7) | 379.2 (81.4) | 400.7 (66.0) |
| Th2 | |||||
| IL-4 | 16.0 (2.6) | 15.7 (3.8) | 17.3 (3.4) | 16.7 (2.6) | 16.7 (2.6) |
| IL-5 | 12.8 (3.4) | 12.3 (1.7) | 13.3 (3.0) | 13.8 (2.7) | 12.7 (2.0) |
| IL-13 | 37.3 (4.5) | 34.6 (5.1) | 37.1 (6.1) | 36.7 (5.0) | 36.6 (8.2) |
| IL-25 | 25.2 (4.5) | 22.8 (3.3) | 23.3 (4.3) | 22.0 (2.8) | 24.5 (2.5) |
| Th17 | |||||
| IL-6 | 38.1 (23.0) | 70.0 (33.0) | 40.2 (11.6) | 37.9 (15.3) | 37.7 (13.7) |
| IL-1beta | 340.3 (328.6) | 1009.2 (1081.3) | 384.3 (214.8) | 283.7 (273.1) | 177.6 (146.3) |
| IL-23A | 90.0 (34.7) | 76.2 (21.6) | 83.1 (15.8) | 77.5 (24.6) | 88.3 (21.8) |
| IL-17 | 31.1 (7.6) | 34.3 (9.1) | 33.7 (6.2) | 32.6 (9.7) | 30.5 (5.4) |
| IL17F | 41.5 (22.2) | 32.6 (5.8) | 32.9 (6.6) | 32.7 (5.4) | 34.6 (6.1) |
| IL21 | 15.2 (2.1) | 15.9 (3.2) | 17.8 (5.5) | 18.9 (3.5) | 17.8 (4.6) |
| IL22 | 17.9 (12.2) | 12.5 (3.4) | 13.3 (5.6) | 11.9 (2.9) | 14.0 (5.0) |
| TNF | 91.1 (20.8) | 100.7 (22.8) | 100.2 (30.6) | 94.8 (27.4) | 95.6 (23.9) |
| Treg | |||||
| TGFbeta | 63.7 (6.8) | 68.3 (6.7) | 65.0 (8.3) | 66.1 (7.7) | 65.4 (14.0) |
| IL-2 | 7.4 (1.1) | 7.0 (1.3) | 7.9 (2.6) | 7.7 (1.3) | 8.4 (1.6) |
| IL-10 | 60.6 (27.7) | 60.3 (17.8) | 54.5 (7.2) | 49.3 (8.1) | 43.3 (7.7) |
Mean and Standard deviation in parenthesis of gene expression from 18 animals/tissues individually analyzed are shown. Significant differences (p≤0.05 vs. Baseline) are shown in red for over-expressed genes and blue for under-expressed genes.
Figure 2.
Cytokine expression levels determined by qRT-PCR. Significant changes in cytokine expression by microarray were validated using qRT-PCR. Total mRNA samples (n=18) from each time point were pooled and cytokine gene expression analyzed using triplicates. Data are expressed as means ± standard deviations. *p≤0.05 when baseline values where compared to the different time points, as determined by Student’s t test.
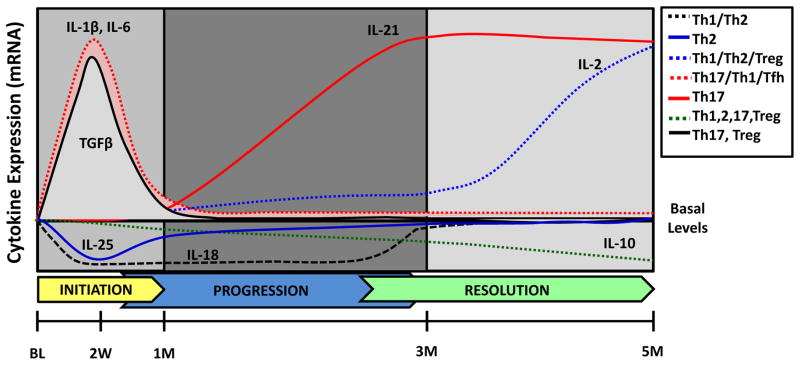
Figure 3.
Schematic of T-helper cytokines gene expression during initiation, progression and resolution of ligature-induced periodontal disease in nonhuman primates. Cytokines whose gene expression showed significant increase or decrease with respect to the basal levels were plotted using arbitrary units. The type of T-helper response(s) to which each cytokine has been previously associated are depicted by different line types and colors.
The expression kinetics of genes related to soft (i.e., MMP2 and MMP9) and bone (i.e., RANKL and CTSK) tissue destruction genes (TDGs) is shown in Table 3. All TDGs, showed significantly elevated expression at 2 weeks and 1 month which returned to basal levels by 3 months. However, expression of MMP9 remained elevated during the entire disease process and did not return to basal levels by the 5th month resolution sample.
Table 3.
Expression analysis of Tissue destruction genes (TDGs) in gingival tissues during initiation (BL and 2W), progression (3M) and resolution (5M) of periodontitis.
| TDGs | BL | 2W | 1M | 3M | 5M |
|---|---|---|---|---|---|
| MMP2 | 978.6 (372.9) | 1535.5 (538.3) | 1175.6 (234.3) | 1051.1 (212.2) | 889.1 (282.9) |
| MMP9 | 143.3 (66.9) | 522.5 (285.9) | 380.2 (136.2) | 315.5 (107.3) | 227.9 (127.2) |
| RANKL | 40.6 (8.0) | 51.2 (14.4) | 47.2 (8.7) | 43.6 (8.5) | 45.6 (8.7) |
| CTSK | 542.3 (196.4) | 759.7 (200.7) | 644.5 (113.0) | 631.2 (112.9) | 502.2 (124.8) |
Mean and Standard deviation in parenthesis of gene expression from 18 animals/tissues individually analyzed are shown. Significant differences (p≤0.05 vs. Baseline) are highlighted in red. MMP2: Matrix metalloproteinase 2, MMP9: Matrix metalloproteinase 9, RANKL: Receptor activator of nuclear factor kappa-B ligand, CTSK: Cathepsin K.
Correlation analyses of T helper-related cytokine genes with TDGs and clinical measures of BOP and PD are shown in Table 4. The expression of the majority of Th17/Treg cytokine genes showed a significant positive correlation with the expression of TDGs. In contrast, the expression of most Th1/Th2 genes was significantly negatively correlated with TDGs. Expression of cytokines associated to the Th17 response cells, such as IL-23A, IL-17F and IL-22 were negatively correlated with TDGs. Finally, expression of IFNγ, IL-4 and IL-21 that overlap Th1, Th2 and Th17 responses showed a negative correlation with MMP2 and MMP9, and a positive correlation with RANKL. Significant negative correlation was observed between IL-18 and IL23A and clinical measures; however, in contrast, IL-21 showed a positive correlation.
Table 4.
Correlation analyses of T helper-related cytokine genes with tissue destruction genes and clinical measures of periodontitis.
| MMP2 | MMP9 | RANKL | CTSK | BOP | PD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Th1 | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value |
| IFNgamma | −0.144 | 0.047 | 0.326 | 0.0015 | −0.102 | −0.007 | 0.151 | |||||
| IL-12A | −0.312 | 0.003 | −0.315 | 0.002 | 0.101 | −0.361 | 0.001 | −0.150 | 0.040 | |||
| IL-12B | −0.285 | 0.008 | −0.054 | 0.193 | −0.281 | 0.007 | −0.016 | −0.125 | ||||
| IL-18 | −0.413 | 6.0E-05 | −0.279 | 0.008 | −0.167 | −0.272 | 0.011 | −0.235 | 0.027 | −0.279 | 0.008 | |
|
| ||||||||||||
| Th2 | ||||||||||||
|
| ||||||||||||
| IL-4 | −0.213 | 0.047 | 0.113 | 0.219 | 0.037 | −0.099 | −0.031 | −0.045 | ||||
| IL-5 | −0.024 | −0.043 | −0.071 | −0.011 | −0.006 | −0.072 | ||||||
| IL-13 | −0.261 | 0.013 | −0.189 | −0.014 | −0.263 | 0.013 | −0.155 | −0.168 | ||||
| IL-25 | −0.072 | −0.317 | 0.002 | −0.093 | −0.165 | −0.076 | −0.073 | |||||
|
| ||||||||||||
| Th17 | ||||||||||||
|
| ||||||||||||
| IL-6 | 0.506 | 2.8E-07 | 0.571 | 1.0E-08 | 0.293 | 0.006 | 0.498 | 6.8E-07 | 0.132 | 0.197 | ||
| IL-1beta | 0.181 | 0.555 | 2.0E-08 | 0.571 | 1.0E-08 | 0.225 | 0.030 | 0.109 | 0.163 | |||
| IL-23A | −0.436 | 1.4E-05 | −0.242 | 0.023 | 0.081 | −0.499 | 6.4E-07 | −0.124 | −0.347 | 0.001 | ||
| IL-17 | 0.234 | 0.029 | 0.295 | 0.004 | 0.257 | 0.013 | 0.253 | 0.018 | 0.165 | 0.207 | ||
| IL17F | −0.225 | 0.029 | −0.116 | 0.067 | −0.280 | 0.008 | −0.156 | −0.163 | ||||
| IL21 | −0.242 | 0.023 | 0.191 | 0.325 | 0.002 | −0.145 | 0.040 | 0.219 | 0.039 | |||
| IL22 | −0.153 | −0.164 | 0.023 | −0.250 | 0.018 | −0.169 | −0.117 | |||||
| TNF | 0.044 | 0.223 | 0.037 | 0.345 | 0.001 | 0.094 | 0.109 | 0.194 | ||||
|
| ||||||||||||
| Treg | ||||||||||||
|
| ||||||||||||
| TGFbeta | 0.13 | 0.36 | 0.001 | 0.19 | 0.23 | 0.030 | 0.097 | 0.047 | ||||
| IL-2 | −0.31 | 0.003 | −0.13 | 0.07 | −0.33 | 0.002 | −0.024 | −0.033 | ||||
| IL-10 | 0.09 | 0.28 | 0.008 | 0.12 | 0.13 | 0.054 | −0.013 | |||||
Significant positive correlations are highlighted in red and significant negative correlations are highlighted in blue (p≤0.05). MMP-2 and MMP9 (matrix metalloproteinases 2 and 9), RANKL (Receptor activator of nuclear factor kappa-B ligand) and CTSK (Cathepsin K). BOP: Bleeding on Probing, PD: Pocket Depth.
DISCUSSION
It has been recognized for several years that variation in the expression profile of individual or a limited number of cytokines reflecting T-helper activities occur in periodontal disease tissues compared to healthy tissues (Preshaw and Taylor, 2011, Gaffen and Hajishengallis, 2008). However, how these changes are related to the pathogenesis of the disease remains unknown, since results derived from human studies cannot document the stage of the disease reflected in the tissues. Here, we described a transcriptional analysis of an array of T-helper cytokines and their relationship with tissue destructive genes involved in soft and bone tissue damage during ligature-induced periodontitis using a prospective nonhuman primate model of progressing periodontitis.
IL-1β and IL-6 were significantly over-expressed during the acute phase of disease at 2 weeks post-ligation. These changes are consistent with an important body of evidence linking increased levels of these two pro-inflammatory cytokines to periodontitis (Okada and Murakami, 1998) and earlier studies reporting similar changes in protein levels of pro-inflammatory mediators including IL-1β using the same model (Smith et al., 1993, Ebersole et al., 2000). Both cytokines have been shown to be critical drivers of the Th17 type of response, which appears to play a critical role in periodontitis-related tissue destruction (Gaffen and Hajishengallis, 2008). Nevertheless, IL-1β has also been related to a “non-classic” Th1 differentiation as well as in the expansion of Th1, Th2 and Th17 cells (Santarlasci et al., 2013). Likewise, IL-6 also appears to induce Th2 and Tfh cell differentiation (Diehl and Rincon, 2002, Eto et al., 2011). However, our findings are consistent with IL-1β and IL-6 mostly driving a Th17 response, which is coincident with the significant increases of IL-21 expression that were noted with progression of the disease at 3 months.
IL-21 is an IL-2 family member that enhances the Th17 differentiation of naïve Th cells. Importantly, the major sources of IL-21 are activated T cells and NKT cells, but not antigen presenting cells (Parrish-Novak et al., 2000), and the largest amounts of IL-21 are produced by Th17 cells, which appear to use IL-21 as a positive feedback loop for maintaining and amplifying the frequency of Th17 precursors when the supply of IL-6 is limited (Korn et al., 2007). Based on this, IL-21 has been suggested as an important player in chronic inflammatory disorders such as inflammatory bowel disease and rheumatoid arthritis (Fina et al., 2008, Niu et al., 2010). The role of IL-21 in periodontal disease remains unknown; however, in agreement with our results, a recent study demonstrated that IL-21 mRNA levels are overexpressed in untreated chronic periodontitis patients and its expression correlated with the expression of Th17 cytokines and clinical parameters of tissue destruction (Dutzan et al., 2012). Although TGFβ engages multiple pathways to control T cell differentiation, increased TGFβ levels accompanied by the presence of elevated IL-6 levels early during disease initiation suggests that it could be playing a role in Th17 rather than in Treg differentiation which would require the presence of IL-2 (Li and Flavell, 2008). Overall these observations are consistent with a cytokine profile driving a Th17 type of response early in the initiation process of periodontitis, followed by a persistence of the Th17 response related with the progression of the disease, in which IL-21 may play an important role in the chronic nature of the disease.
Interestingly, initiation and progression of the disease were also characterized by a significant reduction of IL-18 mRNA levels. IL-18 is a pro-inflammatory cytokine that belongs to the IL-1 cytokine family, and promotes the development of Th1 or Th2 responses in the presence or absence of IL-12 respectively (Biet et al., 2002). The role of IL-18 in periodontal disease remains unclear to date and inconclusive results have been reported regarding variation in its level during disease (Orozco et al., 2007).
Emerging evidence indicates that IL-18 could favor osteoclast formation and participate in the bone destruction observed in rheumatoid arthritis (Zhang et al., 2013, Dai et al., 2004), thus it would be expected that IL-18 levels could be elevated during destructive phases of periodontitis. Nevertheless, here we observed a reduction in the mRNA basal levels of IL-18 during initiation and progression phases of the disease and negative correlation with BOP and PD. Healthy epithelial tissues appear to have higher IL-18 mRNA levels constantly available that are reduced after a rapid translation to protein upon cellular activation (Kampfer et al., 1999). Consistently, IL-18 exhibited a higher mRNA level at base line among all cytokines. Thus, the reduction of mRNA IL-18 basal levels seen during periodontitis may be indicative of an active IL-18 translation and further increase in the IL-18 protein levels; however this will need to be documented in future studies.
Based on the role of IL-18 in Th differentiation, and the absence of significant changes in IL-12 expression, one could hypothesize that if indeed IL-18 mRNA levels are associated with decreased IL-18 protein levels, there would be a reduced likelihood for Th2 responses to occur in early stages of the disease. This hypothesis is in some way supported by the reduced expression levels of IL-25 during the progression of periodontitis observed by microarray and confirmed by qRT-PCR. IL-25 is an IL-17 cytokine family member (IL-17E) that has been related to Th2 cell responses, and enhances the expression of IL-4, IL-5 and IL13, as well as increasing the levels of IgE, IgG1, IgA, blood eosinophilia, and eosinophilic infiltrates in various tissues (Owyang et al., 2006). IL-25 is produced by several cell types including T lymphocytes, mast cells, eosinophils, and basophils (Ikeda et al., 2003, Fort et al., 2001) and its main target cells are monocytes/macrophages that are negatively regulated by IL-25 to reduce the pro-inflammatory cytokine responses induced by Toll-like receptor activation (Caruso et al., 2009). Variations in IL-25 levels associated with periodontal disease have not been previously reported; however the protective role and anti-inflammatory properties of this cytokine make it an interesting target for further studies.
Of note, increased levels of mRNA for IL-2 were significantly associated with the resolution phase of the disease. IL-2 is produced mainly by CD4+ T helper cells in secondary lymphoid organs, and to a lesser extent, by CD8+ T cells, natural killer cells, and natural killer T cells (Malek, 2008). Evidence indicates that IL-2 is involved in T-helper differentiation of Th1, Th2, Th17 and Tregs. For example, the absence of IL-2 is accompanied by a substantial decline in the number of Treg cells, whereas the numbers of Th17 cells increase, leading to enhanced susceptibility to inflammatory disorders. Thus, IL-2 has been suggested to play a role in maintaining the reciprocal balance between Th17 cells and Treg cells (Littman and Rudensky, 2010). In addition, IL-2 enhances Th1 and Th2 responses by increasing the T-bet expression (Th1 transcription factor) and IL4/IL13 transcriptional activation respectively (Boyman and Sprent, 2012). The multiple roles of IL-2 in T-helper subsets differentiation makes it difficult to interpret a potential role for IL-2 during resolution of periodontal disease and further studies will need to clarify this role. Nevertheless, based on the IL-2 effects on T-helper differentiation, resolution of periodontitis may require a recovery of the balance between Th17 and Treg cells which could possibly be altered during the progression of the disease (Okui et al., 2012). Additionally, restoration and amplification of impaired or blocked Th1 and Th2 responses, whereby IL-2 could play a central role, may be crucial ultimately allowing the control of periodontopathogenic bacteria.
A significant reduction in the IL-10 levels was observed during the resolution of periodontitis. IL-10 is an anti-inflammatory cytokine expressed by virtually all T-helper subsets and antigen presenting cells with a crucial role in preventing inflammatory and auto-immune diseases. (Maynard and Weaver, 2008) A fine-tuned balance in the IL-10 levels seems to be critical to efficiently remove pathogens without causing immunopathology. (Belkaid, 2007) Thus, a reduction of IL-10 levels could be important in enabling an efficient clearance or management of pathogenic biofilms by immune cells and ultimately resolving periodontitis.
Growing evidence indicates that some immune cells infiltrating the gingival tissues (especially lymphocytes), as well as some cytokines could be enhancing the production of tissue destructive matrix metalloproteinases (MMPs) and osteoclastogenesis (Taubman et al., 2005). It has been somewhat controversial, which type of T-helper responses are involved in tissue destruction during periodontitis, with both Th1 and Th17 responses suggested to play a major role. Correlation analysis of Th cytokine genes with TDGs indicated that the expression of most Th17/Treg genes showed significant positive correlations with TDGs. In contrast, the expression of the majority of Th1/Th2 genes significantly negatively correlated with TDGs. This observation suggests that soft tissue and bone tissue damage relate more to Th17/Treg cytokines, whereas the Th1/Th2 could actually contribute to protecting from progression of disease. However, the expression of some genes (i.e., IFNγ, IL-4, IL-21) involved in Th1, Th2 and Th17 responses showed a negative correlation with the MMPs but a positive correlation with RANKL. These results suggested that the net resultant expression of Th cytokine genes could be associated with different effects on the expression of tissue protective or destructive genes. Thus, Th1/Th2 responses could be critical for maintaining periodontal health, but once Th17/Treg-related early tissue damage occurs, the Th1/Th2 cell activities could also be involved in bone resorption at later stages of the disease.
Cytokines that belong to the Th17 response such as IL-23A, IL-17F and IL-22 were negatively correlated with TDGs and pocket depth. The effect of IL-17F and IL-22 in activation of MMPs and osteoclastogenesis has not been well described; however these cytokines appear to play important roles in the maintenance of mucosal barrier homeostasis and regulation of inflammation (Chang and Dong, 2007, Sonnenberg et al., 2011). As an example, IL-23p19 inhibits osteoclastogenesis by inducing GM-CSF production by Th17 and γδ T cells (Quinn et al., 2008). This suggests that some Th17 cytokines could be playing a dual role as protective or destructive molecules depending on the cytokine milieu of the tissues. Further studies exploring their active role in soft and bone tissue biology during the course of periodontal disease will be necessary.
In general, initiation, progression and resolution of periodontitis in nonhuman primates involve either up- or down-regulation of cytokine genes transcription related to more than one T-helper subset. Thus, it results difficult to associate an individual T-helper cell response subset or gene to a given phase of the disease. Post-transcriptional regulatory mechanisms are involved in cytokine translation (e.g., RNA binding proteins and miRNAs) (Palanisamy et al., 2012). Therefore, whether or not the transcriptional variations in Th cytokines observed in this study are equivalent to protein levels correlating with the presence of specific T cell subsets remain to be determined. The increasing number of cytokines and their potential interactions through cytokines networks highlight the need of using systems biology approaches, rather than focusing on single mediators for a clearer understanding of the balanced nature of immune responses in health, and loss of this balance with periodontal disease.
Since it would be impossible to reproduce this type of study in humans due to ethical reasons, we think that cytokine changes observed in the ligature-induced model in nonhuman primates is likely reflecting what is occurring in humans, where oscillating periods of initial high immunoinflammatory responses (2W-1M) followed by periods of significant but incomplete resolution (3M) of such responses are taking place. Thus, the frequency of oscillating periods may be determining the severity and extension of the disease. The use of the nonhuman primate translational model combined with cellular and molecular approaches will help to document and characterize the complexity of the dynamic variations of T-helper cell activities and oral microbiome changes related to the pathogenesis of periodontal disease, as well as to identifying potential new molecular biomarkers of disease activity and resolution which could not be closely recapitulated using in vitro or murine models, which despite their helpfulness in developing functional studies, exhibit clear clinical and microbiological differences with respect to humans. Interestingly and similar to what is normally seen in human studies, a great variability in cytokine gene expression was noted. Even though this animal model allows us to control for many extrinsic variables that can modify the host response and therefore the progression of periodontitis, this inter-individual variation could be capitalized in future studies focused towards identifying individual molecular profiles related to resistance or susceptibility for periodontal disease progression and severity, which would help to build evidence that supports the rapidly growing concept of personalized medicine/dentistry.
CLINICAL RELEVANCE.
Scientific rationale for the study
Although evidence supports that cytokines are involved in periodontitis, it remains unclear, what type of T-cell cytokine response(s) are related to initiation, progression and resolution of the disease.
Principal findings
Initiation, progression and resolution of periodontitis involved either up- or down-regulation of cytokine genes related to more than one T-helper subset. Thus, it is difficult to associate an individual T-helper response subset/gene with a given phase of the disease.
Practical implications
Identifying profiles of specific periodontitis-related variations of T-cell cytokines could help to define groups of multiple molecular biomarkers related to disease activity and resolution.
Acknowledgments
SOURCES OF FUNDING: This work was supported by National Institute of Health grants P20GM103538 and UL1TR000117.
We express our gratitude to the Caribbean Primate Research Center (CPRC) supported by grant P40RR03640, and the Microarray Core of University Kentucky for their invaluable technical assistance.
Footnotes
DISCLOSURE
The authors report no conflicts of interest related to this study.
References
- Adibrad M, Deyhimi P, Ganjalikhani Hakemi M, Behfarnia P, Shahabuei M, Rafiee L. Signs of the presence of Th17 cells in chronic periodontal disease. J Periodontal Res. 2012;47:525–531. doi: 10.1111/j.1600-0765.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- Biet F, Locht C, Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med (Berl) 2002;80:147–162. doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- Branch-Mays GL, Dawson DR, Gunsolley JC, Reynolds MA, Ebersole JL, Novak KF, Mattison JA, Ingram DK, Novak MJ. The effects of a calorie-reduced diet on periodontal inflammation and disease in a non-human primate model. J Periodontol. 2008;79:1184–1191. doi: 10.1902/jop.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Cardoso CR, Garlet GP, Moreira AP, Junior WM, Rossi MA, Silva JS. Characterization of CD4+CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leukoc Biol. 2008;84:311–318. doi: 10.1189/jlb.0108014. [DOI] [PubMed] [Google Scholar]
- Caruso R, Stolfi C, Sarra M, Rizzo A, Fantini MC, Pallone F, MacDonald TT, Monteleone G. Inhibition of monocyte-derived inflammatory cytokines by IL-25 occurs via p38 Map kinase-dependent induction of Socs-3. Blood. 2009;113:3512–3519. doi: 10.1182/blood-2008-08-172767. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Dai SM, Nishioka K, Yudoh K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis. 2004;63:1379–1386. doi: 10.1136/ard.2003.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- Dutzan N, Vernal R, Vaque JP, Garcia-Sesnich J, Hernandez M, Abusleme L, Dezerega A, Gutkind JS, Gamonal J. Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients. J Periodontol. 2012;83:948–954. doi: 10.1902/jop.2011.110482. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Cappelli D, Holt SC, Singer RE, Filloon T. Gingival crevicular fluid inflammatory mediators and bacteriology of gingivitis in nonhuman primates related to susceptibility to periodontitis. Oral Microbiol Immunol. 2000;15:19–26. doi: 10.1034/j.1399-302x.2000.150104.x. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Steffen MJ, Gonzalez-Martinez J, Novak MJ. Effects of age and oral disease on systemic inflammatory and immune parameters in nonhuman primates. Clin Vaccine Immunol. 2008;15:1067–1075. doi: 10.1128/CVI.00258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M, Pallone F, Macdonald TT, Monteleone G. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–1048. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: rethinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez OA, Stromberg AJ, Huggins PM, Gonzalez-Martinez J, Novak MJ, Ebersole JL. Apoptotic genes are differentially expressed in aged gingival tissue. J Dent Res. 2011;90:880–886. doi: 10.1177/0022034511403744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K, Vahedi G, Ghoreschi K, Yang XP, Nakayamada S, Kanno Y, O’Shea JJ, Laurence A. Helper T-cell differentiation and plasticity: insights from epigenetics. Immunology. 2011;134:235–245. doi: 10.1111/j.1365-2567.2011.03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- Kampfer H, Kalina U, Muhl H, Pfeilschifter J, Frank S. Counterregulation of interleukin-18 mRNA and protein expression during cutaneous wound repair in mice. J Invest Dermatol. 1999;113:369–374. doi: 10.1046/j.1523-1747.1999.00704.x. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–233. doi: 10.1111/j.1600-065X.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meka A, Bakthavatchalu V, Sathishkumar S, Lopez MC, Verma RK, Wallet SM, Bhattacharyya I, Boyce BF, Handfield M, Lamont RJ, Baker HV, Ebersole JL, Kesavalu L. Porphyromonas gingivalis infection-induced tissue and bone transcriptional profiles. Mol Oral Microbiol. 25:61–74. doi: 10.1111/j.2041-1014.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AJ, Cappelli D, Lantz MS, Holt SC, Ebersole JL. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. J Periodontol. 1998;69:686–697. doi: 10.1902/jop.1998.69.6.686. [DOI] [PubMed] [Google Scholar]
- Mucida D, Cheroutre H. The many face-lifts of CD4 T helper cells. Adv Immunol. 2010;107:139–152. doi: 10.1016/B978-0-12-381300-8.00005-8. [DOI] [PubMed] [Google Scholar]
- Niu X, He D, Zhang X, Yue T, Li N, Zhang JZ, Dong C, Chen G. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum Immunol. 2010;71:334–341. doi: 10.1016/j.humimm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–266. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Takayanagi H. Regulation of bone by the adaptive immune system in arthritis. Arthritis Res Ther. 2011;13:219. doi: 10.1186/ar3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okui T, Aoki Y, Ito H, Honda T, Yamazaki K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res. 2012;91:574–579. doi: 10.1177/0022034512446341. [DOI] [PubMed] [Google Scholar]
- Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin 18 and periodontal disease. J Dent Res. 2007;86:586–593. doi: 10.1177/154405910708600702. [DOI] [PubMed] [Google Scholar]
- Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy V, Jakymiw A, Van Tubergen EA, D’Silva NJ, Kirkwood KL. Control of cytokine mRNA expression by RNA-binding proteins and microRNAs. J Dent Res. 2012;91:651–658. doi: 10.1177/0022034512437372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011;38(Suppl 11):60–84. doi: 10.1111/j.1600-051X.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- Quinn JM, Sims NA, Saleh H, Mirosa D, Thompson K, Bouralexis S, Walker EC, Martin TJ, Gillespie MT. IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J Immunol. 2008;181:5720–5729. doi: 10.4049/jimmunol.181.8.5720. [DOI] [PubMed] [Google Scholar]
- Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T Helper Immune Responses. Front Immunol. 2013;4:182. doi: 10.3389/fimmu.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- Smith MA, Braswell LD, Collins JG, Boyd DL, Jeffcoat MK, Reddy M, Li KL, Wilensky S, Vogel R, Alfano M, et al. Changes in inflammatory mediators in experimental periodontitis in the rhesus monkey. Infect Immun. 1993;61:1453–1459. doi: 10.1128/iai.61.4.1453-1459.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, Dolhain RJ, Lubberts E. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- Zhang W, Cong XL, Qin YH, He ZW, He DY, Dai SM. IL-18 upregulates the production of key regulators of osteoclastogenesis from fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation. 2013;36:103–109. doi: 10.1007/s10753-012-9524-8. [DOI] [PubMed] [Google Scholar]