Abstract
Peptide binding to major histocompatibility complex class II (MHCII) molecules is a key process in antigen presentation and CD4+ T cell epitope selection. This unit describes a fairly simple but powerful fluorescence polarization-based binding competition assay to measure peptide binding to soluble recombinant MHCII molecules. The binding of a peptide of interest to MHCII molecules is assessed based on its ability to inhibit the binding of a fluorescence-labeled probe peptide, with the strength of binding characterized as IC50 (concentration required for 50% inhibition of probe peptide binding). Data analysis related to this method is discussed. In addition, this unit includes a support protocol for fluorescence labeling peptide using an amine-reactive probe. The advantage of this protocol is that it allows simple, fast, and high throughput measurements of binding for a large set of peptides to MHCII molecules.
Keywords: MHCII, Peptide binding, Fluorescence polarization, binding competition, IC50
INTRODUCTION
In this unit, we describe a simple and high-throughput fluorescence polarization-based assay to measure peptide binding to MHCII molecules (Basic Protocol). A probe peptide which has a high affinity to MHCII is labeled with a fluorochrome. Unlabeled target peptide is co-incubated with the probe peptide competing for the binding to MHCII. Incubation is typically 72 hours at 37 °C. The binding of labeled probe peptide to MHCII is measured by fluorescence polarization (FP). A Support Protocol is provided to enable labeling of the probe peptide with Alexa488 fluorochrome. IC50, the concentration of unlabeled target peptide required to inhibit the binding of labeled probe peptide by 50% can be obtained by plotting relative binding versus unlabeled target peptide concentration. IC50 represents a reasonable measurement of peptide binding affinity to MHCII molecules.
BASIC PROTOCOL
MEASUREMENT OF PEPTIDE BINDING TO MHCII MOLECULES AS IC50 USING A FLUORESCENCE POLARIZATION-BASED BINDING COMPETITION ASSAY
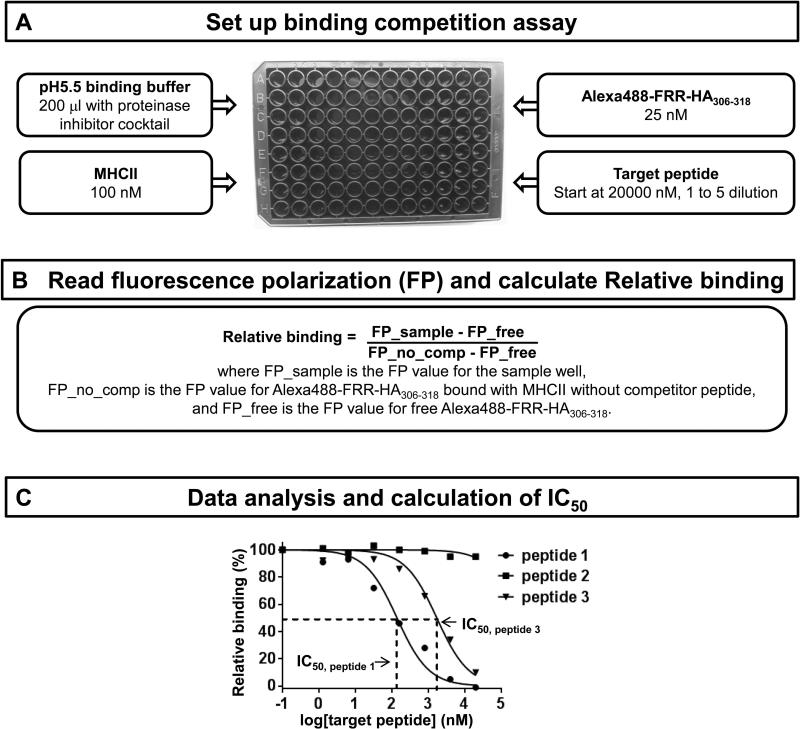
This protocol describes an assay to measure the binding of peptides to MHCII molecules. Fluorescence-labeled probe peptide is incubated with MHCII molecules, together with serial dilutions of unlabeled target peptide at 37 °C for 72 hours. Binding affinity of target peptide is assessed by IC50, which is the concentration of unlabeled target peptide required to inhibit the binding of labeled probe peptide by 50%. IC50 can be determined by plotting the binding competition curve. A flow chart schematizing the assay is presented in Figure 1.
Figure 1. A schematic overview of the steps involved in measuring peptide binding to MHCII molecules by fluorescence polarization.
(A) Set up the binding competition assay as shown in Table I and incubate at 37 °C for 72 hours. (B) Read FP on a 96-well microplate reader, which has been calibrated, and convert the FP values into Relative binding using the equation indicated. (C) Fit the competition curve to obtain IC50. Peptide 1 represents a high affinity peptide, as IC50 is 139nM; peptide 2 has no binding affinity as no competition is observed; peptide 3 has an intermediate affinity with an IC50 of 1709nM.
Materials
Alexa488 labeled influenza hemagglutinin-derived probe peptide (Alexa488-FRR-HA306-318; see support protocol for the labeling)
Unlabeled target peptide Double-distilled water (ddH2O; Millipore)
Dimethylsulfoxide (DMSO)
Purified MHCII molecules (HLA-DR1 in this protocol) (see Unit 18.3) [*Copy ED: Query as to whether HLA-DR1 is available commercially – RC]
Phosphate-buffered saline (PBS), pH7.4 (see recipe)
Binding buffer, pH5.5 (see recipe)
Protease inhibitor cocktail (prepare fresh; see recipe)
1.5 ml black microtube
1.5 ml clear microtube
96-well Polypropylene flat-bottom non-binding black Microplate (Greiner)
Aluminum Sealing Foil for 96-well Microplate (USA Scientific)
37 °C incubator
VICTOR X5 Multilabel Plate Reader (PerkinElmer), POLARstar OPTIMA Plate Reader (BMG LABTECH) or other fluorescence microplate reader equipped for fluorescence polarization
GraphPad Prism 6 graphing and data analyzing software
Prepare peptides
1. Solubilize lyophilized Alexa488-FRR-HA306-318 in a 1.5 ml black microtube with ddH2O to the stock concentration of 200 μM.
See support protocol for the sequence information and labeling procedure of Alexa488-FRR-HA306-318. It is extremely import to keep the fluorescence-labeled peptide in dark and minimize the exposure to light.
2. Solubilize each lyophilized unlabeled target peptide in a 1.5 ml clear microtube with 100% DMSO to the concentration of 20 mg/ml by adding 50μl 100% DMSO to 1mg peptide. Then, dilute the peptide into 50% DMSO to the stock concentration of 400 μM.
The unlabeled target peptides were synthesized at 21st Century Biochemicals with acetylated N-termini and shipped to us as 1mg lyophilized powder in 1.5ml clear microtube. If test peptides are soluble in ddH2O, one could use ddH2O to solubilize each peptide, instead of DMSO here. We have verified that the DMSO contained in peptide stock solution up to 2.5% does not interfere with following peptide binding or FP reading.
Prepare binding competition reaction mix
3. In the 96-well plate, prepare 200 μl binding competition reaction mixture in each well, containing 100 nM MHCII, 25 nM Alexa488-FRR-HA306-318, and series dilution of target peptide, starting at 20uM, 1 to 5 dilution, leaving the last well without competing target peptide for determination of FP value for no inhibition. Control wells with 25 nM Alexa488-FRR-HA306-318 in buffer but without MHCII are also included for determination of FP value for free peptide.
The reaction is performed in pH5.5 binding buffer, with freshly added protease inhibitor cocktail and incubated at 37 °C for 72 hours. Recombinant MHCII is purified as previously described (Frayser et al., 1999; Stern and Wiley, 1992) and stored in PBS. An example of plate set up can be found in Table 1.
Table 1.
Typical set up of the binding competition assay in a 96-well Microplate for measuring peptide binding to MHCII molecules
| Target peptide (nM)a | Target peptide 1 | Target peptide 2 | Target peptide 3 | Target peptide 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + MHCII +Alexa488-FRR-HA306-318 | + MHCII +Alexa488-FRR-HA306-318 | + MHCII +Alexa488-FRR-HA306-318 | + MHCII +Alexa488-FRR-HA306-318 | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 20000 | A | ||||||||||||
| 4000 | B | ||||||||||||
| 800 | C | ||||||||||||
| 160 | D | ||||||||||||
| 32 | E | ||||||||||||
| 6.4 | F | ||||||||||||
| 1.28 | G | ||||||||||||
| 0 | H | FP_no_compb | FP_freec | ||||||||||
Target peptide concentrations starts at 20000 nM with a dilution factor of 5. Assay for each peptide is performed with three replicates. The last row H has no competing target peptide.
Row H, columns 1-6 contain only MHCII and labeled probe peptide, no competing target peptide, allowing for measurement of FP for labeled probe peptide bound with MHCII without competitor peptide (FP_no_comp).
Row H, columns 7-12 contain only labeled probe peptide, no MHCII and competing target peptide, allowing for measurement of FP for free unbound labeled probe peptide (FP_free).
The concentrations for MHCII (100 nM) and labeled probe peptide (25 nM) have been optimized for this assay. The labeled probe peptide is set at lowest concentration for which reliable fluorescence polarization values can be obtained. The MHCII concentration is set by titrating MHCII against fixed labeled peptide concentration, with the selected MHCII concentration which allows for ~50-75% of the maximum binding as judged by polarization assay. It is crucial to optimize the concentrations of MHCII and labeled probe peptide for appropriate FP reading. The starting concentration and dilution factor for serial dilution of target peptides can be changed depending on specific assay configuration.
Detect amount of MHCII-Alexa488-FRR-HA306-318 complex by FP
4. After 72 hr incubation, read FP of the plate using appropriate microplate reader at excitation 485 nm and emission 520 nm.
The key point of this assay is to use FP to measure the fraction of labeled probe peptide bound in complex with MHCII. The FP value for free Alexa488-FRR-HA306-318 is ~35 mP, and for purified MHCII- Alexa488-FRR-HA306-318 complex is ~350. It is important that the plate reader has been calibrated and tested for polarization measurements. Usually, a standard stock solution of fluorescein is used to calibrate the instrument following the manufacturer's recommendations, to give a FP value of 27 mP in aqueous alkali buffer at room temperature. To simulate the FP of the labeled probe peptide in the bound form with MHCII, we solubilize the labeled probe peptide in different percentage of glycerol. In general, 0% glycerol should give the FP value for free form, ~35 mP; 20% glycerol should give the FP value for 50% bound, ~200 mP; and 50% glycerol should give the FP value for 100% bound, ~350 mP. Alternately, purified MHCII- Alexa488-FRR-HA306-318 complex can be used.
Data analysis and calculation of IC50
5. Use the average of the FP values of wells with control free Alexa488-FRR-HA306-318 (no MHCII) as the FP_free. Use the average of the FP values of wells without competing target peptide as FP_no_comp.
6. Calculate Relative binding for the other wells as (FP_sample-FP_free) / (FP_no_comp– FP_free)
7. Plot Relative binding versus concentration of unlabeled target peptide, and fit the curve into equation y=1/(1+[pep]/IC50), where [pep] is the concentration of unlabeled target peptide; and IC50 is the 50% inhibition concentration.
An example of the binding competition curve and calculation of IC50 for peptides with different binding affinities is shown in Figure 1C.
SUPPORT PROTOCOL
LABELING OF THE PROBE PEPTIDE WITH A FLUOROCHROME
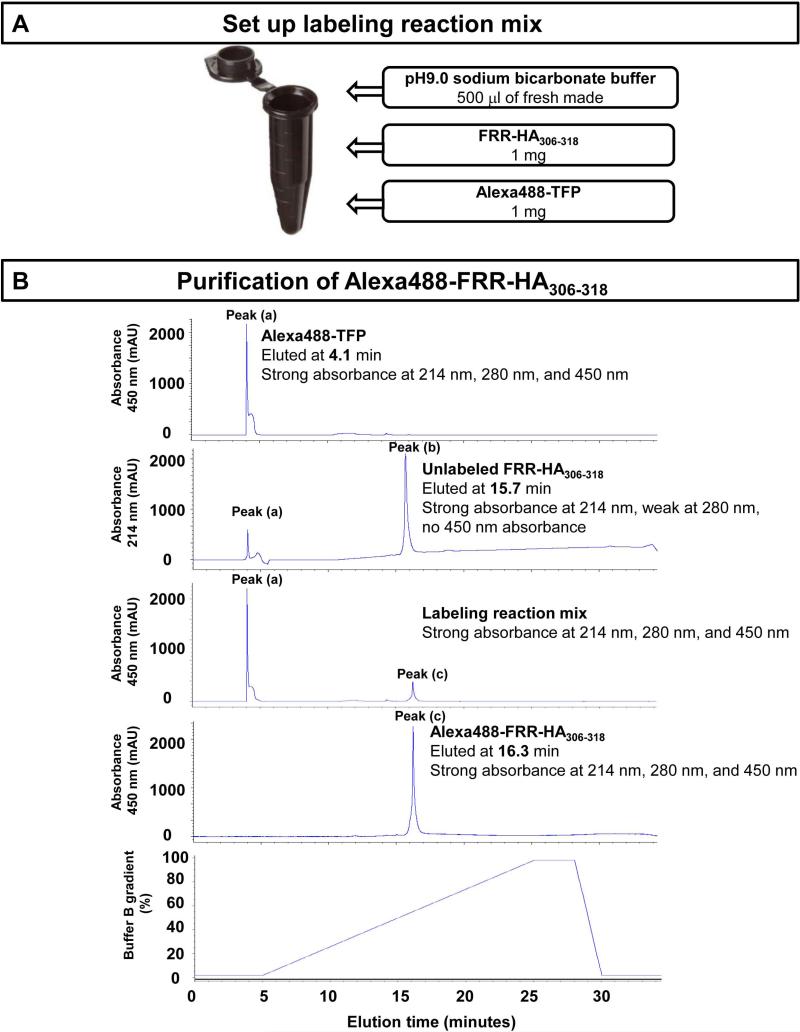
This protocol describes the labeling and purification of a probe peptide FRR-HA306-318 with Alexa488 fluorochrome through amine-reactive probe. FRR-HA306-318 with single lysine at position 310 (K310) is incubated with Alexa488-TFP at room temperature for 1 hour in 150 mM pH9.0 sodium bicarbonate buffer. The labeled peptide is purified using Jupiter 5u C18 column and lyophilized to store as peptide powder. The procedure can be adapted to label other probe peptides with single lysine in the sequence. The labeled probe peptide can be used to study peptide association and dissociation to MHCII molecules, and to measure IC50 for other unlabeled peptides using as probe peptide in binding competition assay. A flow chart schematizing the protocol is presented in Figure 2.
Figure 2. A schematic overview of the steps involved in labeling the probe peptide with a fluorochrome.
(A) Set up the labeling reaction mix and incubate at room temperature for 1 hour. (B) Representative elution profile of fluorochrome Alexa488-TFP (first panel, peak (a)), unlabeled FRR-HA306-318 (second panel, peak (b)), labeling reaction mix (third panel), and Alexa488-labeled FRR-HA306-318 (fourth panel, peak (c)). Peak (a) in the second panel comes from the residual fluorochrome Alexa488-TFP in the last run. The fifth panel shows the Buffer B gradient.
As an alternate approach, peptides containing a single cysteine residue can be labeled efficiently using thiol-specific reagents, such as Alexa488-maleimide. In this case the binding reaction is done at pH7.5 (for example conveniently in phosphate-buffered saline). The presence of free amino groups (N-terminus and lysine) in the peptide or amine-containing compounds in the reaction buffer is immaterial; however it is crucial that the peptide thiol group should be reduced before reaction. In most cases purified synthetic peptides stored after lyophilization from acidic buffer will be fine, but oxidized peptides will need to be reduced (for example using 50 mM dithiothreitol) and repurified before reaction.
Materials
Influenza hemagglutinin (HA) analog peptide FRR-HA306-318 (Ac-PRFVKQNTLRLAT) Alexa Fluor 488 Carboxylic Acid, 2,3,5,6-Tetrafluorophenyl Ester (Alexa488-TFP)
1.5 ml black microtube
150 mM sodium bicarbonate buffer, pH9.0 (or other non-amine buffer) (made fresh)
Double-distilled water (ddH2O)
Trifluoroacetic acid (TFA)
Acetonitrile
Buffer A (5% TFA in ddH2O)
Buffer B (5% TFA in acetonitrile)
Jupiter 5u C18 300A 250x4.6mm column (C18 column; Phenomenex)
Agilent 1200 series Liquid Chromatography system (LC; Agilent Technologies) Lyophilizer
Prepare labeling reaction mix
1. Dissolve 1 mg FRR-HA306-318 in 500 μl 150 mM pH9.0 sodium bicarbonate buffer, and then transfer to a 1.5 ml black microtube containing 1 mg Alexa488-TFP. Reserve 5 μg of FRR-HA306-318 before transfer to Alexa488-TFP for analysis. Also reserve some Alexa488-TFP by dipping a pipette tip into the fluorochrome powder.
It is extremely important that the labeling reaction mix be free of amine-containing substances such as Tris, glycine, ammonium ions. In order to label each peptide with only one fluorochrome, it is crucial that the peptide has only one active amine group, which is the K310 on FRR-HA306-318. If the peptide being labeled or the fluorochrome being used is not soluble in sodium bicarbonate buffer, one can first dissolve it in DMSO or DMF at 10 mg/ml stock solution, and then dilute it into sodium bicarbonate buffer. We observe efficient labeling with molar ratio of fluorochrome to peptide of 2:1 to 5:1. In the protocol described, the ratio is ~2:1. Other fluorescent probes such as fluorescein or tetramethylrhodamine can be used instead of Alexa488, but Alexa488 fluorescence is relatively pH-independent, environment-independent, and relatively stable to photobleaching (Molecular Probes Handbook) that bound and free labeled peptides have clearly distinct FP values. As such it is nearly ideal for this purpose. Other amine-specific modifying groups can be used, but TFP (trifluorophenyl ester) is more stable to hydrolysis in aqueous solution than other commonly used reactive groups such as N-hydroxysuccinimides or imidoesters.
2. Incubate the labeling reaction mix at room temperature for 1 hour with mixing every 10 minutes.
Analysis of labeling by reverse phase LC
3. Run unlabeled probe peptide FRR-HA306-318, free Alexa488-TFP, and a small sample (0.2%) of the labeling reaction mixture over C18 column measuring absorbance at wavelengths to detect both the peptide and the fluorochrome. We use 214 nm, 280 nm and 450 nm. Each sample should be diluted into 100 μl Buffer A before injection onto an equilibrated column.
For unlabeled FRR-HA306-318, dilute the reserved 5 ug into 100 μl Buffer A. For free Alexa488-TFP, transfer the reserved powder into 100 μl Buffer A.
To check the progress of the labeling reaction and to identify the labeled peptide peak from unlabeled peptide and free fluorochrome peaks, we aliquot 1 μl of the 500 μl labeling reaction mix and dilute it into 100 μl Buffer A and inject samples of unreacted peptide, unreacted fluorochrome, and reaction mix. The unlabeled FRR-HA306-318 should have strong absorbance at 214 nm, weak at 280 nm and not at 450 nm. The free Alexa488-TFP should have strong absorbance at 214 nm, 280 nm and 450 nm, with elution time distinct from that of unlabeled FRR-HA306-318. The labeling reaction mix should show a new peak, having absorbance at 214 nm, 280 nm, and 450 nm, which corresponds to the Alexa488-labeled FRR-HA306-318. If so, we can proceed to the purification of Alexa488-FRR-HA306-318. If a substantial peak for unlabeled FRR-HA306-318 still shows up in the labeling reaction mix, we should increase the ratio of fluorochrome to peptide and/or incubate the labeling reaction mix for a longer time. If the peak for Alexa488-FRR-HA306-318 is so close to that of unlabeled FRR-HA306-318, we should adjust the elution gradient until they are separate enough for purification. The finalized Buffer B gradient for elution is listed in Table II, and examples of elution traces for unlabeled FRR-HA306-318, free Alexa488-TFP, reaction mix and Alexa488-labeled FRR-HA306-318 are shown in Figure 2B.
Table 2.
Elution gradient for purification of Alexa488-FRR-HA306-318
| Time (minute) | % Buffer Ba | Flow Rate (ml/minute) |
|---|---|---|
| 0 | 0.0 | 1.0 |
| 5b | 2.0 | 1.0 |
| 25c | 98.0 | 1.0 |
| 28d | 98.0 | 1.0 |
| 30e | 2.0 | 1.0 |
| 35f | 2.0 | 1.0 |
Buffer B is 5% TFA in acetonitrile, and the counterpart Buffer A is 5% TFA in ddH2O.
Use 5 minutes to equilibrium the C18 column in 2% Buffer B.
Run the gradient of Buffer B from 2% to 98% for 20 minutes, which will elute Alexa488-FRR-HA306-318 from the C18 column.
Keep the gradient of Buffer B at 98% for 3 minutes to wash off any remaining substances from the column.
Equilibrium the C18 column back into 2% Buffer B.
Stop the current run at 35 minutes and keep the C18 column in 2% Buffer B for the next run.
Purification of Alexa488-labeled FRR-HA306-318 by reverse phase LC
4. Inject the remaining ~500 μl of the labeling reaction mix and collect fractions for Alexa488-FRR-HA306-318.
We collect 50 fractions during a 10 minutes period, and each vial has 200 μl. One can adjust the number of fractions collected and the amount in each fraction based on the elution profiles.
5. Reinject 1 μl of the pooled fractions of Alexa488-FRR-HA306-318 into C18 column to check the purity.
It is recommended to check the labeled probe peptide by mass spectrometry and functional analysis, such as peptide binding in our case.
6. Measure the concentration of Alexa488-FRR-HA306-318 by measuring the absorbance of the fluorochrome at its excitation maximum and store as lyophilized powder in −20 °C in dark.
We measure the absorbance of Alexa488-FRR-HA306-318 from 200 nm to 600 nm and find that the maximal absorbance is at 495 nm. [Alexa488-FRR-HA306-318]=Absorbance495/71000 (M), where 71000 is the extinction coefficient at maximal wavelength for fluorochrome Alexa488.
REAGENTS AND SOLUTIONS
Binding buffer, pH5.5
100 mM sodium citrate (mix of 0.46%w/v anhydrous citric acid and 2.23%w/v sodium citrate dihydrate)
50 mM sodium chloride
5 mM EDTA
0.1% (w/v) octylglucoside
0.1% (w/v) iodoacetamide
0.1% (w/v) sodium azide
Adjust the pH if necessary to 5.5 using sodium hydroxide and hydrochloric acid Store up to 3 months at room temperature
Phosphate-buffered saline
137 mM sodium chloride
2.7 mM potassium chloride
10 mM disodium hydrogen phosphate
1.8 mM potassium dihydrogen phosphate
Adjust the pH to 7.4 using hydrochloric acid
Store up to 6 months at room temperature
Proteinase inhibitor cocktail (100X)
AEBSF (4-(2-Aminoethyl)benzenesulfonyl fluoride dydrochloride)
Aprotinin
Bestatin hydrochloride
E-64 (N-(trans-Epoxysuccinyl)-L-leucine 4-guanidinobutylamide)
EDTA
Leupeptin hemisulfate salt
This protease inhibitor cocktail is bought from Sigma-Aldrich as a lyophilized powder (catalog number P2714). We dissolve it into 10 ml ddH2O and use it as 100X. Store up to 1 year in small aliquots in −20 °C. Use a fresh vial each time.
COMMENTARY
Background Information [*Copy Ed: Please inform the authors that the editorial board suggested that it would be valuable to have at least one or sentences somewhere in the commentary about the potential applicability (or not) to an MHCI peptide binding assay. Since the off rates of MHCI are longer, it would not be directly comparable. –RC]
Antigen presentation to CD4+ T cells by MHCII molecules plays a central role in the activation and regulation of adaptive immunity. Characterization of peptide binding to MHCII molecules is crucial in antigen presentation and CD4+ T cell epitope selection (Germain, 1994; Roche and Cresswell, 1990b). Measurement of peptide binding is also important in developing, training and evaluation of epitope prediction algorithms based on binding affinity (Greenbaum et al., 2011; Hammer et al., 1992; Peters et al., 2005). Therefore, developing a method capable of measuring binding affinity for a large set of peptides has been one of the key efforts in understanding the molecular mechanism of MHCII antigen presentation, in identifying CD4+ T cell epitopes, and in improving the efficiency of CD4+ T cell epitope prediction algorithms.
The protocol described in this unit allows for a reliable, fast, simple and high throughput measure of peptide binding to MHCII molecules. This FP-based method uses a well-characterized and widely-used influenza hemagglutinin-derived probe peptide (Ferrante et al., 2008; Ferrante and Gorski, 2010; Joshi et al., 2000; Narayan et al., 2007; Roche and Cresswell, 1990a; Stern et al., 1994; Zhou et al., 2009), to study the binding competition by unlabeled target peptides. First introduced in 2006 (De Wall et al., 2006; Nicholson et al., 2006), the FP assay has now become the standard for measuring MHCII-peptide interactions, in studies of T cell epitope selection (Nastke et al., 2012; Yin et al., 2012) and HLA-DM-mediated peptide exchange (Guce et al., 2013; Painter et al., 2011), and in routine use in our laboratory as well as others in the field (Anders et al., 2011; Ferrante and Gorski, 2010; Ferrante and Gorski, 2012; Pos et al., 2012; Zhou et al., 2009).
Other methods have been used previously in MHCII binding experiments to quantify the amount of bound complex, including fluorescence resonance energy transfer (Joshi et al., 2000; Zarutskie et al., 2001), direct fluorescence measurement (Ferrante and Gorski, 2012; Narayan et al., 2007; Rothbard and Busch, 2001), gel filtration using radioactive peptide (Kim et al., 2013; Sidney et al., 2013; Stern and Wiley, 1992), SDS-PAGE gel shift (Ferrante and Gorski, 2012; Kim et al., 2013; Stratikos et al., 2004), ELISA with biotinylated peptide (Tompkins et al., 1993) and surface plasmon resonance(Kim et al., 2013; Narayan et al., 2009). All these methods allow for a direct measurement of binding affinity and estimation of Kd from the association curves. However, the fact that each target peptide has to be labeled for detection in the association reaction and that multiple time points have to be collected to plot the association curves limits the application for measuring binding affinity for a large set of peptides. Compared with these previously used methods, the major advantage of the protocol described in this Unit is that a single labeled probe peptide can be used for binding determination of many different unlabeled peptides and that only single final read is needed to calculate IC50. The protocol we described here uses 96-well microtiter assay plates, and could easily be applied to 384-well or 1536-well format because the basic FP measurement is concentration- and volume-independent.
One potential disadvantage of this protocol is that it measures only relative affinity by comparison with probe peptide, instead of the absolute equilibrium dissociation constant Kd that describes the binding interaction in energetic terms. Under some circumstances, principally simple equilibrium binding reactions where the competing ligands are in large excess over the binding partner, IC50 values can be related to Kd values using the Cheng- Prusoff equation Kd=IC50/(1+[probe peptide]/Kd,probe peptide) (Cheng and Prusoff, 1973). In our case, the concentration of Alexa488-FRR-HA306-318 is 25 nM, and the Kd is expected to be ~10 nM based on measurements of the unmodified peptide (Roche and Cresswell, 1990a), but the MHCII concentration is not negligible, even considering the fact that some of the protein does not participate in the binding reaction (Joshi et al., 2000). In addition, there is considerable kinetic complexity in the interaction of MHCII proteins with peptides (de Kroon and McConnell, 1994; Joshi et al., 2000; Narayan et al., 2007; Pos et al., 2012), including steps not likely to reach equilibrium even during the extended 72 hr incubation time. Thus, relating IC50 and Kd values is not as straightforward in this system. However, in most cases Kd is expected to scale with the IC50, so that the relative Kd and certainly the Kd rank are reflected in the IC50 values. If necessary, the FP approach described here can be adapted to more elaborate assays of the binding reaction, including kinetic studies and HLA-DM dependence (Anders et al., 2011; Yin et al., 2012).
In summary, in this Unit we describe a protocol for fast, simple and high throughput measurement of peptide binding affinity to MHCII molecules, which will facilitate our understanding of the antigen presentation process and improve our ability to screen and predict CD4+ T cell epitopes.
Critical Parameters
Acquisition of the equipment (mainly the microplate reader and LC system) and reagents (mainly purified MHCII, synthesized peptides and fluorochrome) should be considered before the implementation of this procedure. Many microplate readers can be used to measure absorbance, fluorescence, FP and sometimes luminescence and time-resolved fluorescence by changing the detection modes. HPLC systems are widely used in purifying and analyzing biological chemicals by changing the columns. This increased use of microplate reader and LC system in biochemical and biophysical applications has made them a ready tool for immunologists. For measuring FP, it is important to calibrate the instrument before use as described in this protocol and instrument manual. The success of this protocol depends on appropriate measurement of FP. Therefore, it is crucial to optimize the concentrations of MHCII and labeled probe peptide in the competition assay by titration. Because of the usage of fluorescence-labeled material, the entire procedure should be performed with minimum exposure to light.
The MHCII preparation is a critical aspect of this experiment. The reaction depends on labeled probe peptide binding to MHCII protein, either by direct binding to empty molecules or by displacement of weakly associated peptides. Recombinant MHCII proteins produced in insect cells usually are isolated as a mixture of empty molecules and complexes with weakly associated endogenous peptides, depending on the MHCII allele and culture medium (Sloan et al., 1995; Stern and Wiley, 1992; Vollers and Stern, 2008; Zarutskie et al., 1999). By comparison , MHCII proteins isolated from B cells or other native sources where they usually are occupied with a mixture of tight-binding peptides, and less suitable for peptide binding/exchange assays, although in many cases they can be used but at higher concentrations. We have found recombinant soluble DR1 (HLADRB1*01:01), DR2b (HLA-DRB1*15:01), DR3 (HLA-DRB1*03:01), DR4 (HLADRB1*04:01), DR52 (HLA-DRB3*01:01) and DR2a (HLA-DRB5*01:01) suitable for peptide binding experiments (Anders et al., 2011; Hall et al., 2002; Narayan et al., 2007; Yin et al., 2012), and other MHCII proteins expressed in insect cells such as HLA-DQ (Hou et al., 2011; Sidney et al., 2010), also will be suitable. MHCII proteins produced by expression in E. coli inclusion bodies and folded in vitro (Frayser et al., 1999) can be made completely devoid of peptide and work very well for in vitro binding assays, but so far this technique appears to be restricted to HLA-DR1 (DRB1:01). Due to the residual proteolytic activities in many preparations of MHCII, it is extremely important to use proteinase inhibitor cocktail in the binding buffer to prevent degradation of peptides during incubation.
In the support protocol to label probe peptide, the key step is to optimize the elution gradient to separate the labeled from unlabeled peptides. Also important is to make sure the probe peptide being labeled has only one active amine group and the buffer is free of amine-containing substances.
Troubleshooting
It is essential to perform pilot experiments to test whether FP can be properly measured for both free unbound probe peptide and probe peptide in complex with MHCII. Fluorescence-labeled probe peptide in different percentages of glycol or purified MHCII-probe peptide can help evaluate the FP measurement system and determine the FP values for bound labeled probe peptide.
An unexpected problem that we encountered during development of this assay was the dependence of the bound peptide FP value on the position in the peptide used for labeling. In initial studies we used a modified CLIP peptide labeled at the P5 position (De Wall et al., 2006) or the FRR-HA306-318 peptide labeled at the single amino group present lysine at the P3 position (Painter et al., 2011; Yoon et al., 2012). Peptide positions are numbered according the pocket that they occupy in the bound structure, with P1 corresponding to a large hydrophobic group near the N-terminus (Stern et al., 1994). For most peptides, positions P-1 to P10 make substantial contacts with MHCII residues when bound in the peptide binding groove. Another group used a modified MPB peptide labeled at an introduced cysteine residue at the P5 position (Call et al., 2009; Nicholson et al., 2006) for binding to DR2b (HLA-DRB1*15:01). In extending this approach to other peptides, we labeled a different CLIP variant at its amino terminus (corresponding to the P-5) position, but this peptide showed only a small polarization change when bound to DR1 relative to the value for the free peptide. Moving the Alexa probe to the P-1 position in the same sequence provided the expected polarization shift. FP measures the rotational mobility of the fluorochrome on the labeled probe peptide. If the fluorochrome is free to move, it will give a low FP value and if it is fixed, it will give a high FP value. Apparently, fluorochrome linked to peptide positions outside the binding groove are not sufficiently immobilized relative to unbound peptide to provide a useful FP shift. Thus, in adapting this method to new peptide sequences, it is important to know the binding register of the probe peptide and label it accordingly to give maximum difference in FP values between bound and free states of the peptide, or to screen several positions if the register is not known.
The binding reaction is sensitive to pH and care should be taken to the pH of binding buffer to pH5.5. It should be noted that some preparations of peptide contain residual TFA which can cause a significant and peptide-dose dependent pH shift which in some cases can artificially reduce peptide binding, particularly at very high peptide concentration. The high citrate concentration in the binding buffer is intended to guard against such pH shifts, but the pH of peptide dilutions can be spot checked with pH paper or a pH microelectrode if problems are encountered, and if necessary the peptide dissolved in water and lyophilized to reduce residual TFA.
Maintenance of the plate reader and LC systems is a prerequisite for accuracy and duplication of this protocol. The plate reader must be calibrated regularly for polarization measurements. The C18 column needs to be washed each time after purification and stored in solution recommended by the manufacturer.
Anticipated Results
In the basic protocol, after 72 hours incubation at 37°C, the wells with free Alexa488-FRR-HA306-318 should have a FP value ~35 and the wells with no competing target peptide should have a FP value ~350. The unlabeled target peptide should show a dose-dependent inhibition on the binding of Alexa488-FRR-HA306-318 to MHCII. IC50 for unlabeled FRR-HA306-318 (or HA306-318) should be approximately 25 nM.
In the support protocol, we start with 1 mg of unlabeled FRR-HA306-318 and probably obtain 0.23 mg of Alexa488-FRR-HA306-318.
The reproducibility of these results in the basic protocol depends on the quality of purified MHCII, labeled probe peptide and unlabeled target peptides. Using the same batches of those materials, uncertainties between replicate experiments performed on different days usually do not exceed 10%-15% of the calculated IC50.
Time Considerations
The preparation of the binding competition assay in the basic protocol takes about 30 minutes and the incubation is 72 hours, followed by 2 minutes of FP reading of the plate. The fluorescence-labeling of the probe peptide in the support protocol takes about 1 hour for labeling, and 3 hours for purification.
ACKNOWLEDGEMENT
This work was supported by NIH grants R37AI38996, R01AI48833, and U19AI57319.
Literature Cited
- Anders AK, Call MJ, Schulze MS, Fowler KD, Schubert DA, Seth NP, Sundberg EJ, Wucherpfennig KW. HLA-DM captures partially empty HLA-DR molecules for catalyzed removal of peptide. Nat Immunol. 2011;12:54–61. doi: 10.1038/ni.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call MJ, Xing X, Cuny GD, Seth NP, Altmann DM, Fugger L, Krogsgaard M, Stein RL, Wucherpfennig KW. In vivo enhancement of peptide display by MHC class II molecules with small molecule catalysts of peptide exchange. J Immunol. 2009;182:6342–6352. doi: 10.4049/jimmunol.0803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical pharmacology. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- de Kroon AI, McConnell HM. Kinetics and specificity of peptide-MHC class II complex displacement reactions. J Immunol. 1994;152:609–619. [PubMed] [Google Scholar]
- De Wall SL, Painter C, Stone JD, Bandaranayake R, Wiley DC, Mitchison TJ, Stern LJ, DeDecker BS. Noble metals strip peptides from class II MHC proteins. Nature chemical biology. 2006;2:197–201. doi: 10.1038/nchembio773. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Anderson MW, Klug CS, Gorski J. HLA-DM mediates epitope selection by a “compare-exchange” mechanism when a potential peptide pool is available. PLoS One. 2008;3:e3722. doi: 10.1371/journal.pone.0003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A, Gorski J. Cutting edge: HLA-DM-mediated peptide exchange functions normally on MHC class II-peptide complexes that have been weakened by elimination of a conserved hydrogen bond. J Immunol. 2010;184:1153–1158. doi: 10.4049/jimmunol.0902878. [DOI] [PubMed] [Google Scholar]
- Ferrante A, Gorski J. A Peptide/MHCII conformer generated in the presence of exchange peptide is substrate for HLA-DM editing. Sci Rep. 2012;2:386. doi: 10.1038/srep00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayser M, Sato AK, Xu L, Stern LJ. Empty and peptide-loaded class II major histocompatibility complex proteins produced by expression in Escherichia coli and folding in vitro. Protein Expr Purif. 1999;15:105–114. doi: 10.1006/prep.1998.0987. [DOI] [PubMed] [Google Scholar]
- Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Greenbaum J, Sidney J, Chung J, Brander C, Peters B, Sette A. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics. 2011;63:325–335. doi: 10.1007/s00251-011-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guce AI, Mortimer SE, Yoon T, Painter CA, Jiang W, Mellins ED, Stern LJ. HLA-DO acts as a substrate mimic to inhibit HLA-DM by a competitive mechanism. Nat Struct Mol Biol. 2013;20:90–98. doi: 10.1038/nsmb.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FC, Rabinowitz JD, Busch R, Visconti KC, Belmares M, Patil NS, Cope AP, Patel S, McConnell HM, Mellins ED, Sonderstrup G. Relationship between kinetic stability and immunogenicity of HLA-DR4/peptide complexes. Eur J Immunol. 2002;32:662–670. doi: 10.1002/1521-4141(200203)32:3<662::AID-IMMU662>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hammer J, Takacs B, Sinigaglia F. Identification of a motif for HLA-DR1 binding peptides using M13 display libraries. J Exp Med. 1992;176:1007–1013. doi: 10.1084/jem.176.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, Macmillan H, Chen Z, Keech CL, Jin X, Sidney J, Strohman M, Yoon T, Mellins ED. An insertion mutant in DQA1*0501 restores susceptibility to HLA-DM: implications for disease associations. J Immunol. 2011;187:2442–2452. doi: 10.4049/jimmunol.1100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi RV, Zarutskie JA, Stern LJ. A three-step kinetic mechanism for peptide binding to MHC class II proteins. Biochemistry. 2000;39:3751–3762. doi: 10.1021/bi9923656. [DOI] [PubMed] [Google Scholar]
- Kim A, Ishizuka I, Hartman I, Poluektov Y, Narayan K, Sadegh-Nasseri S. Studying MHC class II peptide loading and editing in vitro. Methods in molecular biology. 2013;960:447–459. doi: 10.1007/978-1-62703-218-6_33. [DOI] [PubMed] [Google Scholar]
- Narayan K, Chou CL, Kim A, Hartman IZ, Dalai S, Khoruzhenko S, Sadegh-Nasseri S. HLA-DM targets the hydrogen bond between the histidine at position beta81 and peptide to dissociate HLA-DR-peptide complexes. Nat Immunol. 2007;8:92–100. doi: 10.1038/ni1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan K, Su KW, Chou CL, Khoruzhenko S, Sadegh-Nasseri S. HLA-DM mediates peptide exchange by interacting transiently and repeatedly with HLA-DR1. Mol Immunol. 2009;46:3157–3162. doi: 10.1016/j.molimm.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastke MD, Becerra A, Yin L, Dominguez-Amorocho O, Gibson L, Stern LJ, Calvo-Calle JM. Human CD4+ T cell response to human herpesvirus 6. J Virol. 2012;86:4776–4792. doi: 10.1128/JVI.06573-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson MJ, Moradi B, Seth NP, Xing X, Cuny GD, Stein RL, Wucherpfennig KW. Small molecules that enhance the catalytic efficiency of HLA-DM. J Immunol. 2006;176:4208–4220. doi: 10.4049/jimmunol.176.7.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter CA, Negroni MP, Kellersberger KA, Zavala-Ruiz Z, Evans JE, Stern LJ. Conformational lability in the class II MHC 310 helix and adjacent extended strand dictate HLA-DM susceptibility and peptide exchange. Proc Natl Acad Sci U S A. 2011;108:19329–19334. doi: 10.1073/pnas.1108074108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B, Sidney J, Bourne P, Bui HH, Buus S, Doh G, Fleri W, Kronenberg M, Kubo R, Lund O, Nemazee D, Ponomarenko JV, Sathiamurthy M, Schoenberger S, Stewart S, Surko P, Way S, Wilson S, Sette A. The immune epitope database and analysis resource: from vision to blueprint. PLoS Biol. 2005;3:e91. doi: 10.1371/journal.pbio.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pos W, Sethi DK, Call MJ, Schulze MS, Anders AK, Pyrdol J, Wucherpfennig KW. Crystal structure of the HLA-DM-HLA-DR1 complex defines mechanisms for rapid peptide selection. Cell. 2012;151:1557–1568. doi: 10.1016/j.cell.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche PA, Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990a;144:1849–1856. [PubMed] [Google Scholar]
- Roche PA, Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990b;345:615–618. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- Rothbard JB, Busch R. Binding of biotinylated peptides to MHC class II proteins on cell surfaces. Current protocols in immunology / edited by John E. Coligan ... [et al.] 2001 doi: 10.1002/0471142735.im1801s25. Chapter 18:Unit 18 11. [DOI] [PubMed] [Google Scholar]
- Sidney J, Southwood S, Moore C, Oseroff C, Pinilla C, Grey HM, Sette A. Measurement of MHC/peptide interactions by gel filtration or monoclonal antibody capture. Current protocols in immunology / edited by John E. Coligan ... [et al.] 2013 doi: 10.1002/0471142735.im1803s100. Chapter 18:Unit 18 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney J, Steen A, Moore C, Ngo S, Chung J, Peters B, Sette A. Divergent motifs but overlapping binding repertoires of six HLA-DQ molecules frequently expressed in the worldwide human population. J Immunol. 2010;185:4189–4198. doi: 10.4049/jimmunol.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, Strominger JL, Wiley DC. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994;368:215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Stern LJ, Wiley DC. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992;68:465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- Stratikos E, Wiley DC, Stern LJ. Enhanced catalytic action of HLA-DM on the exchange of peptides lacking backbone hydrogen bonds between their N-terminal region and the MHC class II alpha-chain. J Immunol. 2004;172:1109–1117. doi: 10.4049/jimmunol.172.2.1109. [DOI] [PubMed] [Google Scholar]
- Tompkins SM, Rota PA, Moore JC, Jensen PE. A europium fluoroimmunoassay for measuring binding of antigen to class II MHC glycoproteins. J Immunol Methods. 1993;163:209–216. doi: 10.1016/0022-1759(93)90124-p. [DOI] [PubMed] [Google Scholar]
- Vollers SS, Stern LJ. Class II major histocompatibility complex tetramer staining: progress, problems, and prospects. Immunology. 2008;123:305–313. doi: 10.1111/j.1365-2567.2007.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Calvo-Calle JM, Dominguez-Amorocho O, Stern LJ. HLA-DM constrains epitope selection in the human CD4 T cell response to vaccinia virus by favoring the presentation of peptides with longer HLA-DM-mediated half-lives. J Immunol. 2012;189:3983–3994. doi: 10.4049/jimmunol.1200626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Macmillan H, Mortimer SE, Jiang W, Rinderknecht CH, Stern LJ, Mellins ED. Mapping the HLA-DO/HLA-DM complex by FRET and mutagenesis. Proc Natl Acad Sci U S A. 2012;109:11276–11281. doi: 10.1073/pnas.1113966109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarutskie JA, Busch R, Zavala-Ruiz Z, Rushe M, Mellins ED, Stern LJ. The kinetic basis of peptide exchange catalysis by HLA-DM. Proc Natl Acad Sci U S A. 2001;98:12450–12455. doi: 10.1073/pnas.211439398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarutskie JA, Sato AK, Rushe MM, Chan IC, Lomakin A, Benedek GB, Stern LJ. A conformational change in the human major histocompatibility complex protein HLA-DR1 induced by peptide binding. Biochemistry. 1999;38:5878–5887. doi: 10.1021/bi983048m. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Callaway KA, Weber DA, Jensen PE. Cutting edge: HLA-DM functions through a mechanism that does not require specific conserved hydrogen bonds in class II MHC-peptide complexes. J Immunol. 2009;183:4187–4191. doi: 10.4049/jimmunol.0901663. [DOI] [PMC free article] [PubMed] [Google Scholar]