Abstract
Objectives
To identify infants with hyperinsulinism caused by defects of the β-cell adenosine triphosphate-dependent potassium channel complex and to distinguish focal and diffuse forms of hyperinsulinism caused by these mutations.
Study design
The acute insulin response to intravenous calcium stimulation (CaAIR) was determined in 9 patients <20 years with diffuse hyperinsulinism caused by defective β-cell sulfonylurea receptor (SUR1−/−), 3 patients with focal congenital hyperinsulinism (6 weeks to 18 months), a 10-year-old with insulinoma, 5 with hyperinsulinism/hyperammonemia syndrome caused by defective glutamate dehydrogenase (6 months to 28 years), 4 SUR1+/− heterozygotes with no symptoms, and 9 normal adults. Three infants with congenital focal disease, 1 with diffuse hyperinsulinism, and the child with insulinoma underwent selective pancreatic intra-arterial calcium stimulation with hepatic venous sampling.
Results
Children with diffuse SUR1−/− disease and infants with congenital focal hyperinsulinism responded to CaAIR, whereas the normal control group, patients with hyperinsulinism/hyperammonemia syndrome, and SUR1+/− carriers did not. Selective arterial calcium stimulation of the pancreas with hepatic venous sampling revealed selective, significant step-ups in insulin secretion that correlated anatomically with the location of solitary lesions confirmed surgically in 2 of 3 infants with congenital focal disease and in the child with insulinoma. Selective arterial calcium stimulation of the pancreas with hepatic venous sampling demonstrated markedly elevated baseline insulin levels throughout the pancreas of the infant with diffuse hyperinsulinism.
Conclusions
The intravenous CaAIR is a safe and simple test for identifying infants with diffuse SUR1−/− hyperinsulinism or with focal congenital hyperinsulinism. Preoperative selective arterial calcium stimulation of the pancreas with hepatic venous sampling can localize focal lesions causing hyperinsulinism in children. The combination of these calcium stimulation tests may help distinguish focal lesions suitable for cure by local surgical resection.
Congenital hyperinsulinism, the most common cause of persistent hypoglycemia in infants beyond the first few days of life,1 can result from genetic defects of the pancreatic β-cell including autosomal recessive mutations of the sulfonylurea receptor gene2 or inward-rectifying 6.2-kDa potassium channel,3,4 which together compose the β-cell adenosine triphosphate-de-pendent potassium channel complex. Other defects that can cause congenital hyperinsulinism are autosomal dominant mutations of islet glucokinase5 or glutamate dehydrogenase, the latter causing the hyperinsulinism/hyperammonemia syndrome.6 Recessive SUR mutations (SUR1−/−) compose the most common and most severe form of congenital hyperinsulinism.7,8 Affected infants are usually large for gestational age and have hypoglycemia in the first few postnatal days.1 Children with SUR1−/− hyperinsulinism usually fail to respond to treatment with diazoxide, a drug that acts on the SUR to suppress insulin secretion9 and may require near total pancreatectomy to control hypoglycemia.10
More than a third of patients with congenital hyperinsulinism requiring surgery have focal adenomatous hyperplasia of islet cells.10–12 Surrounded by normal pancreas, these cells express a paternally derived SUR1 mutation in association with specific loss of imprinted maternal alleles of chromosome 11p, the region that includes SUR1.13–15 The focal loss of maternally imprinted 11p tumor suppressor genes may be responsible for proliferation of islet cells, which are disomic for a paternally derived SUR1 mutation (SUR1p–).11,13 It is important to distinguish infants with diffuse SUR1−/− and those with focal SUR1p– lesions before surgery, because the latter are potentially curable with local resection.16,17
The KATP complex is a major pathway for coupling extracellular glucose concentration to insulin secretion. The increase in intracellular adenosine triphosphate concentration after glycolysis leads to adenosine triphosphate binding to SUR1, inducing closure of Kir6.2, which results in membrane depolarization and opens voltage-dependent (L-type) calcium channels. The resultant rise in intracellular ionized calcium concentration induces insulin secretion.18 The acute insulin response to rapid peripheral intravenous infusion of calcium has been used to diagnose insulinomas in adults,19 and selective arterial calcium stimulation of the pancreas with hepatic venous sampling has been used in adults to localize insulinomas.20,21 We hypothesized that children with hyperinsulinism caused by SUR1 defects would also hyper-respond to calcium because of constitutive activation of β-cell L-type calcium channels. The purpose of this study was to determine whether children with focal and diffuse forms of SUR1 hyperinsulinism would respond to calcium and whether calcium ASVS could be used to localize congenital focal (SUR1p–) lesions.
Subjects and Methods
The diagnosis of hyperinsulinism was based on demonstrating fasting hypoglycemia with inadequate suppression of insulin secretion and evidence of increased insulin action such as increased rates of glucose use, inappropriately suppressed plasma free fatty acid and β-hydroxybutyrate concentrations, and inappropriate glycemic response to glucagon stimulation.22,23
The first group (patients with SUR1−/−) ranged in age from 15 months to 20 years (Table I). All had persistent hypoglycemia including 7 of the 9 who had previously undergone 95% to 98% pancreatectomy. The second group consisted of three children with focal congenital hyperinsulinism. The third group with congenital hyperinsulinism consisted of 5 patients with the HI/HA syndrome. The fourth group contained 1 child with new onset of hyperinsulinism caused by an insulinoma.
Table I.
Characteristics of children with different forms of hyperinsulinism
| Patient No. | Age | Sex | Form and cause of hyperinsulinism |
Previous therapy |
|---|---|---|---|---|
| Diffuse hyperinsulinism caused by SUR1−/− | ||||
| 1 | 20 y | F | 3992–9 g → a/delF1388 | Pancreatectomy |
| 2 | 11 y | M | 3992–9 g → a/3992–9 g → a | Pancreatectomy |
| 3 | 13 y | M | 3992–9 g → a/3992–9 g → a | Pancreatectomy |
| 4 | 4 y | M | 3992–9 g → a/3992–9 g → a | Frequent feedings |
| 5 | 15 mo | M | 3992–9 g → a/delF1388 | Pancreatectomy, octreotide, glucagon, frequent feeds |
| 6 | 6 y | F | 3992–9 g → a/delF1388 | Pancreatectomy |
| 7 | 13 y | F | 3992–9 g → a/delF1388 | Pancreatectomy |
| 8 | 7 y | F | Paternal 3992–9 g → a; Maternal genotype pending | Pancreatectomy |
| 9 | 18 mo | F | Genotype pending; diffuse disease on histopathology of surgical specimen | Octreotide (diazoxide-unresponsive) |
| Congenital focal hyperinsulinism | ||||
| 10 | 6 wk | M | Focal adenomatosis encasing pancreatic duct in pancreatic head | Frequent feeds |
| 11 | 5 mo | F | Focal adenomatosis of pancreatic tail | Octreotide, frequent feeds |
| 12 | 18 mo | F | Paternal only SUR1– (six amino acid insertion in exon 5); normal pancreas on multiple biopsies | Octreotide |
| Hyperinsulinism/hyperammonemia syndrome | ||||
| 13 | 10 y | M | H454Y | Diazoxide |
| 14 | 6 mo | F | H454Y | None |
| 15 | 3 y | F | K450E | None |
| 16 | 2 y | F | R977C | None |
| 17 | 28 y | M | R977C | None |
| Insulinoma | ||||
| 18 | 10 y | M | insulinoma | Diazoxide, frequent feeds |
Four parents of patients with SUR1−/−, each an SUR1+/− heterozygous carrier with no symptoms and no history of hypoglycemia, and 9 normal adults served as the control group. The SUR1+/– carriers (1 female, 3 male) were between the ages of 29 and 40 years. The members of the normal adult control group were not obese and ranged in age from 19 to 49 years (5 female, 4 male).
Peripheral Intravenous CaAIR and ASVS
The CaAIR test was performed after an overnight fast by giving 0.1 mEq calcium/1 kg body weight (= 2 mg elemental Ca2+/kg) intravenously within 60 seconds (with 10% calcium gluconate stock diluted to 2% with 4 parts isotonic saline solution). Plasma glucose, serum calcium, and plasma insulin samples were obtained from a contralateral vein at −600, −300, 0, +60, +180, +300, and +600 seconds relative to the start of the calcium infusion. Medications were discontinued before these studies were begun. In children with unstable hypoglycemia, intravenous dextrose was infused continuously as needed to maintain plasma glucose levels between 3.3 to 5.0 mmol/L (60 to 90 mg/dL) before and throughout the study. The CaAIR was calculated as the change in insulin levels between the mean of the baseline values and the mean of the acutely stimulated values at +60 and +180 seconds. The rise in serum calcium was calculated in the same way.
Five children with hyperinsulinism underwent ASVS. ASVS was performed with the patients under general endotracheal anesthesia after an overnight fast with the method of Doppman et al20 and Brown et al.21 In brief, under fluoroscopic visualization by the right internal jugular vein, a diagnostic vascular catheter was advanced selectively into the right hepatic vein for venous blood sampling. Thereafter, by way of retrograde catheterization of the right common femoral artery, another catheter was superselectively negotiated into the right hepatic artery (as a negative control), gastroduodenal artery, superior mesenteric artery, and splenic artery. Contrast was injected solely to confirm catheter placements. Immediately before each intra-arterial Ca2+ stimulation was performed, 50 µg nitroglycerin was infused through the artery under study to prevent vasospasm. A rapid bolus of 0.025 mEq Ca2+/1 kg body weight was infused into each artery over a 15- to 25-second period. Samples of 0.5 mL for plasma insulin were obtained from the right hepatic vein immediately before and then at +30, +60, +90, and +120 seconds after the intra-arterial Ca2+ infusion was completed. We waited a minimum of 10 minutes between selective intra-arterial injections. Intravenous dextrose was infused continuously as required to maintain plasma glucose levels between 3.3 and 5.0 mmol/L (60 to 90 mg/dL) before and throughout the study. A positive ASVS response for focal disease was defined as ≥2-fold rise in right hepatic vein insulin levels within 60 seconds after Ca2+ stimulation.20,21
Assays and Analysis
Plasma glucose was measured with the glucose oxidase method (Yellow Springs Instrument Co, Glucose Analyzer; Yellow Springs, OH). Plasma insulin levels were quantified by a solid phase 2-site enzyme immunoassay with 2 monoclonal antibodies directed against separate antigenic determinants on the human insulin molecule (Insulin ELISA; ALPCO, Windham, NH). The detection limit of this insulin assay is <1 µU/mL. Data were analyzed by unpaired, 2-tailed, Mann-Whitney U tests (InStat program for Macintosh, version 2.00; GraphPad Software Inc, San Diego, CA). All clinical and biochemical studies were carried out in the General Clinical Research Center at The Children’s Hospital of Philadelphia. This research was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Written informed consent was obtained from all subjects >18 years and from parents of children <18 years. Assent was obtained from children >8 years old.
Results
Peripheral Intravenous CaAIR
All groups demonstrated similar rises in serum calcium (Table II). The mean ΔCa for the 3 groups with congenital hyperinsulinism was 1.47 ± 0.11 mg/dL (SUR1−/−; n = 8); 0.98 ± 0.19 mg/dL (congenital focal; n = 3); and 1.20 ± 0.17 mg/dL (HI/HA; n = 5). Excluding patient 9, who had diffuse disease and markedly elevated baseline insulin levels (>145 µU/mL), baseline insulin levels were also similar among all groups studied: 10.5 ± 1.3 µU/mL (SUR1−/−); 9.6 ± 1.8 µU/mL (congenital focal); and 11.2 ± 1.9 µU/mL (HI/HA).
Table II.
Acute insulin responses to peripheral venous Ca stimulation (CaAIR)
| Patient No. | ΔCa (mg/dL) |
Baseline insulin (µU/mL) |
CaAIR (µU/mL) |
|---|---|---|---|
| Diffuse hyperinsulinism caused by SUR1−/− | |||
| 1 | 1.4 | 12.7 | 52.5 |
| 2 | 1.2 | 7.3 | 29.0 |
| 3 | 1.2 | 13.9 | 25.4 |
| 4 | 1.2 | 9.4 | 22.4 |
| 5 | 1.6 | 11.8 | 22.3 |
| 6 | 1.8 | 4.2 | 6.8 |
| 7 | 2.0 | 13.9 | 14.5 |
| 8 | 1.7 | 9.35 | 13.6 |
| 9 | NA | 145 | 26.1 |
| Congenital focal hyperinsulinism | |||
| 10 | 0.7 | 7.2 | 9.2 |
| 11 | 1.4 | 13.1 | 10.5 |
| 12 | 0.9 | 8.5 | 8.9 |
| Hyperinsulinism/hyperammonemia syndrome | |||
| 13 | 1.15 | 16.75 | −3.66 |
| 14 | 0.8 | 8.6 | 1.28 |
| 15 | 1.03 | 14 | −8.4 |
| 16 | 1.2 | 10.2 | −7.9 |
| 17 | 1.8 | 6.5 | −2.38 |
| Mean ± SEM: | 1.2 ± 0.2 | 11.2 ± 1.9 | −4.2 ± 1.8 |
| Insulinoma | |||
| 18 | 1.4 | 10.8 | 2.5 |
| Asymptomatic heterozygous SUR1+/– carriers | |||
| Mean ± SEM (n = 4): Normal adults |
1.3 ± 0.1 | 9.3 ± 1.6 | 2.2 ± 1.4 |
| Mean ± SEM (n = 9): | 1.0 ± 0.2 | 9.4 ± 1.5 | 0.10 ± 0.62 |
NA, Not available.
CaAIRs in the 2 adult control groups were minimal, ranging from –1.90 to +3.88 µU/mL for SUR1+/− carriers and –2.00 to +4.20 µU/mL for normal adults. The 95% CI for the CaAIR of the combined control group (n = 13) was –0.6 to +2.1 µU/mL. The CaAIR for patients with HI/HA ranged from –8.40 to +1.28 µU/mL. The mean CaAIR in patients with HI/HA was significantly lower than the mean CaAIR in the normal adult control group (P < .05), SUR1+/– carriers (P < .04), diffuse SUR1−/− hyperinsulinism (P < .002), and congenital focal hyperinsulinism (P < .04).
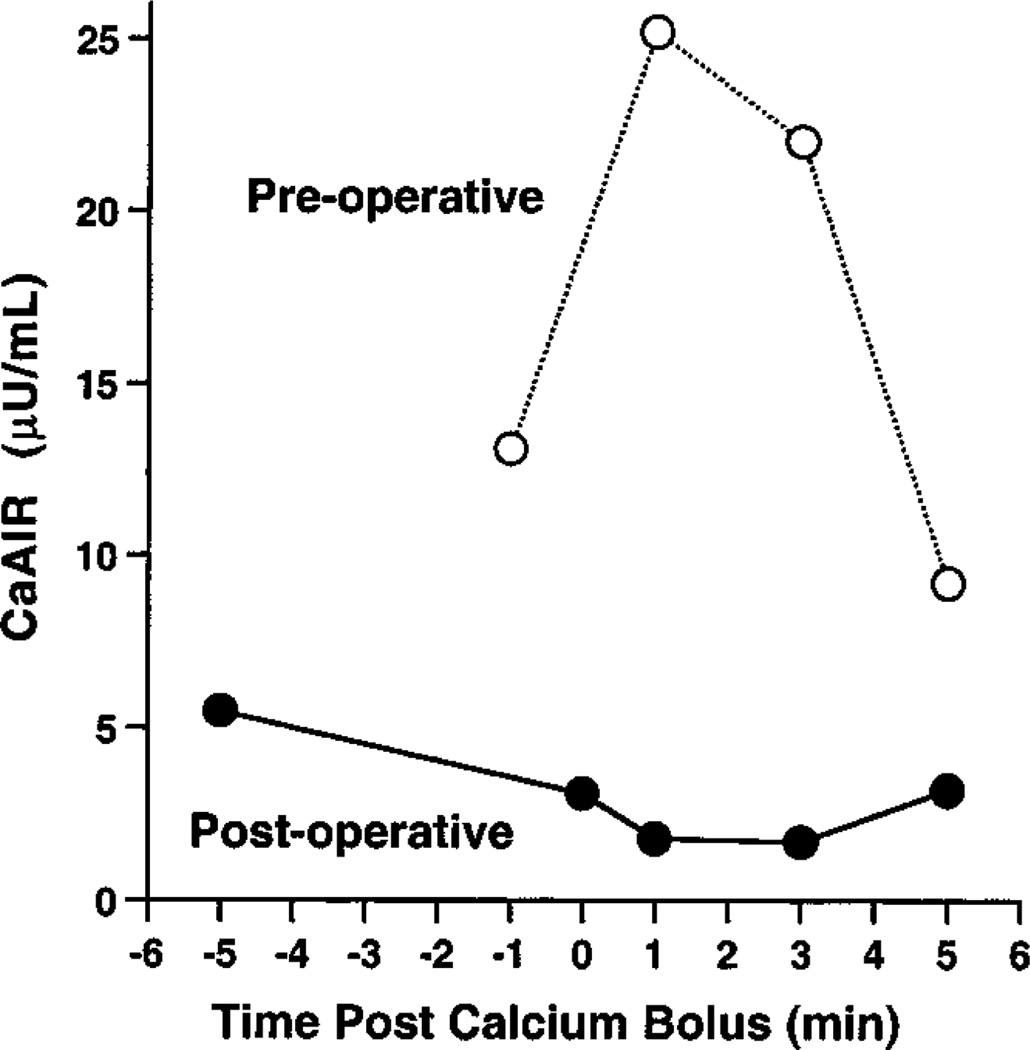
The mean CaAIR for the 9 patients with diffuse SUR1−/− hyperinsulinism was 22.3 ± 4.6 µU/mL, significantly higher than the mean CaAIR in the normal adult control group (P < .0001), SUR1+/– carriers (P < .003), and patients with HI/HA (P < .002). The mean CaAIR for the 3 infants with focal congenital hyperinsulinism was 9.5 ± 0.5 µU/mL, significantly higher than the mean CaAIR of the normal control group (P < .01) and patients with HI/HA (P < .04). All CaAIRs in the patients with diffuse SUR1−/− or congenital focal disease were above the 95% CI for combined adults (n = 13). CaAIRs of patients with diffuse SUR1−/− (6.8–52.5 µU/mL) or congenital focal disease (8.9–10.5 µU/mL) did not overlap with CaAIRS of SUR1+/– carriers (–1.90 to +3.88) or those of patients with HI/HA (–8.40 to +1.28 µU/mL). ΔCa did not correlate to CaAIR magnitude in any group. As shown in Fig 1, the positive CaAIR in patient 11, who had a congenital focal lesion, normalized after surgical resection of her focal adenomatosis was performed. Fig 1 also shows that the Ca-stimulated insulin level returns to baseline by the 300-second time point, which was true for all CaAIRs performed in these patients.
Fig 1.
Preoperative (dashed line) and postoperative (solid line) CaAIRs in patient 11, 5-month-old girl with focal adenomatosis of her proximal pancreatic tail. She was cured after local resection of this congenital focal lesion was performed.
ASVS
Five of the children (patients 9, 10, 11, 12, and 18) underwent ASVS in an attempt to delineate a focal, potentially resectable lesion. Limited contrast injections during catheter placements revealed no lesion in these patients.
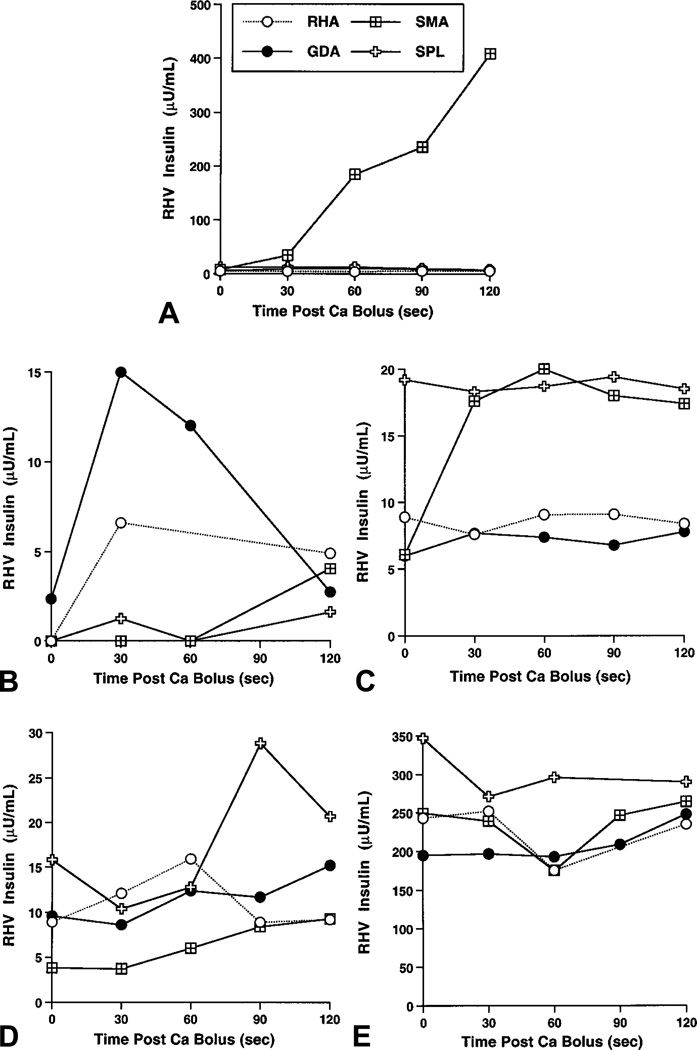
In patient 18, magnetic resonance imaging and 111In-octreotide scan did not show the lesion (an insulinoma) before surgery. The lesion was suspected on spiral computed tomography and was located at surgery in the uncinate process of his pancreas. In response to ASVS, a 52-fold step-up in insulin level was noted in the region supplied by the SMA (Fig 2, A); no other vessel demonstrated a rise greater than 2-fold. A postoperative fasting study confirmed that his hyperinsulinism was cured.
Fig 2.
ASVS results. A, Patient 18 with insulinoma; B, patient 12 with paternal only (SUR1p–) mutation; C, patient 10 with focal adenomatosis of pancreatic head; D, patient 11 with focal adenomatosis of proximal tail; E, patient 9 with diffuse disease unresponsive to diazoxide and octreotide. During infusion of Ca at right hepatic artery for patient 12 (B), small backwash into gastroduodenal artery by collateral vessel was noted. Right hepatic artery, open circle; gastroduodenal artery, solid circle; SMA, crossed box; SpA, open cross SMA, Superior mesenteric artery; SpA, splenic artery.
In patient 12, an 18-month-old girl thought to have congenital focal hyperinsulinism because she carried only a paternally derived SUR1 mutation, ASVS demonstrated a selective 5.6-fold insulin step-up at the gastroduodenal artery, consistent with focal disease in the head of the pancreas (Fig 2, B). Although she had a 2.7-fold rise at the right hepatic artery, this was due to retrograde filling of the gastroduodenal artery as was seen with contrast injection. Partial (30%) pancreatectomy in the region of the pancreatic head and biopsies throughout the entire pancreas revealed only normal pancreatic tissue, consistent with a focal lesion being present in the remaining pancreatic head. She remained hypoglycemic after surgery, and her parents declined further surgery.
In patient 10, a 6-week-old boy, ASVS demonstrated a selective 2.3-fold rise in insulin after selective injection into the SMA was performed (Fig 2, C). Baseline insulin values before SPL injection were high but followed SMA injection and thus may have been due to a second-pass effect (insufficient pause between SMA and SPL injections) or reflux of Ca resulting from over-rapid injection of his small vessels. Stimulation of the SPL showed no step-up. At surgery an area of focal adenomatosis was found encasing his pancreatic duct in the pancreatic head. Two additional partial pancreatectomies (totaling 95%) failed to remove all of the lesion. The rest of the pancreas showed normal histologic characteristics, consistent with focal adenomatosis. He continues to have hypoglycemia that requires treatment with continuous subcutaneous octreotide infusion and frequent feedings. His parents refused further surgery (Whipple pancreaticoduodenectomy).
In patient 11, ASVS at 5 months of age revealed a 2.4-fold step-up (by 90 seconds after Ca) at the SPL, which supplied the distal body/proximal tail of the pancreas, probably by way of the dorsal pancreatic artery24 (Fig 2, D). Subtotal pancreatectomy disclosed a small focal adenomatosis lesion of her pancreatic tail but an otherwise normal pancreas. Cure was subsequently confirmed by fasting without hypoglycemia for >16 hours and the loss of sensitivity to intravenous calcium (Fig 1).
Patient 9, an 18-month-old girl, had diffuse islet hyperplasia demonstrated at 99% pancreatectomy. As seen during her CaAIR and ASVS tests (Fig 2, E), her baseline insulin levels were extraordinarily high (195 to 347 µU/mL), and there was no significant rise after intra-arterial calcium stimulation.
Discussion
These studies demonstrate that children with diffuse SUR1−/− and focal SUR1p– forms of congenital hyperinsulinism hyper-respond to intravenous calcium stimulation. The smallest CaAIR observed in these 2 groups of patients (6.8 µU/mL for patient 6) exceeded the range (–1.90 to +4.2 µU/mL) of the combined control group of normal adults and SUR1+/– carriers. The abnormal responsiveness to intravenous calcium persisted in children with diffuse SUR1−/− disease who had previously been treated with subtotal pancreatectomy. Patients with HI/HA did not have abnormal hyper-responsiveness to calcium, suggesting that calcium responsiveness may be specific to hyperinsulinism caused by defects of the β-cell KATP complex (SUR1 or Kir6.2).
SUR1-mediated insulin exocytosis requires a rise in cytoplasmic Ca2+ within the β-cell.25 Mice lacking β-cell potassium channel activity (Kir6.2−/−) demonstrate elevated basal intracellular calcium concentration.26 Islets isolated from a 14-month-old girl with congenital diffuse hyperinsulinism showed similar elevations of intracellular calcium concentration in vitro both at baseline and after an extracellular Ca2+ load.27 A rise in total serum calcium as low as 0.7 mg/dL was sufficient to induce a positive CaAIR in our SUR1-defective patients (with either focal or diffuse disease). Thus our in vivo data support recent in vitro evidence that the SUR1-defective β-cell vigorously secretes insulin in response to a rise in extracellular calcium concentration, because its L-type Ca2+ channels remain constitutively open irrespective of ambient glucose concentration.27
All the patients with SUR1−/− studied here (except patient 9, whose genotype remains unknown) carried the same 3992-9 mutation of the in-tron 32 splice site. We expect more severe mutations, which further reduce SUR1 activity, will yield larger CaAIRs. All mutations that generate truncated SUR1 protein that fails to migrate to the cell surface28 would be expected to yield CaAIR results similar to those reported here.
CaAIRs of infants with congenital focal disease overlap those of patients with diffuse SUR1−/− disease. The 95% CI for CaAIR in our patients with congenital focal disease (8.9 to 10.5 µU/mL) was more restricted than the interval in patients with diffuse SUR1−/− disease (6.8 to 52.5 µU/mL). Our data suggest that a child with congenital hyperinsulinism who has a CaAIR >5 µU/mL should be suspected of having focal or diffuse β-cell KATP disease. Patients with a CaAIR >15 µU/mL may be unlikely to benefit from ASVS. Nevertheless, until the CaAIR test has been evaluated in more patients with congenital focal hyperinsulinism, the decision to proceed with ASVS in patients responsive to peripheral calcium is best made on their response to intravenous tolbutamide stimulation rather than the magnitude of their CaAIR.29
Blind 95% to 99% pancreatectomy carries significant morbidity,10 so it is crucial to identify focal lesions causing hyperinsulinism before surgery. Focal pancreatic lesions causing hyperinsulinism in children are usually below the lower limit of resolution for ultrasonography,30 magnetic resonance imaging, computed tomography, and contrast angiography.31 Because SUR1+/– carriers had normal CaAIRs, the CaAIR test cannot be used to identify SUR1+/– carrier states. Gaeke et al32 first observed in 1975 that adults with insulinomas secrete insulin in response to a continuous infusion of calcium. Their method was improved by Brunt et al,19 who first reported a large acute insulin response to rapid intravenous infusion of calcium in adults with insulinoma. Doppman20,21,33 and others have shown that intra-arterial calcium stimulates insulin secretion from insulinomas in adult patients.
Reports in adults suggest that ASVS is both simpler and superior to transhepatic portal venous sampling for localizing insulinomas.34 Transhepatic portal venous sampling has been used successfully in children35; however, it is technically difficult, requires the maintenance of hypoglycemia to suppress insulin release from normal pancreas, and consumes large volumes of blood because of the frequent monitoring of central and peripheral levels of insulin and glucose. By contrast, none of our subjects became hypoglycemic (defined as blood glucose <50 mg/dL) during CaAIR or ASVS testing. Among the 33 CaAIR and 5 ASVS tests in 31 subjects, no subject required extra dextrose infusion during the critical sampling (baseline through +180 seconds for CaAIR or +120 seconds for ASVS), and no subject had adverse side effects. Moreover, the CaAIR and ASVS tests required minimal phlebotomy volumes: <15 mL of blood for both tests per patient (line flushes were returned to the patient). Although performed in our study for improved scientific rigor, CaAIR samples drawn beyond +180 seconds are not necessary for informative studies.
ASVS accurately localized the focal lesion in 2 infants whose lesions were confirmed surgically (patients 10 and 11). ASVS also gave results consistent with diffuse disease in patient 9 and consistent with the lack of diffuse disease in patient 12. In 1 older child with an acquired insulinoma (patient 18), the ASVS response successfully localized the tumor. The ASVS response in this child resembled that seen with insulinoma in adults. Despite his large insulin response to ASVS, he had a minimal response to peripheral intravenous calcium on 2 separate tests. This anomaly may be due to a lack of local factors affecting the response of the tumor to intravenous calcium. Perhaps the environment of an insulinoma formed within mature pancreas is different (lacks neuroendocrine factors, for example) than that of a congenital focal lesion that develops simultaneously within developing pancreas. Current ASVS technique performed on adults uses proximal and mid-splenic injections to distinguish lesions from the body and tail. Localization is more precise, although accuracy is not increased.20 If laparoscopic surgery is contemplated for older children with insulinoma, such precise localization can be significant.
In contrast to SUR1-defective patients, children with HI/HA had no acute insulin response to calcium. On clinical evaluation HI/HA is a milder disease than SUR1p– or SUR1−/− hyperinsulinism, suggesting that β-cells in patients with HI/HA have fewer calcium channels constitutively open to respond to calcium stimulation. The lack of the positive CaAIR together with their high plasma ammonium concentrations helps to distinguish patients with HI/HA from those with SUR1 mutations. It is not known how insulinomas can respond to calcium, whereas glutamate dehydrogenase–defective β-cells do not. We speculate that this phenotypic difference arises from as yet unappreciated defective potassium channel function in insulinomas.
In conclusion, the peripheral venous CaAIR test effectively identifies children with diffuse and focal forms of hyperinsulinism caused by defective β-cell SUR1. It is likely that infants with HI caused by Kir6.2 mutations would respond in a similar manner. The CaAIR distinguishes patients with hyperinsulinism caused by defective SUR1 from patients with HI/HA caused by glutamate dehydrogenase gene defects and from SUR1+/– carriers. ASVS appears to be a promising method for identifying focal lesions in infants with hyperinsulinism. Whether other genetic defects causing hyperinsulinism such as glucokinase mutations will confer calcium responsiveness remains to be studied.
Acknowledgments
Supported in part by National Institutes of Health grants R01 DK53012 and R01 DK56268 (to C.A.S.) and T32 DK07314 (to R.J.F., A.K., A.G.), GCRC grant M01 RR00240 (to C.A.S.), American Diabetes Association Research Grants (to C.A.S., L.A.B.), Houston Endowment (L.A.B.), Baylor College of Medicine (L.A.B.), Israel Ministry of Health Grant No. 4201 (B.G), a grant from the Israel Science Foundation founded by the Academy of Sciences and Humanities (B.G.), and Lawson Wilkins Pediatric Endocrine Society Research Fellowships (Pharmacia & Upjohn Award to R.J.F. and Eli Lilly & Co. Award to A.G.).
Glossary
- ASVS
Selective arterial calcium stimulation of the pancreas with hepatic venous sampling
- CaAIR
Acute insulin response to peripheral intravenous calcium stimulation
- HI/HA
Hyperinsulinism/hyperammonemia syndrome
- KATP
Adenosine triphosphate-dependent potassium channel complex formed by SUR1 with Kir6.2
- Kir6.2
Inward-rectifying 6.2-kDa potassium channel
- SMA
Superior mesenteric artery
- SPL
Splenic artery
- SUR1
Sulfonylurea receptor gene
Footnotes
Presented in part at the Eighty-first Annual Endocrine Society Meeting, San Diego, California, June 1999, and at the Maurice Attie Memorial Lecture of The Philadelphia Endocrine Society, Philadelphia, Pennsylvania, May 1999.
References
- 1.Stanley CA. Hyperinsulinism in infants and children. Pediatr Clin North Am. 1997;44:363–374. doi: 10.1016/s0031-3955(05)70481-8. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev. 1999;20:101–135. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- 3.Thomas P, Ye YY, Lightner E. Mutations of the pancreatic islet inward rectifier KIR6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5:1809–1812. doi: 10.1093/hmg/5.11.1809. [DOI] [PubMed] [Google Scholar]
- 4.Nestorowicz A, Inagaki N, Gonoi T, Schoor KP, Wilson BA, Glaser B, et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes. 1997;46:1743–1748. doi: 10.2337/diab.46.11.1743. [DOI] [PubMed] [Google Scholar]
- 5.Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, Buchs A, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338:226–230. doi: 10.1056/NEJM199801223380404. [DOI] [PubMed] [Google Scholar]
- 6.Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338:1352–1357. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- 7.Kane C, Shepherd RM, Squires PE, Johnson PR, James RF, Milla PJ, et al. Loss of functional KATP channels in pancreatic beta-cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nature Med. 1996;2:1344–1347. doi: 10.1038/nm1296-1344. [DOI] [PubMed] [Google Scholar]
- 8.Nestorowicz A, Wilson BA, Schoor KP, Inoue H, Glaser B, Landau H, et al. Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet. 1996;5:1813–1822. doi: 10.1093/hmg/5.11.1813. [DOI] [PubMed] [Google Scholar]
- 9.Kane C, Lindley KJ, Johnson PR, James RF, Milla PJ, Aynsley-Green A, Dunne MJ. Therapy for persistent hyperinsulinemic hypoglycemia of infancy. Understanding the responsiveness of beta cells to diazoxide and somatostatin. J Clin Invest. 1997;100:1888–1893. doi: 10.1172/JCI119718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovvorn HN3rd, Nance ML, Ferry RJ, Jr, Stolte L, Baker L, O’Neill JA, Jr, et al. 35-year perspective of congenital hyperinsulinism. J Pediatr Surg. 1999;34:786–793. doi: 10.1016/s0022-3468(99)90374-3. [DOI] [PubMed] [Google Scholar]
- 11.de Lonlay-Debeney P, Poggi-Travert F, Fournet JC, Sempoux C, Vici CD, Brunelle F, et al. Clinical features of 52 neonates with hyperinsulinism. N Engl J Med. 1999;340:1169–1175. doi: 10.1056/NEJM199904153401505. [DOI] [PubMed] [Google Scholar]
- 12.Glaser B, Ryan F, Donath M, Landau H, Stanley CA, Baker L, et al. Hyperinsulinism caused by paternal-specific inheritance of a recessive mutation in the sulfonylurea-receptor gene. Diabetes. 1999;48:1652–1657. doi: 10.2337/diabetes.48.8.1652. [DOI] [PubMed] [Google Scholar]
- 13.Fournet JC, Verkarre V, De Lonlay P, Rahier J, Brunelle F, Robert JJ, et al. Loss of imprinted genes and paternal SUR1 mutations lead to hyperinsulinism in focal adenomatous hyperplasia. Ann Endocrinol (Paris) 1998;59:485–491. [PubMed] [Google Scholar]
- 14.Verkarre V, Fournet JC, de Lonlay P, Gross-Morans MS, Devillers M, Rahier J, et al. Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest. 1998;102:1286–1291. doi: 10.1172/JCI4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser B, Ryan F, Donath M, Landau H, Stanley CA, Baker L, et al. Hyperinsulinism caused by paternal-specific inheritance of a recessive mutation in the sulfonylurea-receptor gene. Diabetes. 1999;48:1652–1657. doi: 10.2337/diabetes.48.8.1652. [DOI] [PubMed] [Google Scholar]
- 16.Craver RD, Hill CB. Cure of hypoglycemic hyperinsulinism by enucleation of a focal islet cell adenomatous hyperplasia. J Pediatr Surg. 1997;32:1526–1527. doi: 10.1016/s0022-3468(97)90584-4. [DOI] [PubMed] [Google Scholar]
- 17.Rahier J, Sempoux C, Fournet JC, Poggi F, Brunelle F, Nihoul-Fekete C, et al. Partial or near-total pancreatectomy for persistent neonatal hyperinsulinaemic hypoglycaemia: the pathologist’s role. Histopathology. 1998;32:15–19. doi: 10.1046/j.1365-2559.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 18.Owada S, Larsson O, Arkhammar P, Katz AI, Chibalin AV, Berggren PO, et al. Glucose decreases Na+/K+-ATPase activity in pancreatic beta-cells. An effect mediated via Ca2+-independent phospholipase A2 and protein kinase C-dependent phosphorylation of the alpha-subunit. J Biol Chem. 1999;274:2000–2008. doi: 10.1074/jbc.274.4.2000. [DOI] [PubMed] [Google Scholar]
- 19.Brunt LM, Veldhuis JD, Dilley WG, Farndon JR, Santen RJ, Leight GS, et al. Stimulation of insulin secretion by a rapid intravenous calcium infusion in patients with beta-cell neoplasms of the pancreas. J Clin Endocrinol Metab. 1986;62:210–216. doi: 10.1210/jcem-62-1-210. [DOI] [PubMed] [Google Scholar]
- 20.Doppman JL, Chang R, Fraker DL, Norton JA, Alexander HR, Miller DL, et al. Localization of insulinomas to regions of the pancreas by intra-arterial stimulation with calcium. Ann Intern Med. 1995;123:269–273. doi: 10.7326/0003-4819-123-4-199508150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Brown CK, Bartlett DL, Doppman JL, Gorden P, Libutti SK, Fraker DL, et al. Intraarterial calcium stimulation and intraoperative ultrasonography in the localization and resection of insulinomas. Surgery. 1997;122:1189–1193. doi: 10.1016/s0039-6060(97)90226-9. [DOI] [PubMed] [Google Scholar]
- 22.Stanley CA, Baker L. Hyperinsulinism in infancy: diagnosis by demonstration of abnormal response to fasting hypoglycemia. Pediatrics. 1976;57:702–711. [PubMed] [Google Scholar]
- 23.Finegold DN, Stanley CA, Baker L. Glycemic response to glucagon during fasting hypoglycemia: an aid in the diagnosis of hyperinsulinism. J Pediatr. 1980;96:257–259. doi: 10.1016/s0022-3476(80)80817-1. [DOI] [PubMed] [Google Scholar]
- 24.Woodburne RT, Olsen LL. The arteries of the pancreas. Anat Rec. 1951;111:255–270. doi: 10.1002/ar.1091110209. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Barrado MJ, Jonas JC, Gilon P, Henquin JC. Sulphonylureas do not increase insulin secretion by a mechanism other than a rise in cytoplasmic Ca2+ in pancreatic B-cells. Eur J Pharmacol. 1996;298:279–286. doi: 10.1016/0014-2999(95)00806-3. [DOI] [PubMed] [Google Scholar]
- 26.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abernethy LJ, Davidson DC, Lamont GL, Shepherd RM, Dunne MJ. Intraarterial calcium stimulation test in the investigation of hyperinsulinaemic hypoglycaemia. Arch Dis Child. 1998;78:359–363. doi: 10.1136/adc.78.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma N, Crane A, Clement JP4th, Gonzalez G, Babenko AP, Bryan J, Aguilar-Bryan L. The C terminus of SUR1 is required for trafficking of KATP channels. J Biol Chem. 1999;274:20628–20632. doi: 10.1074/jbc.274.29.20628. [DOI] [PubMed] [Google Scholar]
- 29.Grimberg A, Ferry RJ, Jr, Kelly A, Koo-McCoy S, Glaser B, Permutt A, et al. Acute insulin responses (AIRs) to tolbutamide and glucose distinguish diffuse versus focal forms of congenital hyperinsulinism (HI) due to sulfonylurea receptor (SUR) mutations [oral abstract]; Program and Abstracts of the Eighty-first Annual Meeting of The Endocrine Society, The Endocrine Society; June 2–15, 1999; San Diego California, Bethesda, MD. 1999. p. 78. [Google Scholar]
- 30.Beccaria L, Bosio L, Burgio G, Paesano PL, Del Maschio A, Chiumello G. Multiple insulinomas of the pancreas: a patient report. J Pediatr Endocrinol Metab. 1997;10:309–314. doi: 10.1515/jpem.1997.10.3.309. [DOI] [PubMed] [Google Scholar]
- 31.Kuzin NM, Egorov AV, Kondrashin SA, Lotov AN, Kuznetov NS, Majorova JB. Preoperative and intraoperative topographic diagnosis of insulinomas. World J Surg. 1998;22:593–597. doi: 10.1007/s002689900440. [DOI] [PubMed] [Google Scholar]
- 32.Gaeke RF, Kaplan EL, Rubenstein A, Starr J, Burke G. Insulin and proinsulin release during calcium infusion in a patient with islet-cell tumor. Metabolism. 1975;24:1029–1034. doi: 10.1016/0026-0495(75)90096-7. [DOI] [PubMed] [Google Scholar]
- 33.O’Shea D, Rohrer-Theurs AW, Lynn JA, Jackson JE, Bloom SR. Localization of insulinomas by selective intraarterial calcium injection. J Clin Endocrinol Metab. 1996;81:1623–1627. doi: 10.1210/jcem.81.4.8636378. [DOI] [PubMed] [Google Scholar]
- 34.Pereira PL, Roche AJ, Maier GW, Huppert PE, Dammann F, Farnsworth CT, et al. Insulinoma and islet cell hyperplasia: value of the calcium intraarterial stimulation test when findings of other preoperative studies are negative. Radiology. 1998;206:703–709. doi: 10.1148/radiology.206.3.9494488. [DOI] [PubMed] [Google Scholar]
- 35.Dubois J, Brunelle F, Touati G, Sebag G, Nuttin C, Thach T, et al. Hyperinsulinism in children: diagnostic value of pancreatic venous sampling correlated with clinical, pathological and surgical outcome in 25 cases. Pediatr Radiol. 1995;25:512–516. doi: 10.1007/BF02015782. [DOI] [PubMed] [Google Scholar]