Abstract
Background
Prescription opioid use and overdose deaths are increasing in the U.S. Among under-age-65, disabled Medicare beneficiaries, the rise in musculoskeletal conditions as qualifying diagnoses suggests opioid analgesic use may be common and increasing, raising safety concerns.
Methods
From a 40% random-sample Medicare denominator, we identified fee-for-service beneficiaries under-age-65 and created annual enrollment cohorts 2007-2011 (6.4 million person-years). We obtained adjusted, annual opioid use measures: any use, chronic use (≥6 prescriptions), intensity of use (daily morphine equivalent dose (MED)), opioid prescribers per user. Geographic variation was studied across Hospital Referral Regions (HRRs).
Results
Most measures peaked in 2010. The adjusted proportion with any opioid use was 43.9% in 2007, 44.7% in 2010 and 43.7% in 2011. The proportion with chronic use rose from 21.4% in 2007 to 23.1%, in 2011. Among chronic users: mean MED peaked at 81.3 mg in 2010, declining to 77.4 mg in 2011; in 2011, 19.8% received ≥ 100 mg MED; 10.4% received ≥200 mg. In 2011 HRR-level measures varied broadly (5th to 95th percentile): any use: 33.0% to 58.6%, chronic use: 14.0% to 36.6%; among chronic users, mean MED ranged from 45 mg to 125 mg; mean annual opioid prescribers from 2.4 to 3.7.
Conclusions
Among these beneficiaries, opioid use was common. While intensity stabilized, the population using opioids chronically grew. Variation shows a lack of standardized approach and reveals regions with mean MED at levels associated with overdose risk. Future work should assess outcomes, chronic use predictors and policies balancing pain control and safety.
Background
As prescription opioid consumption rises in the U.S., use by the under age-65, disabled population warrants careful examination.1 Growing numbers of Americans are applying for and receiving Social Security Disability Insurance (SSDI).2,3 This SSDI program expansion includes a rapid rise in eligibility due to musculoskeletal conditions, often treated with prescription analgesics. In 2011, musculoskeletal conditions such as back pain, were the most common SSDI-qualifying diagnoses, accounting for 33.8% of program participants (up from 20% in 1996).2 This shift in the composition of disabling conditions, combined with national trends of increasing prescription opioid use and prescription opioid overdose deaths, suggests the potential for substantial opioid use in the SSDI population and raises concern for the overall health and safety of these injured and ill workers.
Although the best approach to pain management and opioid analgesic prescribing, in particular, are debated, intense chronic opioid analgesics use for non-malignant pain is increasingly recognized as ineffective and potentially hazardous to individuals and to the public.1,4-7 In state and national-level analyses, higher use of prescription opioids has been linked to higher drug overdose death rates among patients and, due to drug diversion, non-patients as well. 5,6,8-10 Despite these warnings, the use of prescription opioids in the U.S. continues to climb.11-13 The SSDI population may be at high risk for chronic, intense and potentially hazardous prescription opioid use, but the analgesic consumption of this large and growing national population of patients supported by federal disability insurance has not been studied.
We examined the opioid prescription fill patterns of under 65-year-old Medicare beneficiaries. Disabled workers become eligible for Medicare benefits, regardless of age, two years after qualifying for SSDI, and SSDI recipients make up nearly all under-65-year-old Medicare beneficiaries.3 Our aim was to quantify their use of prescription opioids over time and across geographic areas.
Methods
Study Population
Using a 40% Medicare random sample denominator for each of five calendar years, 2007-2011, we identified patients under age 65, continuously enrolled in fee-for-service Medicare Parts A, B and D (inpatient, outpatient and prescription benefits). We analyzed opioid use separately for each calendar year from 2007-2011 using all beneficiaries in our 40% sample. Beneficiaries were only included in a calendar year if they were continuously enrolled for the entire 12 months. We obtained individual-level covariates using the Medicare Beneficiary Summary File (denominator file), MedPAR, Carrier, Outpatient and Hospice files.14 To eliminate study of opioids used for cancer-related pain or end-of-life pain management, we excluded patients with any hospice use or any ICD-9 diagnosis code for cancer (except non-melanoma skin cancer) occurring at any time in the enrollment year. To focus our cohort on disabled workers, we also excluded patients qualifying for Medicare due to end-stage renal disease.
Main Measures
We determined individual-level counts of opioid analgesic fill events from the Medicare Part D Prescription Drug Event (PDE) file. We calculated individual annual pills (or volume of elixir products (< 1% of fills)) received. To achieve opioid use measures comparable across products, individual daily morphine equivalent dose (MED) was calculated by dividing total annual morphine equivalents received by 365 (Appendix Table 1,Supplemental Digital Content 1, http://links.lww.com/MLR/A786 ).15,16 Annualized, one additional daily morphine equivalent approximately equals an additional 5 mg of oxycodone each week. Among chronic opioid users (6 or more fills), we used encrypted unique prescriber identification to calculate the number of unique opioid prescribers. We calculated population measures of opioid analgesic use including: proportion of the population with any use (one or more fill), proportion of the population with chronic use, total number of opioid pills and morphine equivalents per user; among chronic users we calculated number of unique opioid prescribers. Because of high-profile abuse of oxycodone, we measured proportion of all units dispensed as oxycodone products.9,17
Covariates
For each beneficiary in each study year we obtained demographic characteristics from the Medicare Beneficiary Summary File, including age, sex, race/ethnicity (categorized as black, Hispanic, white or other) and Part D low-income subsidy (LIS) status (a poverty indicator). Year-specific inpatient and outpatient claims were used to obtain Charlson comorbidities, as well as diagnoses important in this population: musculoskeletal disorders (using Social Security Administration range of diagnosis codes) depression and serious mental illness (bipolar disorder, schizophrenia, schizoaffective disorder and other non-organic psychoses).18-20 Appendix 2(Supplemental Digital Content 2, http://links.lww.com/MLR/A787 ) lists diagnostic codes. We determined individual Prescription Hierarchical Clinical Condition (Rx-HCC) scores.21 Rx-HCC is the system used to risk adjust Part D plan payments for health status, and we constructed them using index year data.22 ZIP code was used to assign each patient to a state and one of 306 Dartmouth Atlas of Healthcare Hospital Referral Regions (HRR).23
Analysis
We used descriptive statistics to quantify opioid analgesic fills and pills as well as morphine equivalents. To compare national mean population use measures across years, we estimated linear regression models that included age, gender, LIS status, race/ethnicity, comorbidities, an indicator variable for musculoskeletal disease, and year indicators, using 2007 as our reference. Comorbidity counts were used for year-to-year comparisons, rather than Rx-HCC because Rx-HCC is recalibrated periodically and thus not valid for longitudinal comparison. We used quantile regression to obtain adjusted median values for opioid measures. To obtain 2011 adjusted HRR measures, the same analytic approach was used but Rx-HCC was employed in place of comorbidity count. We prefer the Rx-HCC to morbidity counts for within year comparisons because it includes a large number of diagnoses hierarchically and was designed specifically to predict prescription spending, a correlate of prescription use.21,22 As musculoskeletal diseases are included broadly in the Rx-HCC, our indicator for these disease was not included in calculation of these 2011 HRR-level measures. In sensitivity analyses, we repeated measures across years, without the indicator variable for musculoskeletal disease.
Results
Primary Analyses
The study from 2007 to 2011 included 6.4 million person years; annual cohorts ranged from 1.21 million (2007) to 1.37 million (2011) people, with substantial year to year cohort overlap; on average each unique individual was present in 3.3 of the 5 years studied, creating an 83 to 84% cohort overlap between consecutive years. Demographic, economic status, health status, and eligibility category did not change appreciably from year to year. In 2011, mean age was 49.2; 49.9% were female; 20.2% were Black and 8.6% were Hispanic; 87.5% received Part D low-income subsidy, and 74.0% were eligible for Medicaid; 100% were eligible for Medicare due to Disability Insurance. Mean Charlson comorbidity count was 1.64 (standard deviation (SD) 1.85), mean Rx-HCC score was 1.10 (SD 0.61); overall 65.5% had musculoskeletal disease, 7.1% had serious mental illness diagnoses; 25.2% had depression diagnosis. Demographics, comorbidity counts and Rx-HCC scores varied substantially across groups defined by opioid use. Compared to non-users, users with less than six fills had higher proportions of females, more morbidities, more musculoskeletal disease and more depression. These differences were more pronounced in the comparison of non-users to chronic users, particularly in the proportion with musculoskeletal disease. Chronic users were more likely to be white compared to non-users and users with les than 6 fills. (Table 1) (Appendix Table 3, Supplemental Digital Content 3, http://links.lww.com/MLR/A788 shows characteristics by year)
Table 1. Medicare Beneficiaries Under Age 65, 2007-2011: Demographics and Select Characteristics Overall and by Opiate Use Category.
| Overall | Opiate Non-Users | Opiate Users | ||
|---|---|---|---|---|
| Less than six fills | Six or more fills | |||
| N | % | % | % | |
| Person years 2007-2011 | 6,375,633 | 54.3 | 22.2 | 23.5 |
| 2007 | 1,213,680 | 56.1 | 22.6 | 21.4 |
| 2008 | 1,231,506 | 54.9 | 22.5 | 22.7 |
| 2009 | 1,254,370 | 53.8 | 22.4 | 23.8 |
| 2010 | 1,308,389 | 53.3 | 22.1 | 24.6 |
| 2011 | 1,367,688 | 53.8 | 21.3 | 24.9 |
| 2011 Population Characteristics | ||||
| Age Mean (SD) | 49.2 (10.6) | 48.4 (11.1) | 49 (10.7) | 51 (8.9) |
| Female % | 49.9 | 44.1 | 56.1 | 56.2 |
| White % | 67.7 | 64.7 | 64.7 | 74.7 |
| Black % | 20.2 | 21.4 | 22.5 | 16.9 |
| Hispanic % | 8.6 | 9.8 | 9.5 | 5.9 |
| Low-income subsidy (% with any) | 87.5 | 87.8 | 87.3 | 87.2 |
| Medicare & Medicaid Dually eligible (%) | 74 | 74.1 | 74.3 | 74.5 |
| RxHCC Mean (SD) | 1.10 (0.61) | 1.03 (0.58) | 1.14 (0.62) | 1.22 (0.62) |
| Charlson comorbidity count Mean (SD) | 1.64 (1.85) | 1.2 (1.53) | 1.93 (1.91) | 2.36 (2.13) |
| Musculoskeletal Disease (%) | 65.5 | 47 | 78.4 | 94.4 |
| Serious mental illness diagnosis (%) | 7.1 | 7.3 | 7.3 | 6.5 |
| Depression diagnosis (%) | 25.2 | 18 | 29.2 | 37.5 |
RxHCC stands for Rx Hierarchical Clinical Condition scores based on index year diagnoses on inpatient and outpatient claims. Charlson Comorbidities from 1987 Journal of Chronic Disease. Low-income subsidy is Medicare Part D low-income subsidy, an indicator or income ≤ 150% of poverty. Serious mental illness is bipolar disorder, schizophrenia, schizoaffective disorder and other non-organic psychoses. All diagnoses occur in the year of study for each annual cohort. The annual cohorts include only patients enrolled in fee-for-service Medicare Parts A, B and D for the full calendar year.
Nearly all opioid use measures peaked in 2010, with modest declines in 2011 (Table 2). The proportion of the population with any opioid use was 43.9% in 2007, 44.7% in 2010 and 43.7% in 2011; chronic use rose from 21.4% in 2007 to 23.1% in 2011. Overall, median MED increased from 9.8 mg in 2007 to 11.0 mg in 2010 and the returned to 9.9 mg in 2011. Among patients with chronic use: median MED rose from 32.9 mg in 2007 to 36.2 mg in 2010, mean from 77.1 mg to 81.3 mg; from 2010 to 2011, both measures decreased to near 2007 levels. Proportion of chronic users with MED ≥ 100 mg and MED ≥ 200 mg was stable over this period at, essentially, 20% and 10% respectively. Two measures continued to grow through 2011: median annual pill count increased 30.7%, from 239 in 2007 to 312 in 2011, suggesting, with relatively stable MED, that higher numbers of lower dose pills were being dispensed. Oxycodone, the second most commonly prescribed opioid (after hydrocodone), rose from 18.8% of all pills dispensed in 2007 to 24.4% in 2011.
Table 2. Prescription opiate fill patterns 2007-2011, adjusted for year-to-year differences in age, sex, race, comorbidities (including musculoskeletal disease), and Part D low income subsidy status.
| 2007 | 2008 | 2009 | 2010 | 2011 | |
|---|---|---|---|---|---|
| Overall | |||||
| Percent of beneficiaries with any opiate fill | 43.9 | 44.5 | 44.7 | 44.7 | 43.7 |
| Percent with six or more opiate fills | 21.4 | 22.2 | 22.8 | 23.2 | 23.1 |
| Median annual pills dispensed per user per year | 239.1 | 254.5 | 279.6 | 295.6 | 312.4 |
| Median morphine equivalents per day per user | 9.8 | 10.2 | 10.8 | 11.0 | 9.9 |
| Percent of units dispensed as oxycodone products | 18.8 | 19.6 | 20.5 | 22.3 | 24.4 |
| Chronic Opiate Users (6 or more opiate fills per year): | |||||
| Mean morphine equivalents per day | 77.1 | 77.7 | 80.7 | 81.3 | 77.4 |
| Median morphine equivalents per day | 32.9 | 33.4 | 35.7 | 36.2 | 33.9 |
| Mean unique opiate prescribers per year | 3.01 | 3.08 | 3.03 | 2.99 | 2.93 |
| Mean opiate prescription fill events per year | 15.6 | 15.7 | 15.6 | 15.5 | 15.4 |
| Percent taking more than 100 mg morphine equivalents per day | 20.9 | 20.9 | 21.6 | 21.6 | 19.8 |
| Percent taking more than 200 mg morphine equivalents per day | 10.0 | 10.2 | 10.7 | 10.9 | 10.4 |
Sensitivity analyses repeating all adjusted annual measures without the musculoskeletal disease indicator variable revealed nearly identical values.
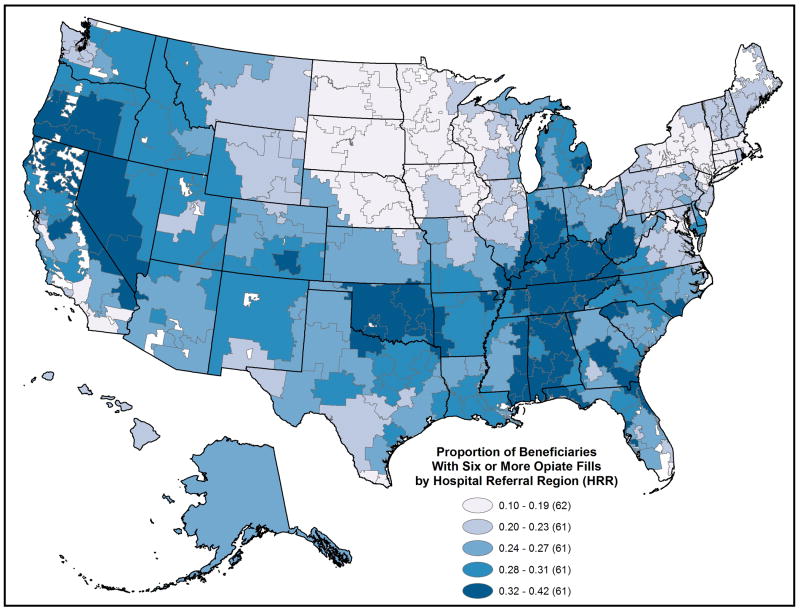
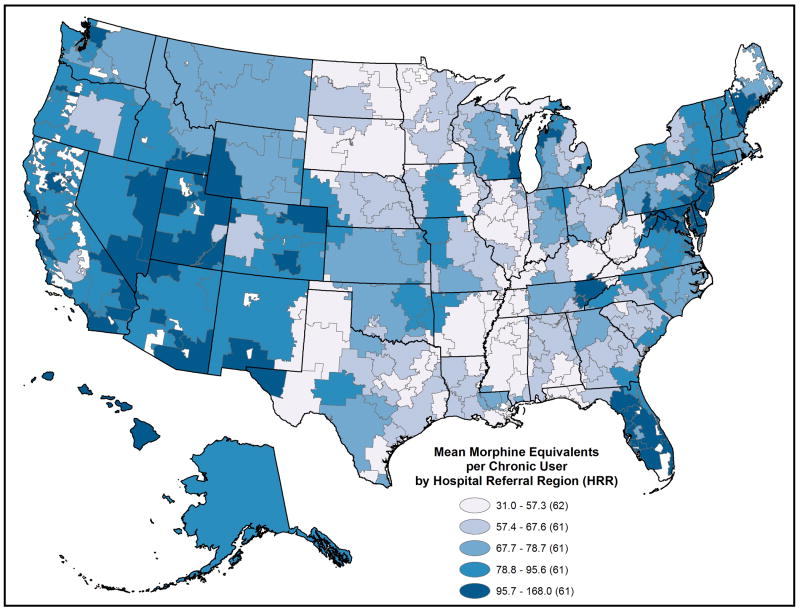
Adjusted prescription opioid use varied substantially across the 306 HRRs in 2011. (Figures 1 and 2 and Appendix Table 4, Supplemental Digital Content 4, http://links.lww.com/MLR/A789 ) The proportion of these beneficiaries per HRR with any opioid fill ranged from 24.9% to 63.0% (5th to 95th percentile, 33.0% to 58.6%); the proportion with chronic use ranged from 9.5% to 42.4% (5th to 95th percentile, 14.0% to 36.6%). Among chronic users: MED varied broadly, median from 19.1 mg to 84.6 mg (5th to 95th percentile 22.8 to 52.8), mean from 31.5 mg to 167.9 mg (5th to 95th percentile 44.9 to 124.5), while median number of opioid fills per chronic user varied little, 10.6 to 15.6 (5th to 95th percentile 12.5 to 13.9); mean number of opioid prescribers ranged from 2.1 to 4.2. (5th to 95th percentile 2.4 to 3.7). Overall, the proportion of pills dispensed as oxycodone ranged from 1.7% to 60.5% (5th to 95th percentile 4.0% to 48.6%).
Figure 1.
2011 Proportion of the under age 65, disabled Medicare beneficiaries filling six or more opioid prescriptions, by Hospital Referral Region.
Figure 2.
2011 Prescription opioid use intensity among under age 65, disabled Medicare beneficiaries filling six or more opioid prescriptions, by Hospital Referral Region. Opioid use intensity is measured as mean daily morphine equivalents per user.
State-level patterns also emerged. When HRRs were ranked on adjusted 2011 mean MED per chronic user, Florida HRRs made up 8 of the top-10; all had values above 135 mg. When ranked on proportion of the population with six or more fills, four of the top-10 HRRs were in Tennessee. When HRRs were ranked by oxycodone use, Newark, NJ was at the top (60.5%); eight of the remaining top-10 clustered on the eastern seaboard (New Jersey, Maryland, and Delaware); 18 of the 20 lowest HRRs were in Texas. (Figures 1 and 2 and Appendix Table 4, Supplemental Digital Content 4, http://links.lww.com/MLR/A789)
Discussion
Prescription opioid use is common among disabled, under age 65 Medicare beneficiaries. Each year, nearly half filled at least one opioid prescription; almost one in four filled six or more prescriptions. Although the increase in opioid intensity observed from 2007 to 2010 was not sustained through 2011, average daily dose remained intense in the growing population of chronic users; 20% received 100 mg morphine equivalents per day or more, 10% reached or exceeded 200 mg daily. 1,5,24-26 Broad HRR-level variation persisted after adjustment for patient-level factors. Wide ranges of opioid use, in this population of patients meeting national disability eligibility criteria, and universally insured through fee-for-service Medicare, suggest strong regional determinants of the patterns we observed.
We document the intense burden of musculoskeletal disease and the analgesic treatment experience of disabled workers over five years. Our findings are consistent with growing evidence of increasing opiate use in the U.S, but provides granular population and geographic detail. Overall, among disabled Medicare beneficiaries, the presence of some diagnosed musculoskeletal condition is normative (65%); among those using opioids chronically, it was ubiquitous (94%). By comparison, 50% of adults in the general, U.S. population reported having some musculoskeletal disease (back, neck or joint pain) in 2008.27 A study of two integrated delivery system health plans in the Western U.S. reported in that 20% of all enrollees in 2005 used some opioid analgesics while 5% were “long term users.”12,27 In a population more comparable to disabled Medicare beneficiaries, a systematic review of studies from 2000 to 2012 estimated 20% to 40% of workers' compensation patients used some opioids.28 For a subset of these patients in one state (Ohio) for whom claims data were analyzed, mean daily opioid dose was slightly higher (MED 58 mg) than the average we observed among 2011 beneficiaries with any opioid use (MED 43 mg, overall, MED 22.2-42.5 in Ohio regions ); the difference in daily dose likely is due in part to population differences. The Medicare population we studied suffers diverse disabling conditions that are not necessarily work related.
Although overall prevalence of any opioid use in this population changed little between 2007 and 2011, the proportion of the population with chronic use grew steadily. What might explain this increase in chronic use? The prevalence of one or more musculoskeletal diseases increased slowly over the span of our study, from 61.1% in 2007 to 65.5% in 2011(Appendix table 3,Supplemental Digital Content 3, http://links.lww.com/MLR/A788 ). We adjusted for this growth in our annual opioid use measures and nonetheless observed a steady increase in chronic opioid use (from 21.4% in 2007 to 23.1% in 2011). The growth may simply reflect, to some extent, the effect of time on the experience of patients with substantial disease burden in the U.S, where opioid use is increasingly common. 11-13 It may also reflect population health state changes we have not accounted for in our measures, despite the very inclusive models. The regional variation we observed, and discuss further below, suggests clinician practice patterns likely explain some of this trend. Whatever the explanation, the large and increasing proportion of this population chronically using opioids at a mean MED of at least 77mg is worrisome in light of established and growing evidence that intense opioid use to treat non-malignant pain may not be effective and may confer important risks.29-31
The level of opioid use we observed among the 20% of chronic users with the most intense daily dose (MED 100 mg to 200 mg) is especially concerning. Opioid use of this intensity has been associated with risk of overdose death in the general U.S. population.1,5,24,25 In a study specifically examining disabled workers in Washington state, investigators found a high risk of opioid overdose death when the daily dose averaged 100-120 morphine equivalents.26 This suggests the cohorts we studied may be at significant risk of opioid overdose death.
Characteristics we observed among chronic opioid users echo others' findings and raise further concern. Although white men are still the most common victims of opioid overdose death in the U.S., the Centers for Disease Control and Prevention has reported growth in opioid overdose deaths among women.10 In the SSDI population, we find that women were at greater risk than men of being chronic opioid users. Another common condition diagnosed in chronic opioid users was depression (38%). The relationship between opioid use and depression is complex and directionality uncertain. Depression may be experienced as physical pain prompting opioid use, and depression may blunt responsiveness to analgesics reinforcing increased or prolonged use.32,33 A recent study of U.S. veterans showed opioid initiation and continuation was strongly associated with greater risk of developing depression, and the association appeared dose dependent.34 Such evidence is worrisome given the relationship between depression and prescription opioid use in this population, and more generally, the risk of overdose and overdose death due to opioid misuse, suicidality, or hazardous combination of opioids and other sedatives commonly prescribed for mental illness.12,25,35
The variation we observed at HRR and state-levels is consistent with a lack of standardized approach to use of opioid analgesics for the management of non-malignant pain, and reveals regions with mean MED at levels associated with overdose risk. The diversity of approach is highlighted not only by the broad range of any use and chronic use observed but also the lack of substantial overlap between regions at the high end of daily dose among chronic users and those with higher rates of chronic use overall (Figures 1 & 2). That chronic users received prescriptions from a mean of 3 unique prescribers (and as many as 4 in some HRRs) suggests a fragmented approach that may lack the level of coordination and oversight commonly understood as important for safe management of persistent, high dose opioid use.4,26,36 The very high intensity of opioid use by some also suggests many prescribers are not heeding warnings on the risk associated with chronic high dose opioid use, warnings published in prominent journals and by the CDC since the early 2000's.1,4,5,29,36-39 The decrease in mean daily dose observed between 2010 and 2011 that followed as a steady climb of this measure, may reflect efforts to curb the riskiest use of prescription opioids by the Drug Enforcement Agency and by states as well expectations around formal risk mitigation programs sponsored by drug manufacturers that were discussed (but not fully implemented) during this study period.13,40 Our findings of regional variability and growing chronic use highlight the importance of further developing policies at all levels that balance risks with the need for pain control for the population served by SSDI and Medicare.
Our observations regarding the high-profile and potent opioid, oxycodone, exemplify the discordant approaches to opioid analgesic management experienced by these beneficiaries.17 Use of this analgesic varied from 1.7% to 60.5% of pills dispensed across HRRs. Oxycodone was prominent on the eastern seaboard while Texas prescribers all but eschewed these products. Texas laws create hurdles to prescribing controlled substances (tamper-resistant prescriptions and record keeping) but the state is not unique in such regulation, and many eastern coastal states have enacted such requirements.41 Additionally, many states contain HRRs with both high and low opioid use (with varied oxycodone use specifically). (Figures 1 and 2 and Appendix Table 4) Differences in state laws are thus unlikely to fully explain the broad range in opioid use we observed. Other factors that deserve research include patient and prescriber level factors influencing opioid use intensity and product choice.
Limitations
Our claims-based analysis has important limitations. For our annual enrollment cohorts, we do not know the medical condition or combination of conditions that resulted in qualification for disability insurance. Because disability assessments precede Medicare enrollment by two years, Medicare claims are not reliable for determining cause of disability. However, current diagnoses do reflect the conditions experienced by beneficiaries at the time of opioid use once enrolled in Medicare. The lack of qualifying disability diagnosis limits our ability to control for disease state differences beyond common morbidities and the presence of musculoskeletal disease measured broadly such that residual confounding could explain some observed variation in opiate use. And, while all providers and states are subject to the same federal criteria for disability qualification, interpretation of and adherence to these criteria may not be uniform over space or time.3 Thus, the disabled population we study may be less homogeneous in terms of function and illness than the unifying benefits program suggests.
Additionally, our measure of unique prescribers relies on the number of unique encrypted prescriber identifiers appearing on each patient's claims in the Part D data; the validity of this approach has not been proven and cannot yet be tested as actual identity remains suppressed in this dataset. Invalid prescriber identifiers were recognized as a problem in the first few years of the Part D program.42 It is not clear if this problem persists or how this would affect our measure of unique prescribers.
Conclusion
Opioid use is common in this national population of disabled, under-age-65 Medicare beneficiaries. Little controversy surrounds intermittent, short-term use of opioid analgesics for acute, severe pain. The overall rise in prescription opioid consumption however appears driven not by an increase in the proportion of disabled beneficiaries using any opiates, but rather by the proportion using opioids chronically, at least 6 and generally 13 prescriptions per year. The effectiveness of such sustained, high dose is supported by scant evidence for effectiveness and the subject of growing evidence of hazards.4,25,29,34 We are not suggesting all chronic opioid use is more harmful than beneficial, but instead, that the common and increasing chronic use we observed seems inconsistent with the uncertainties surrounding such prescribing practice. That regional factors strongly influence this clinically unsettled practice further highlights the need to examine both the determinants and outcomes of the opioid use we observed.
Our findings call attention to the complex and potentially unique health care needs of under-65-year-old disabled workers. They suffer a high burden of illness and injury, low incomes and now, a high burden of opioid use. Medicare administrators and clinicians must respond to the importance of high-quality pain management in this population. Approaches might include development and implementation of quality measures for chronic opioid management, measures of prescribing continuity (number of unique opioid prescribers per user), active monitoring through office visits, consultation with pain specialists for patients above a specified daily dose, surveillance for signs of addiction and ample coverage of addiction services.43 While such policies and programs might be complex and costly, evidence suggests inaction will also come at a substantial cost.
Supplementary Material
Acknowledgments
Funding and support: NIH/NIA P01 AG019783 Skinner, Bynum and Zhou
NIH/NIA U01 AG046830 Skinner, Bynum, Colla, Meara, Munson, and Zhou
NIH/NIA K23 AG035030 - 01A1 Morden
Robert Wood Johnson Foundation Dartmouth Atlas Project 059491 Morden
SYNERGY at Dartmouth and Dartmouth Hitchcock Medical Center Morden, Munson and Colla
Funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Contributor Information
Nancy E. Morden, Associate Professor of Community and Family Medicine and The Dartmouth Institute for Health Policy & Clinical Practice, 35 Centerra Parkway Room 3088, Lebanon NH 03766, Nancy.e.morden@dartmouth.edu, 603.653.0831.
Jeffrey C. Munson, 35 Centerra Parkway, Lebanon, NH 03766, Jeffrey.C.Munson@Hitchcock.org, 603-650-6685.
Carrie H. Colla, 35 Centerra Parkway, Lebanon, NH 03766, Carrie.H.Colla@Dartmouth.edu, 603- 650-3521.
Jonathan S. Skinner, 35 Centerra Parkway, Lebanon, NH 03766, Jonathan.S.Skinner@Dartmouth.edu, 603-646-2535.
Julie P.W. Bynum, 35 Centerra Parkway, Lebanon NH 03766, julie.bynum@dartmouth.edu, 603-653-0827.
Weiping Zhou, 35 Centerra Parkway, Lebanon NH 03766, Weiping.Zhou@dartmouth.edu, 603-653-0811.
Ellen R. Meara, 35 Centerra Parkway, Lebanon NH 03766, ellen.r.meara@dartmouth.edu, 603-653-0899.
References
- 1.Centers for Disease C, Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morbidity and mortality weekly report. 2011 Nov 4;60(43):1487–1492. [PubMed] [Google Scholar]
- 2.U.S. Social Security Administration. Annual Statistical Report on the Social Security Disability Insurance Program, 2012. [Accessed July 1, 2013];2011 http://www.socialsecurity.gov/policy/docs/statcomps/di_asr/2011/index.html.
- 3.Autor DH, Duggan MG. The growth in the Social Security Disability rolls: a fiscal crisis unfolding. The journal of economic perspectives : a journal of the American Economic Association. 2006 Summer;20(3):71–96. doi: 10.1257/jep.20.3.71. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne JC, Mao J. Opioid therapy for chronic pain. The New England journal of medicine. 2003 Nov 13;349(20):1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 5.Paulozzi LJ, Ryan GW. Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006 Dec;31(6):506–511. doi: 10.1016/j.amepre.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008 Dec 10;300(22):2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 7.Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid dose and risk of road trauma in Canada: a population-based study. JAMA internal medicine. 2013 Feb 11;173(3):196–201. doi: 10.1001/2013.jamainternmed.733. [DOI] [PubMed] [Google Scholar]
- 8.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013 Feb 20;309(7):657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 9.Substance Abuse and Mental Health Services Administration. Substance Abuse and Mental Health Services Administration, , Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2011. [Google Scholar]
- 10.Centers for Disease Control and Prevention. Vital Signs: Overdoses of Prescription Opioid Pain Relievers and Other Drugs Among Women — United States, 1999-2010. [Accessed July 29, 2013];2013 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6226a3.htm?s_cid=mm6226a3_w. [PMC free article] [PubMed]
- 11.Meier B, Marsh B. The Soaring Cost of the Opioid Economy. The New York Times. 2013 Jun 22; 2013. [Google Scholar]
- 12.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiology and drug safety. 2009 Dec;18(12):1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okie S. A flood of opioids, a rising tide of deaths. The New England journal of medicine. 2010 Nov 18;363(21):1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 14.Research Data Assistance Center. Find a CMS Data File. [Accessed May 12, 2014];2014 http://www.resdac.org/cms-data/search?f%5B0%5D=im_field_privacy_level%3A42.
- 15.Washington State Agency Medical Directors' Group. Opiod Dose Calculator. [Accessed July 4, 2013];2010 http://agencymeddirectors.wa.gov/mobile.html.
- 16.Medcalc.com. Narcotic Equivalence Converter. [Accessed July 4, 2013];2001 http://www.medcalc.com/narcotics.html.
- 17.Centers for Disease C, Prevention. Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004-2008. MMWR Morbidity and mortality weekly report. 2010 Jun 18;59(23):705–709. [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Panis Constantijn, Euller Roald, Grant Cynthia, et al. SSA Program Data User's Manual. 2000 Jun; 2000. [Google Scholar]
- 21.Centers for Medicare & Medicaid Services. 2011 RxHCC Model Software. [Accessed November 4, 2012];2012 http://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors-Items/Risk2006-2011.html?DLPage=1&DLSort=0&DLSortDir=descending.
- 22.Robst J, Levy JM, Ingber MJ. Diagnosis-based risk adjustment for medicare prescription drug plan payments. Health Care Financ Rev. 2007 Summer;28(4):15–30. [PMC free article] [PubMed] [Google Scholar]
- 23.Wennberg JE, Skinner J, Goodman DC, Fisher E. Tracking the Care of Patients with Chronic Illness: The Dartmouth Atlas of Health Care 2008. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice; 2008. [PubMed] [Google Scholar]
- 24.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011 Apr 6;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 25.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Annals of internal medicine. 2010 Jan 19;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franklin GM, Mai J, Wickizer T, Turner JA, Fulton-Kehoe D, Grant L. Opioid dosing trends and mortality in Washington State workers' compensation, 1996-2002. American journal of industrial medicine. 2005 Aug;48(2):91–99. doi: 10.1002/ajim.20191. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Orthopaedic Surgeons. Rosemont, IL: 2011. [Google Scholar]
- 28.Dembe A, Wickizer T, Sieck C, Partridge J, Balchick R. Opioid use and dosing in the workers' compensation setting. A comparative review and new data from Ohio. American journal of industrial medicine. 2012 Apr;55(4):313–324. doi: 10.1002/ajim.21021. [DOI] [PubMed] [Google Scholar]
- 29.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008 Jul-Aug;24(6):469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 30.Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009 Feb;10(2):147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937. [DOI] [PubMed] [Google Scholar]
- 32.Nekovarova T, Yamamotova A, Vales K, Stuchlik A, Fricova J, Rokyta R. Common mechanisms of pain and depression: are antidepressants also analgesics? Frontiers in behavioral neuroscience. 2014;8:99. doi: 10.3389/fnbeh.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Archives of internal medicine. 2006 Oct 23;166(19):2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 34.Scherrer JF, Svrakic DM, Freedland KE, et al. Prescription opioid analgesics increase the risk of depression. Journal of general internal medicine. 2014 Mar;29(3):491–499. doi: 10.1007/s11606-013-2648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLellan AT, Turner BJ. Chronic noncancer pain management and opioid overdose: time to change prescribing practices. Annals of internal medicine. 2010 Jan 19;152(2):123–124. doi: 10.7326/0003-4819-152-2-201001190-00012. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease C, Prevention. CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR Morbidity and mortality weekly report. 2012 Jan 13;61(1):10–13. [PubMed] [Google Scholar]
- 37.Paulozzi LJ. Opioid analgesic involvement in drug abuse deaths in American metropolitan areas. Am J Public Health. 2006 Oct;96(10):1755–1757. doi: 10.2105/AJPH.2005.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulozzi LJ, Annest JL. US data show sharply rising drug-induced death rates. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention. 2007 Apr;13(2):130–132. doi: 10.1136/ip.2006.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulozzi LJ, Xi Y. Recent changes in drug poisoning mortality in the United States by urban-rural status and by drug type. Pharmacoepidemiology and drug safety. 2008 Oct;17(10):997–1005. doi: 10.1002/pds.1626. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategy (REMS) for Extended-Release and Long-Acting Opioids. 2012 http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm163647.htm.
- 41.Centers for Disease Control and Prevention. Prescription Drug Overdose: State Laws, Selected State Legislative Strategies Governing Prescription Drug Abuse and Diversion in the US. [Accessed July 4, 2013];2012 http://www.cdc.gov/HomeandRecreationalSafety/Poisoning/laws/index.html.
- 42.Office of the Inspector General. OIG Report: Invalid Prescriber Identifiers on Medicare Part D Drug Claims CMS Requirements. 2011 http://www.ncpanet.org/pdf/leg/aug11/medicare/oig_npi_report.pdf, 2014.
- 43.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-Assisted Therapies - Tackling the Opioid-Overdose Epidemic. The New England journal of medicine. 2014 Apr 23; doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.