Abstract
Performance in an adjusting delay discounting procedure is predictive of drug abuse vulnerability; however, the shared underlying specific prefrontal neural systems linking delay discounting and increased addiction-like behaviors are unclear. Rats received direct infusions of methylphenidate (MPH; 6.25, 25.0, or 100 μg), amphetamine (AMPH; 0.25, 1.0, or 4.0 μg), or atomoxetine (ATO; 1.0, 4.0, or 16.0 μg) into either medial prefrontal cortex (mPFC) or orbitofrontal cortex (OFC) immediately prior to performance in an adjusting delay task. These drugs were examined because they are efficacious in treating impulse control disorders. Because dopamine (DA) and serotonin (5-HT) receptors are implicated in impulsive behavior, separate groups of rats received microinfusions of the DA receptor-selective drugs SKF 81297 (0.1 or 0.4 μg), SCH 23390 (0.25 or 1.0 μg), quinpirole (1.25 or 5.0 μg), and eticlopride (0.25 or 1.0 μg), or received microinfusions of the 5-HT receptor-selective drugs 8-OH-DPAT (0.025 or 0.1 μg), WAY 100635 (0.01 or 0.04 μg), DOI (2.5 or 10.0 μg), and ketanserin (0.1 or 0.4 μg). Impulsive choice was not altered significantly by MPH, AMPH, or ATO into either mPFC or OFC, indicating that neither of these prefrontal regions alone may mediate the systemic effect of ADHD medications on impulsive choice. However, quinpriole (1.25 μg) and eticlopride infused into mPFC increased impulsive choice, whereas 8-OH-DPAT infused into OFC decreased impulsive choice. These latter results demonstrate that blockade of DA D2 receptors in mPFC or activation of 5-HT1A receptors in OFC increases impulsive choice in the adjusting delay procedure.
Keywords: adjusting delay discounting, dopamine transporter, norepinephrine transporter, dopamine receptor, serotonin receptor, rat
1. Introduction
Delay discounting refers to a decrease in subjective value of a reinforcer as the delay to its delivery is increased and can be measured in both humans and laboratory animals. Typically, a subject is allowed to choose between an immediate, small reward and a delayed, larger reward. Subjects are considered more impulsive if they choose the small, immediate reward over the larger, delayed reward (Ainslie, 1975). Delay discounting is associated with substance abuse, as individuals diagnosed with substance use disorders show steeper discounting of monetary rewards relative to controls (e.g., Bickel et al., 1999; Coffey et al., 2003; Hoffman et al., 2006; Madden et al., 1997; Mitchell, 1999; Vuchinich and Simpson, 1998). Preclinical studies also show that increased sensitivity to delay is a predictor of drug abuse vulnerability, as measured in several operant and non-operant paradigms (Anker et al., 2009; Diergaarde et al., 2008; Marusich and Bardo, 2009; Perry et al., 2005, 2008a; Poulos et al., 1995; Yates et al., 2012; but see Diergaarde et al., 2012 for an exception). Thus, elucidating the neural substrates underlying impulsive choice and addiction is important for the development of novel therapies to improve treatment outcomes.
Some lesion studies have implicated different regions of the prefrontal cortex in delay discounting. The prefrontal cortex includes medial prefrontal and orbital frontal cortices (mPFC and OFC, respectively) and is involved in the acquisition and relapse of drug use (see Perry et al., 2011 for a review). Inactivation of mPFC increases sensitivity to delayed reinforcement in a T-maze paradigm (Churchwell et al., 2009), although lesions to this area do not alter delay discounting performance in an operant procedure (Cardinal et al., 2001). Evidence suggests a role for OFC in impulsive decision making, although available results are somewhat inconsistent. Lesions to OFC have been reported to increase (Mobini et al., 2002; Kheramin et al., 2002, 2004; Rudebeck et al., 2006), decrease (Winstanley et al., 2004), or have no effect on discounting (Abela and Chudasama, 2013; Churchwell et al., 2009; Stopper et al., in press). These inconsistent findings may relate to the extent and anatomical specificity of the lesion site across studies (e.g., Mar et al., 2011).
At the neurochemical level, evidence suggests that dopaminergic (DA) and serotonergic (5-HT) activity within mPFC and OFC are involved in discounting behavior. Animals high in impulsive choice show reduced electrically-evoked DA release in mPFC relative to low impulsive animals (Diergaarde et al., 2008), and an elevation of intra-mPFC 5-HT efflux is observed in animals performing a progressive delay discounting procedure (Winstanley et al., 2006). Furthermore, decreasing DA levels in mPFC increases preference for a small, immediate reinforcer (Loos et al., 2010; Pardey et al., 2013). Similarly, antagonism of 5-HT2A/C or DA D2-like receptors within OFC increases impulsive choice (Pardey et al., 2013; Wischhof et al., 2011; Zeeb et al., 2010), although these effects may be dependent on baseline levels of discounting (e.g., Zeeb et al., 2010). However, none of these studies have examined the role of DA or 5-HT receptors in mPFC or OFC using an adjusting delay discounting procedure.
The goal of the present study was to determine the role of DA and norepinephrine transporters (DAT and NET, respectively), as well as DA and 5-HT receptors in mPFC and OFC, in impulsive choice. In the current experiments, we used an adjusting delay procedure to calculate a mean adjusting delay (MAD) score, a measure of indifference between a small, immediate reinforcer and a large, delayed reinforcer. Lower MAD scores indicate increased impulsive choice, whereas higher MAD scores indicate decreased impulsive choice. We used this procedure because it has been shown to be a predictor of distinct stages of the addiction process (Anker et al., 2009; Marusich and Bardo, 2009; Perry et al., 2005, 2008a; Yates et al., 2012). In Experiment 1, rats received intra-mPFC infusions of the ADHD medications methylphenidate (MPH), amphetamine (AMPH), and atomoxetine (ATO), which exert their therapeutic effects by blocking DAT and/or NET (see Biederman, 2005 for a review). Furthermore, individuals with ADHD show greater discounting of delayed reinforcement relative to controls (Anouk et al., 2013; Demurie et al., 2012; Scheres et al., 2010). In Experiment 2, rats received intra-mPFC infusions of the DA receptor-selective drugs SKF 81297 (D1-like agonist), SCH 23390 (D1-like antagonist), quinpirole (D2-like agonist), and eticlopride (D2-like antagonist). In Experiment 3, rats received intra-mPFC infusions of the 5-HT receptor-selective drugs 8-OH-DPAT (5-HT1A agonist), WAY 100635 (5-HT1A antagonist), DOI (5-HT2A agonist), and ketanserin (5-HT2A antagonist). Experiments 4–6 were similar to Experiments 1–3, except that rats received intra-OFC infusions.
2. Results
2.1. Baseline MAD Scores
Figure 1 shows MAD scores during the final three sessions before guide cannulae implantation and during the final three sessions before receiving the first microinfusion. A 2 × 3 × 2 × 3 ANOVA showed that rats that received bilateral cannulae into OFC had higher baseline MAD scores relative to rats that received cannulae into mPFC (F(1, 36) = 10.40, p < .05), perhaps due to a difference in the type of pellet reward used across these experiments (see Procedures). However, there were no main effects of surgery, experiment, or session. Therefore, data for each experiment were collapsed in the statistical analyses. Overall, MAD scores were stable for rats receiving inta-mPFC and intra-OFC infusions.
Fig. 1.
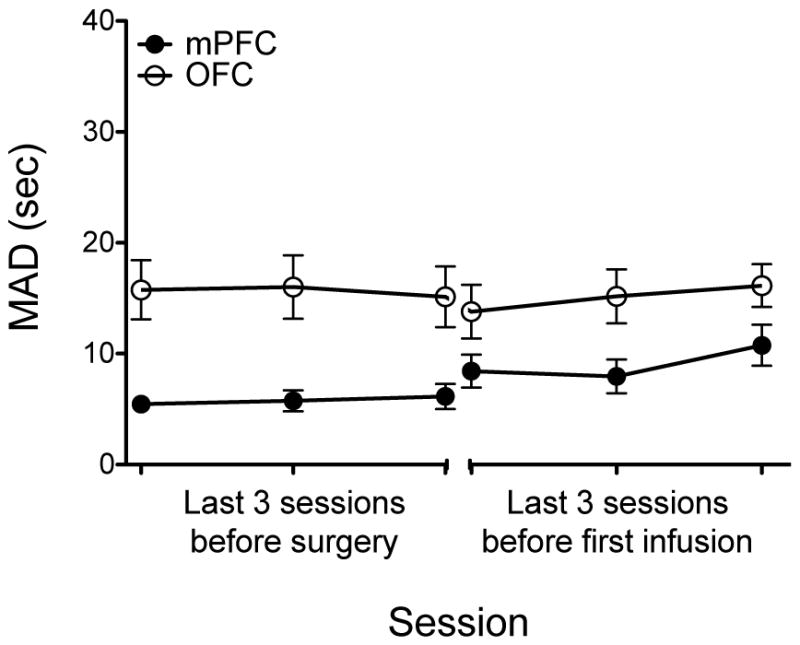
Mean (± SEM) MAD scores during the last 3 sessions before guide implantation surgery and during the last 3 sessions before the first microinfusion. Note that data for experiments 1–3 (mPFC) were collapsed and experiments 4–6 (OFC) were collapsed.
2.2. ADHD medications
There were trends for MPH to increase MAD scores in mPFC and OFC (mPFC: t(10) = 2.18, p = .06, effect size = .90; OFC: t(10) = 1.98, p = .08, effect size = .75; Fig. 2a), which suggests a decrease in impulsive choice. Within individual doses, MPH (100 μg) infused into OFC tended to increase MAD scores (t(20) = 2.47, p = .07; Fig. 2a). AMPH or ATO infusions into mPFC or OFC did not significantly alter MAD scores (p’s > .13, effect sizes < .68; Figs. 2b and 2c).
Fig. 2.
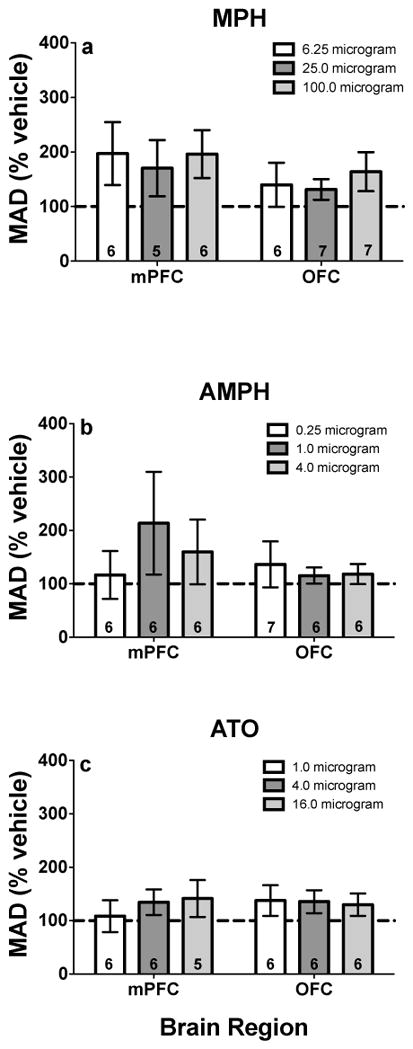
MAD scores (% vehicle; ± SEM) following mPFC and OFC microinfusions of a MPH, b AMPH, and c ATO. Values in individual bars indicate the sample size. Note, values above 100% indicate decreased impulsive choice, whereas values below 100% indicate increased impulsive choice.
2.3. DA-selective drugs
There was no overall significant effect for SKF 81297 or SCH 23390 infusions into mPFC or OFC (p’s > .14, effect sizes < .62; Figs. 3a and 3b). Quinpirole infused into mPFC tended to increase impulsive choice (t(20) = −2.04, p = .054, effect size = .84; Fig. 3c). Subsequent analysis showed that quinpirole (1.25 μg) infused into mPFC significantly decreased MAD scores (t(37) = −2.35, p < .05; Fig. 3c). Eticlopride infused into mPFC also significantly decreased MAD scores (t(20) = −2.68, p = .01, effect size = 1.10; Fig. 3d), although there were no significant differences between individual doses of eticlopride and vehicle. Administration of quinpriole or eticlopride into OFC did not significantly alter MAD scores (p’s > .14, effect sizes < .56; Figs. 3c and 3d).
Fig. 3.
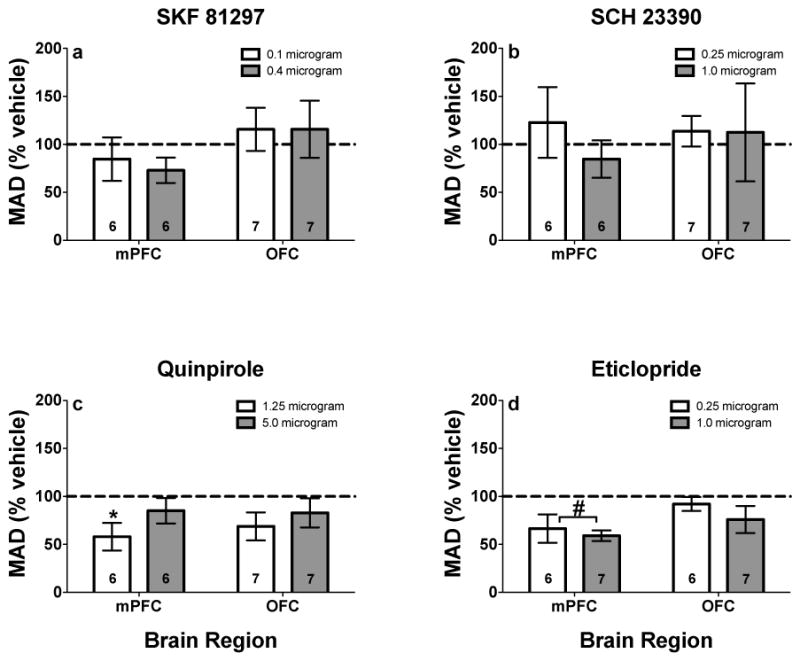
MAD scores (% vehicle; ± SEM) following mPFC and OFC microinfusions of a SKF 81297, b SCH 23390, c quinpirole, and d eticlopride. *Represents significant difference from vehicle for individual dose, p < .05. #Represents significant overall drug effect in the brain region designated, p < .05. Values in individual bars indicate the sample size. Note, values above 100% indicate decreased impulsive choice, whereas values below 100% indicate increased impulsive choice.
2.4. 5-HT-selective drugs
8-OH-DPAT infused into OFC significantly increased MAD scores (t(26) = 2.35, p = .03, effect size = .79; Fig. 4a), although there were no significant differences between individual doses of 8-OH-DPAT and vehicle. 8-OH-DPAT infused into mPFC did not significantly alter MAD scores (effect size = .17; Fig. 4a). WAY 100635, DOI or ketanserin infusions into mPFC or OFC did not significantly alter impulsive choice (p’s > .39; effect sizes < .30; Figs. 4b–4d).
Fig. 4.
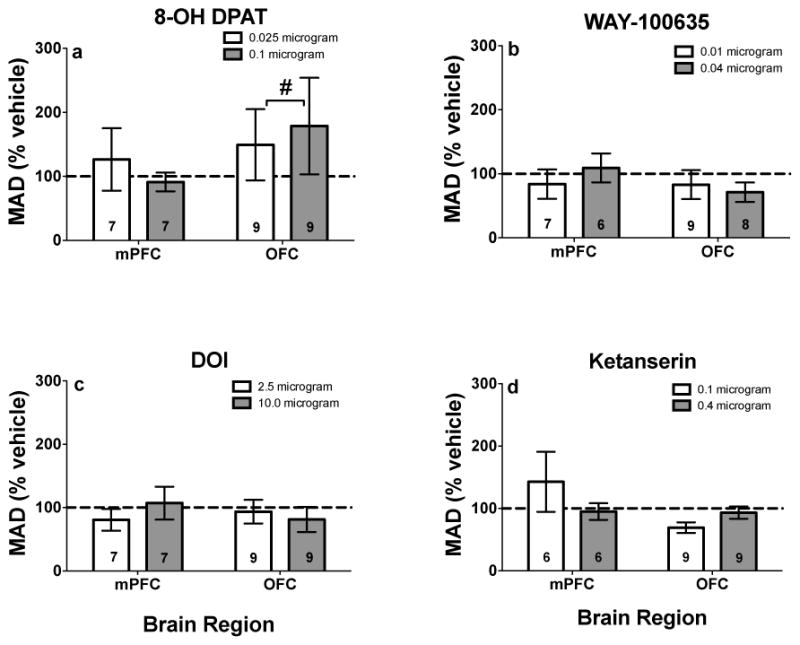
MAD scores (% vehicle; ± SEM) following mPFC and OFC infusions of a 8-OH-DPAT, b WAY-100635, c DOI, and d ketanserin. #Represents significant overall drug effect in the brain region designated, p < .05. Values in individual bars indicate the sample size. Note, values above 100% indicate decreased impulsive choice, whereas values below 100% indicate increased impulsive choice.
3. Discussion
There were three key findings in the current experiments. First, infusions of the ADHD medication drugs (MPH, AMPH and ATO) into either mPFC or OFC did not reliably alter delay discounting performance. Second, the DA D2-like agonist quinpirole (1.25 μg) and antagonist eticlopride infused into mPFC increased impulsive choice. Third, infusion of the 5-HT1A selective agonist 8-OH-DPAT into OFC decreased impulsive choice. Thus, DA D2-like receptors in mPFC and 5-HT1A receptors in OFC are involved in impulsive choice measured by performance in the adjusting delay discounting procedure.
Impulsive choice (as measured with delay discounting) and impulsive action (as measured with the five choice serial reaction time task) are predictive of distinct stages of the addiction process, such as acquisition (Perry et al., 2005, 2008a), maintenance (Diergaade et al., 2008; Marusich and Bardo, 2009), escalation (Anker et al., 2009; Dalley et al., 2007), and reinstatement (Diergaarde et al., 2008; Economdou et al., 2009) of drug self-administration. Because impulsivity is implicated in the addiction process, it is important to understand the underlying neural mechanisms of impaired decision making to develop treatment options for those with impulse control disorders and/or substance use disorders. However, because we only examined delay discounting in the current experiments, comparing and contrasting the underlying neural mechanisms of impulsive choice and impulsive action is beyond the scope of this discussion (see Dalley et al., 2008; Winstanley, 2011 for discussions on the anatomical and neurochemical substrates of impulsivity).
Given their efficacy in treating ADHD, we predicted that MPH, AMPH, and ATO would decrease impulsive choice when infused into either mPFC and/or OFC. In humans, MPH and AMPH decrease delay discounting (de Wit et al., 2002; Pietras et al., 2003). In rats, ADHD medications administered systemically decrease sensitivity to delay in the adjusting delay procedure (Perry et al., 2008b) and in the progressive delay procedure (Pitts and McKinney, 2005; Robinson et al., 2008; van Gaalen et al., 2006; Wade et al., 2000; Winstanley et al., 2003; Winstanley et al., 2005; but see Broos et al., 2012; Evenden and Ryan, 1996), although the effects of MPH and AMPH on the adjusting delay procedure are dependent on baseline levels of impulsive choice (Perry et al., 2008b). Due to the relatively small sample size (N=6–8 per group), separately examining the effects of these drugs in rats with low and high baseline levels of discounting was not feasible. Blocking NET did not alter impulsive choice in the current study, which is consistent with previous reports (Baarendse and Vanderschuren, 2012; van Gaalen et al., 2006; but see Robinson et al., 2008). Furthermore, intra-mPFC and intra-OFC infusions of guanfacine, an α2-adrenergic receptor agonist which is effective in treating ADHD (Biederman et al., 2008), do not alter delay discounting (Pardey et al., 2013), although guanfacine infused into dorsal prelimbic cortex increases impulsive action (Bari et al., 2011). Overall, the current results revealed no significant effects of MPH, AMPH or ATO infused into either mPFC or OFC, perhaps suggesting that other brain regions may play more important roles; indeed, mPFC and OFC contribute to a network system involving multiple regions, including basolateral amygdala and nucleus accumbens core (see Cardinal, 2006 for a comprehensive review), which are known to contribute to impulsive choice (e.g., Cardinal et al., 2001; Winstanley et al., 2004). Furthermore, recent evidence has implicated the anterior cingulate cortex as critically involved in behavioral disinhibition among ADHD populations (Bledsoe et al., 2013) and in delay discounting and behavioral disinhibition among stimulant abusing populations (see Crunelle et al., 2012 for a review), although it is important to note that lesions to anterior cingulate cortex do not alter impulsive choice in rats (Cardinal et al., 2001). Further studies are needed to elucidate the exact neural mechanisms involved in the ability of ADHD medications to reduce impulsive choice.
DA systems are thought to play a critical role in impulsive choice, especially in delay discounting (see Winstanley, 2011 for a review). In the current study, there was a dissociation of D1 and D2 receptor involvement in mPFC and OFC. While the D1-like drugs did not significantly alter performance, both the D2-like agonist quinpirole and antagonist eticlopride injected into mPFC increased impulsive choice. The lack of effect with D1-like drugs into mPFC or OFC is consistent with previous work showing that systemic SCH 23390 does not alter performance in an adjusting amount procedure (Wade et al., 2000). However, other work has shown that systemic or intra-mPFC administration of SCH 23390 increases impulsive choice in a progressive delay discounting procedure (Koffarnus et al., 2011; Loos et al., 2010; van Gaalen et al., 2006; Zeeb et al., 2010). Interestingly, SCH 23390 also has been reported to decrease choice for a large magnitude reinforcer when no delay is imposed (van Gaalen et al., 2006), thus complicating interpretation of the role of D1-like receptors in delay discounting with a choice between two different reinforcer magnitudes.
This is the first study to demonstrate an increase in impulsive choice following antagonism of D2-like receptors in mPFC but not in OFC. This finding contrasts with a study by Zeeb et al. (2010), who reported that eticlopride increased the preference for a small, immediate reward when infused into OFC. However, Zeeb et al. (2010) found that eticlopride increased impulsive choice only when the delay to the larger reward was cued. In the current experiment, the houselight remained illuminated during the delay to the larger reward, and it was used instead to signal the start of each trial. Thus, the houselight may have been an ambiguous cue, which may account for the lack of effect observed following intra-OFC infusions of eticlopride.
Although blockade of D2-like receptors in mPFC increased delay discounting, activation of these receptors with the agonist quinpirole also decreased preference for the larger, delayed reward. In addition to activating D2 receptors, quinpirole also binds to D3 receptors (Gehlert et al., 1992), and activation of these receptors decreases the preference for a larger, probabilistic reinforcer (St Onge and Floresco, 2009). Further, quinpirole may have stimulated D2 autoreceptors preferentially over D2 heteroreceptors at the lower concentration (1.25 μg), which would be expected to result in a functional reduction in extracellular DA release. This effect was not observed with the higher concentration of quinpirole (5.0 μg), however, perhaps due to a more direct stimulation of D2 heteroreceptors. Future studies are needed to elucidate the role of D2 autoreceptors and D3 receptors in delay discounting.
It is important to note that impulsive choice can be conceptualized as a form of decision making. In delay discounting, two factors influence an animal’s choice between two reinforcers: delay to reinforcement and reinforcer magnitude (see Ho et al., 1999). One limitation of the delay discounting procedure used in the current experiments is that this task confounds sensitivity to reinforcer amount and sensitivity to delayed reinforcement. Thus, we cannot rule out the possibility that intra-mPFC infusions of quinpirole and eticlopride impaired the discriminability of the small and large magnitude reinforcers. However, choice for the large magnitude reinforcer when its delivery is immediate is not altered following intra-OFC infusions of eticlopride (Zeeb et al., 2010). Therefore, the increase in impulsive choice observed in the current experiment appears to reflect increased intolerance to delayed reinforcement as opposed to an inability to discriminate reinforcer magnitudes.
The serotonergic system has been implicated in impulsive choice as measured with the adjusting delay procedure, as 5-HT depletion increases sensitivity to delay (Mobini et al., 2000; Wogar et al., 1993). The current study revealed a decrease in impulsive choice following 8-OH-DPAT into OFC. Since the 5-HT1A receptor is generally considered to be an autoreceptor (Albert et al., 1996), a microinjection of the agonist 8-OH-DPAT would be expected to decrease 5-HT signaling in OFC, thus suggesting that the decrease in impulsive choice was associated with decreased 5-HT heteroreceptor activation in OFC. This finding contrasts with previous results showing that systemic administration of 8-OH-DPAT increases impulsive choice in the adjusting delay procedure (Blasio et al., 2012), as well as in the progressive delay discounting task (Stanis et al., 2008; Winstanley et al., 2005). Similar to the effects observed with DA D1 antagonists, 8-OH-DPAT and related 5-HT1A agonists affect sensitivity to reinforcer magnitude (Liu et al., 2004; Stanis et al., 2008; Winstanley et al., 2005). Thus, the discrepant results observed in the current study with 8-OH-DPAT may reflect differential alterations in sensitivity to reinforcer amount and/or sensitivity to delayed reinforcement. Further, the discrepancy observed in the current study and previous reports may be explained by differential involvement of subregions of OFC in impulsive choice. For example, damage to lateral OFC increases delay discounting, whereas damage to medial OFC decreases delay discounting (Mar et al., 2011). Future studies should examine the potential dissociable effects of 5-HT1A receptors within subregions of OFC. Despite the discrepancy, the present results suggest that simulating 5-HT1A receptors within OFC, but not mPFC, decreases sensitivity to delayed reinforcement using an adjusting delay procedure.
The present study observed no significant alteration in impulsive choice following activation or blockade of 5-HT2A receptors within mPFC or OFC. In one previous study, DOI increased impulsive choice in a T-maze paradigm (Wischhof et al., 2011). However, one important procedural difference between studies is that the delay to the larger reinforcer (3 pellets) was titrated according to an animal’s responses during free-choice trials in the current study, whereas the delay to the large reward (10 pellets) was always set to 10 sec in the T-maze experiment (Wischhof et al., 2011). In any case, blockade of 5-HT2A receptors with ketanserin did not significantly affect delay discounting behavior, which is consistent with at least one previous report (Talpos et al., 2006). Thus, it appears that 5HT1A receptors in OFC, but not 5HT2A receptors in either OFC or mPFC, contribute to impulsive choice.
There are at least two caveats to the current study that need to be mentioned. First, MAD scores between rats that received intra-mPFC and intra-OFC infusions differed significantly. It is important to note that the mPFC experiments were conducted before the OFC experiments, and a couple of parameters were altered between mPFC and OFC experiments. The food reinforcer used for mPFC and OFC differed, with grain-based food pellets used in the mPFC experiments and sucrose pellets used in the OFC experiments. Considering sucrose pellets are usually more reinforcing compared to grain-based pellets (Bouton et al., 2013), this may have contributed to the increased MAD scores in the OFC experiments relative to the mPFC experiments. In addition, the initial delay for mPFC experiments (6 sec) also differed from OFC experiments (0 sec). The delay was altered to ensure that every animal experienced the immediate delivery of the large magnitude reinforcer. This procedural discrepancy may have also contributed to the discrepancies observed in MAD scores across the mPFC and OFC experiments.
A second caveat to the current study was that there was no true anatomical control (e.g., direct infusions into an area not associated with impulsive choice). Although 19 rats were excluded from the current experiments, it is important to emphasize that there were six different experiments. Thus, the number of excluded rats in each experiment was relatively low (n = 2–4), which did not make it feasible to use misplaced cannula sites as an anatomical control group. Furthermore, the finding that infusions of DA D2-like receptor ligands into mPFC, but not OFC, increased impulsive choice demonstrates that alterations in MAD scores anatomically distinct.
3.1. Conclusions
Overall, the present results support a role for dopaminergic circuitry within the mPFC and serotonergic circuitry within the OFC in impulsive choice. At the clinical level, genetic association studies have shown that polymorphisms of genes that encode for the DA transporter and D2, D4, and D5 receptors are linked to ADHD, impulsive choice, and addiction (Eisenberg et al., 2007; Kreek et al., 2005). In addition, a polymorphism in the gene encoding for the 5-HT transporter has been implicated in impulsivity as measured in a continuous performance task, with individuals having two short alleles being more impulsive than individuals having two long alleles or one short/one long allele (Walderhaug et al., 2010). The C(−1019)G polymorphism, which regulates 5-HT1A gene expression in presynaptic raphe neurons, has been associated with impulsivity as assessed with the Barratt Impulsiveness Scale (Benko et al., 2010). The current results suggest further that individuals who are high in trait-based impulsive choice may have dopaminergic hypo-functioning in mPFC and serotonergic hyper-functioning in OFC.
4. Experimental Procedure
4.1. Animals
A total of 63 experimentally-naïve adult male Sprague Dawley rats (Harlan Industries, Indianapolis, IN, USA) were used in the present experiments (N = 9, Experiments 1–3; N=12, Experiments 4–6; each rat was used in only one experiment). Upon arrival in the laboratory, rats initially had ad libitum access to food and water in their home cage for a 5-day acclimation period. After the acclimation period, rats continued to have ad libitum access to water but were food restricted to approximately 85% free feed weight. Rats were housed in temperature- and humidity-controlled rooms that were maintained on a light-dark cycle in which lights were on from approximately 06:00 to 20:00 h. All procedures were in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
4.2. Apparatus
Operant chambers (28 × 21 × 21 cm; ENV-008; MED Associates, St. Albans, VT) located inside sound-attenuating chambers (ENV-018M; MED Associates) were used for the adjusting delay task. The front and back walls of the experimental chambers were made of aluminum, while the side walls were made of Plexiglas. There was a recessed food tray (5 × 4.2 cm) located 2 cm above the floor in the bottom-center of the front wall. Retractable levers (4.5 cm) were located 6 cm above the floor on each side of the food tray on the front wall. A 28-V white cue light was located 6 cm above each response lever. A white houselight was mounted in the center of the back wall of the chamber. All responses and scheduled consequences were recorded and controlled by a computer interface. A computer controlled the experimental session using Med-IV software.
4.3. Procedure
4.3.1. Adjusting delay task
Rats were tested on an adjusting delay task using procedures similar to those described previously (Perry et al., 2005, 2008a). Daily sessions were conducted during the light phase and ended following the completion of 60 trials or 2 h, whichever occurred first. Each session included 15 blocks of 4 trials, in which the first 2 trials were forced-choice trials and the last 2 trials were free-choice trials. During forced-choice trials, only one lever (left or right; counterbalanced across trials) was extended, and the white stimulus light above the extended lever was illuminated. During free-choice trials, both levers were extended, and the stimulus lights above each lever were illuminated. Following a lever press response, one or three grain-based 45 mg pellets (PJA1-0045, Research Diets Inc., New Brunswick, NJ; Experiments 1–3) or sucrose-based 45 mg pellets (F0021 dustless precision pellet, Bio-Serve, Frenchtown, NJ; Experiments 4–6) were delivered immediately or after a delay, respectively (the location of the lever associated with delivery of immediate or delayed reinforcers alternated daily). In Experiments 1–3, the initial delay for the 3-pellet reinforcer was 6 sec. To ensure that every animal experienced the large, delayed reinforcer when no delay was imposed, the initial delay was set to 0 sec in Experiments 4–6. The delay to the 3-pellet reinforcer was adjusted according to responses during the third and fourth trials in each block (i.e., the free-choice trials). Responses for the 3-pellet reinforcer increased the delay to the larger, delayed reinforcer by 1 sec. A response for the small, immediate reinforcer resulted in a 1 sec decrease in delay to the larger, delayed reinforcer. A minimum delay of 0 sec and a maximum delay of 45 sec to the 3-pellet reinforcer were imposed. During the delay, the stimulus lights were turned off, although the house light remained illuminated until the delivery of the 3 pellets. Following reinforcement, an adjusting inter-trial interval (ITI) occurred, such that each trial lasted 60 sec. During the ITI, lights were extinguished, and lever responses had no programmed consequence. After 60 sec elapsed, the next trial began. The delay on the final free-choice trial during each session was used as the initial delay on the next session. The main outcome measure, mean adjusted delay (MAD) score, was calculated at the end of each session by averaging all adjusting delays on free-choice trials. Thus, high MAD scores reflected decreased impulsive choice, whereas low MAD scores reflected increased impulsive choice.
4.3.2. Intracranial infusions
After initial training on the adjusting delay procedure (9.2 ± 0.7 sessions), rats were anesthetized with ketamine (80 mg/kg, i.p.) and diazepam (5 mg/kg, i.p.) and implanted with bilateral intracranial cannulae (26 gauge, Small Parts, Inc, Miramar, FL) into mPFC (AP: +2.7, ML: ±1.2, DV: −2.6 below the dura, at the 10° angle off the midline; Paxinos and Watson 1998) or OFC (AP: +3.7, ML: ±2.4, DV: −3.4 below the dura, at the 10° angle off the midline; Paxinos and Watson 1998). Each cannula was fitted with a 33 gauge stylet and embedded in a cap of dental acrylic affixed at the top of the skull with stainless-steel jeweler’s screws. Rats were allowed 7 recovery days before adjusting delay sessions resumed.
The adjusting delay procedure resumed as described above to ensure that the surgery did not significantly alter MAD scores (11.7 ± 1.6 sessions). Intracranial infusions were administered immediately prior to adjusting delay sessions, and there was at least 1 baseline adjusting delay session between each infusion in which no drug was administered. On infusion days, stylets were removed from the cannulae, and stainless-steel injection cannulae (33 gauge, Small Parts, Inc) were inserted 1 mm below the tip of the guide cannulae. The injection cannulae were connected to 10 ul Hamilton syringes (Hamilton Company, Reno, NV) via PE50 tubing (Small Parts, Inc, Miramar, FL). The Hamilton syringes were mounted on an infusion pump (KDS Scientific, Holliston, MA), and all drugs were infused bilaterally at a volume of 0.5 ul/side over 2 min. Injection cannulae were left in place for 1 min after the infusion, at which time the injection cannulae were removed and the stylets were replaced. A within-subjects design was used for each experiment; the various doses of each drug were administered to each rat in random order, such that no rat received the same dosing order as another rat. In the ADHD medication experiments, rats were given MPH (6.25, 25, 100 μg), AMPH (0.25, 1.0, 4.0 μg), ATO (1.0, 4.0, 16.0 μg), or phosphate buffered saline (PBS) into either the mPFC (Experiment 1) or OFC (Experiment 4). In the DA receptor-selective drug experiments, SKF 81297 (0.1, 0.4 μg), SCH 23390 (0.25, 1.0 μg), quinpirole (1.25, 5.0 μg), and eticlopride (0.25, 1.0 μg) were administered into either the mPFC (Experiment 2) or OFC (Experiment 5); saline was used as the vehicle comparison for SCH 23390, while PBS was the vehicle for SKF 81297, quinpirole, and eticlopride. In the 5-HT receptor-selective drug experiments, 8-OH-DPAT (0.025, 0.1 μg), WAY 100635 (0.01, 0.04 μg), DOI (2.5, 10.0 μg), and ketanserin (0.1, 0.4 μg) were administered into either the mPFC (Experiment 3) or OFC (Experiment 6). Because ketanserin did not readily go into solution with PBS, dilute HCl (0.1 N) was added to PBS to dissolve ketanserin into solution and then the pH of the solution was raised to 6 using 0.1 N NaOH. PBS+HCl was used as the vehicle comparison for ketanserin, while PBS alone was used as the vehicle for 8-OH-DPAT, WAY 100635, and DOI. The concentration ranges selected for each drug were based on previous reports (Beyer and Steketee, 2000; Beyer and Steketee, 2002; Carli et al., 2006; Floresco et al., 2006; Granon et al., 2000; Herremans et al., 1996; Hooks et al., 1992; Kozlov et al., 2001; Passetti et al., 2003; Prasad et al., 1999; Willins and Meltzer, 1997).
4.3.3. Histology
At the end of each experiment, rats were euthanized and cannulae placements were verified. The brain was removed and 40 μm frozen serial sections were taken and stained with cresyl violet. The sections were examined under a light microscope, and the most ventral point of each injection track was mapped onto the appropriate place from the rat brain atlas of Paxinos and Watson (1998).
4.3.4. Drugs
Methylphenidate hydrochloride was purchased from Mallinckrodt (St. Louis, MO). d-Amphetamine sulfate, atomoxetine hydrochloride, R(+)-SKF 81297 hyrdrobromide, R(+)-SCH 23390 hydrochloride, (−)-quinpirole hydrochloride, (±)-8-hydroxy-2-(di-N-propylamino) tetraline hydrobromide (8-OH-DPAT), WAY-100635 maleate, (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI), ketanserin tartate were purchased from Sigma-Aldrich (St. Louis, MO). S(−)-eticlopride hydrochloride was purchased from Research Biochemicals (Natick, MA).
4.4. Data Analyses
To determine whether MAD scores were stable during the final 3 sessions before surgery and during the final 3 sessions before the first microinfusion, a 2 × 3 × 2 × 3 analysis of variance (ANOVA) with region (mPFC vs. OFC) and experiment (3 experiments) as between subject factors and surgery (before vs. after) and session as within-subjects factors was conducted using version 19 of SPSS (SPSS Inc., Armonk, NY). A p-value less than 0.05 defined statistical significance.
A linear mixed model was used to analyze MAD scores following microinfusions in each experiment using Version 9.3 of SAS (SAS Institute, Cary, NC). A linear mixed model is a generalization of repeated measures ANOVA that can accommodate covariates and partially missing data from some subjects, while still accounting for within-subject correlations on repeated measurements (see Gueorgieva and Krystal, 2004; Young et al., 2009 for a discussion on mixed modeling). The linear mixed model included both treatment and dose as explanatory variables, and MAD scores were normalized to vehicle.
Using contrasts from the linear mixed model (thus, still accounting for within-subject correlations and the different doses that were administered), we derived T test statistics for the primary null hypotheses that the MAD score across MPH concentrations was 100% of vehicle, the MAD score across AMPH concentrations was 100% of vehicle, and the MAD score across ATO concentrations was 100% of vehicle. When one of the results from these hypothesis tests was statistically significant (p-value less than 0.05) or trended toward statistical significance (p-value between 0.05 and 0.10), we used additional contrasts from the linear mixed model to derive T test statistics for the secondary null hypotheses that the MAD scores at the individual doses of the corresponding treatment were 100% of vehicle. A Bonferroni adjustment was used, such that an overall significance level of 0.05 was maintained across all comparisons for a particular treatment. Effect size was defined as an estimate of ([absolute value(mean response – 100%)/standard deviation of response)]/number of drug doses), where response was defined for each subject and each drug as the subject’s MAD (as a percentage of vehicle) across the doses of that drug. Effect sizes below 0.50 were defined as small, effect sizes between 0.50 and 1.00 were defined as medium, and effect sizes above 1.00 were defined as large.
Figure 5 shows correct probe placements for rats used for data analyses. Rats were excluded from data analysis if one or both probe placements were outside of the mPFC or OFC. Three rats in Experiment 1, three rats in Experiment 2, two rats in Experiment 3, four rats in Experiment 4, four rats in Experiment 5, and three rats in Experiment 6 had one or both probe placements outside of the mPFC and OFC, and thus were excluded from data analyses. For the mPFC experiments, incorrect probe placements were observed in various brain regions, including the anterior cingulate cortex, the dorsal peduncular cortex, the forceps minor of the corpus callosum, and the secondary motor cortex. For the OFC experiments, incorrect probe placements were observed in several brain regions, including the forceps minor of the corpus callosum, the periform cortex, and the ventral tenia tecta. Overall, 44 rats were included in the present analyses (Experiment 1 N = 6, Experiment 2 N = 6, Experiment 3 N = 7, Experiment 4 N = 8, Experiment 5 N = 8, Experiment 6 N = 9).
Fig. 5.
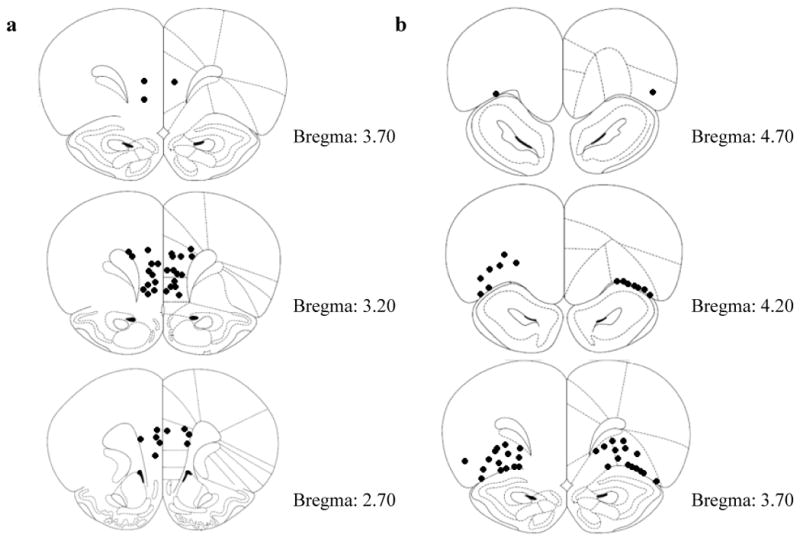
a Diagram showing locations for bilateral intra-mPFC cannula injection sites for rats in Experiments 1–3 (mPFC). b Diagram showing locations for bilateral intra-OFC cannula injection sites for rats in Experiments 4–6 (OFC).
Highlights.
Administration of ADHD medications into mPFC or OFC does not alter impulsive choice.
Stimulating or blocking DA D2 receptors within mPFC increases impulsive choice.
Stimulating 5-HT1A receptors within OFC increases impulsive choice.
Acknowledgments
We would like to thank Josh Cutshall, Blake Dennis, Jason Ross, Emily Denehy, Kate Fischer, and Travis McCuddy for technical assistance.
Footnotes
This work was supported by USPHS grants P50 DA05312 and T32 DA007304.
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abela AR, Chudasama Y. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. Eur J Neurosci. 2013;37:640–647. doi: 10.1111/ejn.12071. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5-HT1A receptor: signaling, desensitization, and gene expression. Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anouk S, Tontsch C, Thoeny AL. Steep temporal reward discounting in ADHD-combined type: acting upon feelings. Psychiatry Res. 2013;209:207–213. doi: 10.1016/j.psychres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Baarendse PJJ, Vanderschuren LJMJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology. 2012;219:313–326. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko A, Lazary J, Molnar E, Gonda X, Tothfalusi L, Pap D, Mirnics Z, Kurimay T, Chase D, Juhasz G, et al. Significant association between the C(−1019)G functional polymorphism of the HTR1A gene and impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2009;153B:592–599. doi: 10.1002/ajmg.b.31025. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Intra-medial prefrontal cortex injection of quinpirole, but not SKF 38393, blocks the acute motor-stimulant response to cocaine in the rat. Psychopharmacology. 2000;151:211–218. doi: 10.1007/s002139900345. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Cocaine sensitization: Modulation by dopamine D2 receptors. Cereb Cortex. 2002;12:526–535. doi: 10.1093/cercor/12.5.526. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective review. Biol Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiebot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology. 1999;146:400–412. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- Blasio A, Narayan AR, Kaminski BJ, Steardo L, Sabino V, Cottone P. A modified adjusting delay task to assess impulsive choice between isocaloric reinforcers in non-deprived male rats: Effects of 5-HT2A/C and 5-HT1A receptor agonists. Psychopharmacology. 2012;219:377–386. doi: 10.1007/s00213-011-2517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe JC, Semrud-Clikeman M, Pliszka SR. Anterior cingulate cortex and symptom severity in attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2013;122:558–565. doi: 10.1037/a0032390. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Miles OW, León SP, Epstein LH. Within- and between-session variety effects in a food-seeking habituation paradigm. Appetite. 2013;66:10–19. doi: 10.1016/j.appet.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos N, Schmaal L, Wiskerke J, Kostelijk L, Lam T, Stoop N, Weierink L, Ham J, de Geus EJ, Schoffelmeer AN, van den Brink W, Veltman DJ, de Vries TJ, Pattij T, Goudriaan AE. The relationship between impulsive choice and impulsive action: A cross-species translational study. PLoS One. 2012;7:e36781. doi: 10.1371/journal.pone.0036781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Networks. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Veltman DJ, Booij J, Emmerik-van Oortmerssen K, van den Brink W. Substrates of neuropsychological functioning in stimulant dependence: A review of functional neuroimaging research. Brain Behav. 2012;2:499–523. doi: 10.1002/brb3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peña Y, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Temporal discounting of monetary rewards in children and adolescents with ADHD and autism spectrum disorders. Dev Sci. 2012;15:791–800. doi: 10.1111/j.1467-7687.2012.01178.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattiji T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, de Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer AN, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addict Biol. 2012;17:576–587. doi: 10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT, Mackillop J, Modi M, Beauchemin J, Dang D, Lisman SA, Lum JK, Wilson DS. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 Taql A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Seeman P, Schaus J. Autoradiographic localization of [3H]quinpirolepirole binding to dopamine D2 and D3 receptors in rat brain. Eur J Pharmacol. 1992;211:189–194. doi: 10.1016/0014-2999(92)90528-c. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Herremans AH, Hijzen TH, Welborn PF, Olivier B, Slangen JL. Effects of infusion of cholinergic drugs into the prefrontal cortex area on a delayed matching to position performance in the rat. Brain Res. 1996;711:102–111. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- Ho M-Y, Mobini S, Chiang T-J, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology. 1999;146:362–372. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Liem BJ, Justice JB., Jr Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intra-cranial amphetamine infusions. Ann N Y Acad Sci. 1992;654:444–447. doi: 10.1111/j.1749-6632.1992.tb25993.x. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Ho M-Y, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: A quantitative analysis. Psychopharmacology. 2004;175:206–214. doi: 10.1007/s00213-004-1813-y. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Mobini S, Ho M-Y, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of quinolinic acid-induced lesuons of the orbital frontal cortex on inter-temporal choice: A quantitative analysis. Psychopharmacology. 2002;165:9–147. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behav Pharmacol. 2011;22:300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict Behav. 2003;28:1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Kozlov AP, Druzin MY, Kurzina NP, Malinina EP. The role of D1-dependent dopaminergic mechanisms of the frontal cortex in delayed responding in rats. Neurosci Behav Physiol. 2001;31:405–411. doi: 10.1023/a:1010488612338. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Geneticlopride influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Liu YP, Wilkinson LS, Robbins TW. Effects of acute and chronic buspirone on impulsive choice and efflux of 5-HT and dopamine in hippocampus, nucleus accumbens and prefrontal cortex. Psychopharmacology. 2004;173:175–185. doi: 10.1007/s00213-003-1726-1. [DOI] [PubMed] [Google Scholar]
- Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, Spijker S, van Gaalen MM. Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb Cortex. 2010;20:1064–1070. doi: 10.1093/cercor/bhp167. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Exp Clin Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–454. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The effect of delay and of intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; 1987. pp. 53–73. [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang T-J, Ho M-Y, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology. 2005;182:508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Pardey MC, Kumar NN, Goodchild AK, Cornish JL. Catecholamine receptors differentially mediate impulsive choice in the medial prefrontal and orbitofrontal cortex. J Psychopharmacol. 2013;27:203–212. doi: 10.1177/0269881112465497. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, Robbins TW. Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology. 2003;165:136–145. doi: 10.1007/s00213-002-1227-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: Translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacology. 2008a;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008b;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Pietras CJ, Cherek DR, Lane SD, Tcheremissine OV, Steinberg JL. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology. 2003;170:390–398. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- Pitts RC, McKinney AP. Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav. 2005;83:297–314. doi: 10.1901/jeab.2005.47-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Prasad BM, Hochstatter T, Sorg BA. Expression of cocaine sensitization: Regulation by the medial prefrontal cortex. Neuroscience. 1999;88:765–774. doi: 10.1016/s0306-4522(98)00183-3. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biol Psychiatry. 2010;67:641–648. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Depend. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Stopper CM, Green EB, Floresco SB. Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cereb Cortex. doi: 10.1093/cercor/bhs297. in press. [DOI] [PubMed] [Google Scholar]
- Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacol. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacology. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Walderhaug E, Herman AI, Magnusson A, Morgan MJ, Landrø NI. The short (S) allele of the serotonin transporter polymorphism and acute tryptophan depletion both increase impulsivity in men. Neurosci Lett. 2010;473:208–211. doi: 10.1016/j.neulet.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Brit J Pharmacol. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology. 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: Therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Hollensteiner KJ, Koch M. Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav Pharmacol. 2011;22:805–813. doi: 10.1097/FBP.0b013e32834d6279. [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology. 1993;111:239–243. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]
- Yates JR, Marusich JA, Gipson CD, Beckmann JS, Bardo MT. High impulsivity in rats predicts amphetamine conditioned place preference. Pharm Biochem Behav. 2012;100:370–376. doi: 10.1016/j.pbb.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Clark MH, Goffus A, Hoane MR. Mixed effects modeling of Morris water maze data: advantages and cautionary notes. Learn Mem. 2009;40:160–177. [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: Interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology. 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]


