Abstract
Rhizomelic chondrodysplasia punctata (RCDP) is a genetically heterogeneous autosomal recessive syndrome characterized by congenital cataracts, shortening of the proximal limbs, neurological abnormalities, seizures, growth delays, and severe intellectual disability. Most RCDP children die in the first decade of life due to respiratory complications. Mutations in alkylglycerone phosphate synthase (AGPS) cause RCDP type 3 (RCDP3). We've previously established that cataracts and male infertility in blind sterile 2 (bs2) mice are caused by a spontaneous hypomorphic mutation in Agps. As a part of this study, we set out to further explore the bs2 phenotypes and how they correlate to the clinical presentations of RCDP3 patients. Our results show that ~ 50% bs2 mice die embryonically and surviving bs2 mice exhibit growth delays that they overcome by adulthood. The X-ray analysis of adult bs2 mice revealed significant humeral, but not femoral shortening. Clinical and histological eye evaluations revealed that bs2 lenses undergo normal development with first opacities developing at P21 that by P28 rapidly progress to mature cataracts. Evaluation of testes determined that infertility in bs2 mice is due to the aberrant formation of multicellular cellular clusters that undergo apoptosis. Given that the bs2 locus is a hypomorphic Agps mutation, we set out to generate Agps knockout mice utilizing the Knockout Mouse Project (KOMP) resource. Our results showed that ~ 85% of Agps knock-out mice die embryonically whereas surviving adult Agps knock-out mice phenotypically exhibit cataracts and testicular abnormalities similar to those observed in bs2 mice. Given that the majority of Agps knock-out mice die embryonically, this presented a challenge for further analyses of Agps deficiency in mouse models. Although not done as a part of this study, Agps-KOMP mice or ES cells can be further modified with FLP recombinase to generate mice suitable for subsequent matings with a transgenic Cre strain of choice, thereby providing an opportunity to study conditional Agps deficiency in a specific tissue or desired developmental time points without Agps deficiency-mediated embryonic lethality.
Abbreviations: RCDP, rhizomelic chondrodysplasia punctata; GNPAT, glyceronephosphate O-acyltransferase; AGPS, alkylglycerone phosphate synthase; PTS, peroxisomal targeting signal; KOMP, Knockout Mouse Project; PEX7, peroxisomal biogenesis factor 7
Keywords: Mutation, AGPS, RCDP3, Mouse, Phenotype, Hypomorphic
1. Introduction
Rhizomelic chondrodysplasia punctata (RCDP) is a malformation syndrome characterized by bilateral congenital cataracts, severe shortening of the humerus and femur, stippled epiphyses, vertebral coronal clefts, microcephaly, myelination defects, growth and developmental delays, and severe intellectual disability [1], [2]. Only about 50% of RCDP children survive up to 6 years of age and less than 10% reach teenage years [3]. Genetic studies have determined that RCDP is a heterogeneous disorder with three distinct forms: RCDP type 1 (RCDP1) [OMIM 215100], RCDP type 2 (RCDP2) [OMIM 222765], and RCDP type 3 (RCDP3) [OMIM 600121] [1], [4], [5]. RCDP1 is caused by mutations in PEX7[6], [7], [8], RCDP2 is caused by mutations in glyceronephosphate O-acyltransferase (GNPAT) [9], [10], and RCDP3 is caused by mutations in alkylglycerone phosphate synthase (AGPS) [11], [12]. Although genetically distinct, biochemical studies have determined that the depletion of plasmalogens causes all three forms of RCDP [13] and as such all three RCDP forms are clinically indistinguishable.
Plasmalogens are a class of ether lipids where acylation of dihydroxyacetone phosphate at the sn-1 position is mediated by the GNPAT enzyme followed by the exchange of the acyl group for an alkyl group by the AGPS enzyme [13], [14]. It has been shown previously that catalytically active AGPS promotes the catalytic activity of GNPAT [15]. Therefore, functional loss of either GNPAT or AGPS disrupts plasmalogen synthesis and results in plasmalogen deficiency in RCDP2 and RCDP3. RCDP1, however, is caused by the functional loss of PEX7, a protein which is the peroxisomal targeting signal 2 (PTS2) receptor mediating import of proteins containing the PTS2 consensus sequence into peroxisomes [16]. Although the majority of peroxisomal proteins contain the PTS1 consensus sequence and are imported into peroxisomes via the PTS1/PEX5 receptor mechanism, AGPS, phytanoyl-CoA hydroxylase, and 3-ketoacyl thiolase contain the PTS2 signal and are imported into the peroxisomes via PTS2/PEX7 receptor mechanism [5]. While PTS2 disruption affects all three proteins, it has been established that plasmalogen deficiency in RCDP1 patients is caused by the failure of AGPS peroxisomal import, and the consequent AGPS functional loss during synthesis of plasmalogens [6], [17].
How plasmalogen deficiency results in RCDP clinical phenotypes is largely unknown. RCDP mouse models provide an excellent resource for addressing this question. Pex7−/− and Gnpat−/− mice exhibit plasmalogen deficiency as well as skeletal, testicular, brain, and eye abnormalities, recapitulating some phenotypes observed in RCDP patients [18], [19]. Recently, our lab showed that blind sterile 2 (bs2) mice exhibiting cataracts and male infertility [20] are caused by a spontaneous mutation in Agps[21]. Our analysis identified a G to A substitution at the + 5 position of Agps intron 14 that alters splicing resulting in an Agps∆exon14 transcript lacking exon 14, an additional aberrant Agpsexon∆13–14 transcript lacking both exons 13 and 14, and residual levels of the full-length Agps transcript [21]. Both aberrant Agps∆exon14 and Agpsexon∆13–14 transcripts encode putative truncated catalytically inactive AGPS proteins whereas residual levels of the full-length Agps encode putative full-length catalytically active AGPS protein, but at severely reduced levels of about 15% of that observed in WT mice. Mass spectrometry analysis of lipid species from bs2 confirmed severely reduced levels of plasmalogens; therefore, the bs2 mouse was established as a hypomorphic Agps mutation [21].
As a part of this study, we focused on further evaluation of the bs2 mouse phenotypes. Our results showed that about half of the bs2 mice die embryonically and the surviving bs2 mice exhibit delayed growth, shortening of the humerus, cataracts, and male infertility associated with seminiferous tubule abnormalities. We also set out to create Agps knock-out mice utilizing resources from the Knockout Mouse Project (KOMP) [22]. We show that ~ 85% of Agps knock-out mice die embryonically which has hindered detailed studies of phenotypes associated with Agps deficiency. However, we recovered few adult Agps knock-out mice and our analysis showed that phenotypically these mice exhibit growth delays, cataracts, and testicular abnormalities similar to those identified in the bs2 mice.
2. Materials and methods
2.1. Mice and genotyping
bs2, B6.FVB-Tg(EIIa-Cre)C5379Lmgd/J (referred to in the text as ElIa-Cre) and C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Genotyping of the bs2, rd8, and EIIa-Cre alleles was done as described previously [21], [23], [24] using primers summarized in Table 1S. The bs2 mice were maintained on C57BL/6J × CastEi/J mixed F2 background as previously described by brother to sister breedings [21]. Mice heterozygous for the Agpstm1a(KOMP)Wtsi allele (referred to in the text as Agps-KOMP) were obtained from the Knockout Mouse Project (KOMP) [22] repository at the University of California, Davis. The Agps-KOMP allele was genotyped utilizing primers summarized in Table 1S. All primers were synthesized by Integrated DNA Technologies (Iowa City, IA), and used with Platinum Taq polymerase (Invitrogen).
Table 1.
A list of primers used in the study.
| Primer | Sequence | Purpose |
|---|---|---|
| mAgps cDNA 38F | CCGCTCTGAGTGACCTTTCAC | Agps RT-PCR |
| mAgps cDNA 681R | ATCAGGAATCCGCTCAAACATCC | Agps RT-PCR |
| mAgps cDNA 390F | GGTTGAGCTGACTGGGAAAA | Agps RT-PCR, Agps semiquantitative RT-PCR |
| mAgps cDNA 1024R | TCATGCCTGACGCTCGAGTA | Agps RT-PCR, Agps semiquantitative RT-PCR |
| mAgps cDNA 884F | CAGCTCACGTAGAGGCTGGGATA | Agps RT-PCR |
| mAgps cDNA 1579R | GCAAATAACCCCTCTGACCGTTA | Agps RT-PCR |
| mAgps cDNA 1477F | GACCGTGAGAAGGTTCTTCAGCA | Agps RT-PCR |
| mAgps cDNA 2176R | GCACAGAACAAAAACCAACTCATTT | Agps RT-PCR |
| mAgps cDNA 1204F | AGACCAACCCCTGAGTACCAGAA | Agps RT-PCR |
| mAgps cDNA 1768R | TCTGTGTCACCCTGCACGTAGAA | Agps RT-PCR |
| Gapdh cDNA F | CTTTGGCATTGTGGAAGGG | Gapdh RT-PCR |
| Gapdh cDNA R | CCTCTCTTGCTGCAGTGTC | Gapdh RT-PCR |
| mAgps Exon 14F | CTTTCTGCAGGCCAGGCAGTG | bs2 allele |
| mAgps Exon 14R | CAAATTTTGGAGTAAGAAGAGTTTTT | bs2 allele |
| Cre-F | GCGGTCTGGCAGTAAAAACTATC | Cre allele |
| Cre-R | GTGAAACAGCATTGCTGTCACTT | Cre allele |
| rd8-F | GGTGACCAATCTGTTGACAATCC | rd8 allele |
| rd8-R | GCCCCATTTGCACACTGATGAC | rd8 allele |
| Agps KOMP F | GCAGTGGCTCTCAACCTTCCTAAA | Agps KOMP allele |
| Agps KOMP R1 | TGGGAAAGGGTTCGAAGTTCCTA | Agps KOMP allele |
| Agps KOMP R2 | CCAGAGTTCCCAGATCCTACTTCCA | Agps KOMP allele |
2.2. Clinical examination
Weights of WT (n = 8) and bs2 (n = 8) postnatal mice were measured and recorded in littermates from bs2/+ × bs2/+ crosses between P0.5 and 4 months of age. Age-matched bs2 (n = 4), AGPS-KOMP mice (n = 4), Agps-KOMP EIIa-Cre (n = 2) and control (n = 4) mice were X-ray imaged at 4 months of age. Exposures were recorded at a peak kilovoltage of 50 kVp and a charge of 0.50 mAs (milliampere seconds). The same mice were also evaluated with a Topcon SL-D8Z slit lamp biomicroscope with a Nikon SLR-based Photo Slit Lamp imaging system following mydriasis with 1% Atropine Sulfate (Bausch & Lomb). WT (n = 6) and bs2 (n = 6) testes weights were measured in age-matched pairs between 4 and 8 weeks of age. Significance for all measurements was calculated via two-tailed t-test (GraphPad), where P < 0.05 was considered significant.
2.3. Histology and immunohistochemistry
All tissues were collected, paraffin embedded, and H&E stained as previously described [25]. For evaluations of cataracts, eyes from age-matched bs2 and control mice were collected at P0.5 (n = 2), P5 (n = 2), P14 (n = 2), P21 (n = 2), P28 (n = 2) and 4 months of age (n = 2) as well AGPS-KOMP mice (n = 2), Agps-KOMP EIIa-Cre (n = 2) and control (n = 2) mice were collected at 4 months of age. For immunohistochemistry, we used antibodies: E-cadherin (Cell Signaling), MIP (Milipore), DAZL (Abcam), and TRA54 (B-Bridge) as primary antibodies and DyLight 488 goat anti-rat or goat anti-rabbit (Abcam) as secondary antibodies following manufacturers' recommendations. Peanut agglutinin lectin (PNA) staining was done with the Lectin PNA-Alexa-488 conjugate (Life Technologies) according to the manufacturers' recommendations. For proliferation studies, EdU was injected intraperitoneally at a concentration of 100 mg/kg 3 h prior to euthanizing the mice; EdU detection was performed with the Click iT EdU Alexa Fluor 488 Imaging Kit and counterstained with DAPI (Life Technologies) according to the manufacturer's recommendations. TUNEL staining was performed utilizing the Chemicon ApopTag Plus In Situ Apoptosis Fluorescein Detection Kit (Millipore) according to the manufacturer's recommendations. All slides were mounted with Vectashield and imaged with a Nikon DS-Fi1 camera on a Nikon Eclipse 80i microscope with NIS-Elements software (Nikon). All cell-counting measurements were performed on sections from a minimum of three separate genotypes with at least ten sections per genotype. Significance was calculated via two tailed t-test (GraphPad), where P < 0.05 was considered significant.
2.4. RT-PCR
Lenses from eyes of WT P5, P14, P21, and P28 mice were collected for RNA analysis. Spleen, kidney, liver, and testes were collected from Agps-KOMP, Agps-KOMP EIIa-Cre, and control mice at 4 months of age. RNA was isolated and reverse transcribed as previously described [21]. Subsequent PCR products were generated using primers in Table 1S, electrophoresed, gel-purified, and sequenced as previously described [25]. Comparative sequence analysis was performed using DNAStar software. For semi-quantitative analysis of Agps transcript levels, RT-PCR products were generated while in the exponential phase of PCR amplification using Gapdh as an internal control as previously described [21]. PCR band intensities were quantified using ImageJ software (http://rsbweb.nih.gov/ij/) and are expressed relative to Gapdh. The results represent at least three independent experiments performed in triplicates. Comparison between genotype groups was analyzed by two-tailed t-test and the data is expressed as mean ± SEM. A value of P < 0.05 was designated as being statistically significant.
3. Results
3.1. Growth delays
At birth, bs2 pups appeared indistinguishable from WT littermates, but as bs2 mice progressed through postnatal development, they appeared smaller in size than WT littermates. To further investigate this phenotype, we measured postnatal weights of bs2 and WT littermates. At P0.5, the weights of bs2 pups did not significantly differ from WT (not shown). Between P5 and six weeks of age bs2 mice weighed significantly less than WT littermates; however, by 4 months of age weights of bs2 mice no longer significantly differed from WT littermates (Fig. 1).
Fig. 1.

Growth delays in bs2 mice. Evaluation of the weights of bs2 (white bars; n = 8) and WT (black bars; n = 8) throughout early postnatal development revealed that bs2 mice weigh significantly less than WT, but bs2 catch up to WT littermates by four months of age. Statistical significance was determined by two-tailed t-test and error bars represent ± SEM. Double asterisk indicates statistical significance with P < 0.001, a single asterisk indicates statistical significance with P < 0.05 and N.S. indicates no statistical significance.
3.2. Shortening of the proximal limb phenotypes
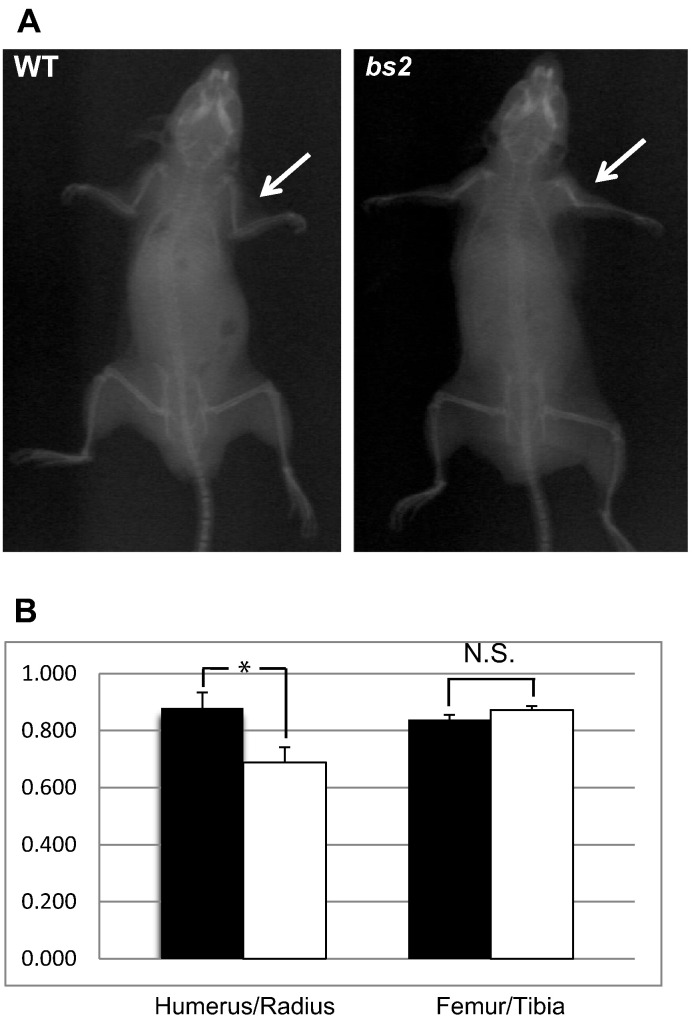
A profound feature of RCDP patients is the shortening of the proximal limbs [3]. Therefore, we proceeded to evaluate if adult bs2 mice exhibit shortening of the humerus and femur. X-ray images of 4-month-old WT and bs2 mice identified shorter humeri, although no shortening of the femur was observed (Fig. 2A). Measurements of the humerus:radius length ratios confirmed a significantly shorter humerus in bs2 mice whereas no differences were observed in the femurs when comparing femur:tibia ratios of bs2 to WT (Fig. 2B).
Fig. 2.
Humeral shortening in bs2 mice. X-ray imaging (A) of WT and bs2 mice at 4 months of age reveals shortening of the humerus, indicated by white arrows. As quantified in (B), there is a significant shortening of the humerus/radius ratio in bs2 (white bar) when compared to WT (black bar) mice (n = 4), while there is no difference in the femur/tibia ratios. Statistical significance was determined by Student's t test and error bars represent ± SEM. Asterisk indicates statistical significance with P < 0.05 and N.S. indicates no statistical significance.
3.3. Cataracts
Our previous study identified severe cataracts in bs2 mice [21]. Congenital cataracts are a clinical feature in RCDP3 children [15]. Therefore, we focused on determining the onset and progression of the bs2 cataracts. A slit-lamp evaluation of bs2 lenses starting at P14 did not identify any opacities prior to P21 (not shown); however at P21 bs2 lenses exhibited mild nuclear opacities that rapidly progressed to severe total opacities by P28 (Fig. 3A). At later time points (12–24 months), the bs2 cataracts did not differ from those observed at P28 (not shown). Histological evaluation of bs2 eyes supported the clinical findings; postnatal eyes (P0.5, P5, P14) did not identify any bs2 morphological abnormalities (not shown). At P21 the initial abnormality noted was the swelling of the cells in the bow region and by P28, bs2 lens fiber cells were disorganized, swollen, and detached from their apical–apical connections with lens epithelial cells (Fig. 3B). Immunostaining for E-cadherin (epithelial cells), and MIP (lens fiber cells) in WT and bs2 lenses prior to the onset of cataracts did not identify any difference between WT and bs2 mice at P14 (not shown), indicating that lens epithelial cells were undergoing normal development and differentiation. To determine if the cataract onset in bs2 mice was associated with the temporal expression of Agps in the lens, we performed RT-PCR from RNA collected from postnatal WT lenses. Our results identified that Agps is expressed throughout postnatal development (Fig. 3C). These findings indicated that in mice Agps is not required for lens development, but is required for lens homeostasis.
Fig. 3.
Cataracts in bs2 mice. (A) Clinical image of mild nuclear cataracts in bs2 evident at P21 (top right panel) that rapidly progress to mature cataracts by P28 (bottom right panel); no lens abnormalities were noted in WT littermates at P21 (top left panel) or P28 (bottom left panel). (B) Histological analysis prior to P21 did not identify any abnormalities in bs2 lenses. The initial bs2 lens abnormalities were observed at P21 characterized by swelling of cells in the transitional zone (black arrow). By P28, bs2 lenses exhibited detachment of the apical–apical connections between epithelial and fiber cells, as well as swelling and degeneration of fiber cells. Scale bar = 25 μm. (C) RT-PCR analysis using total RNA from postnatal (P) lenses of Agps (top panel) and Gapdh (bottom panel) revealed expression of Agps at all postnatal lenses evaluated.
3.4. Testicular abnormalities
Our previously published data identified that bs2 males exhibit smaller testes characterized by the absence of elongated spermatids and spermatozoa [21]. Thus, we proceeded to further characterize bs2 male testes. The weights of bs2 adult testes were significantly smaller when compared to the WT controls (Fig. 4A). In addition, the bs2 seminiferous tubules contained significantly fewer cells (Fig. 4B). Large multicellular clusters were present in bs2 (Fig. 4D) that were not present in WT tubules (Fig. 4C). To evaluate this phenotype further, we immunostained WT and bs2 tubules for deleted in azoospermia-like (DAZL), a marker for spermatogonia and Sertoli cells [26]. Our analysis did not identify any significant difference in the number of DAZL-positive cells between the two genotypes (Fig. 4G) although in bs2 tubules DAZL-positive cells appeared disorganized (Fig. 4F) in contrast to highly organized DAZL-positive cells in WT tubules (Fig. 4E). Next, we focused on the evaluation of the number of proliferating spermatogonia in bs2 and WT testes. The percentage of EdU positive spermatogonia in WT (Fig. 4H) and bs2 (Fig. 4I) mice did not significantly differ (Fig. 4J). Immunostaining of WT tubules with TRA54, which is a haploid sperm cell-specific antigen, revealed a punctate staining characteristic of spermatocytes [27] (not shown) and crescent-shaped staining pattern marking the round spermatids [27] (Fig. 4K). In the bs2 tubules TRA54 positive punctate and crescent-shaped staining were also present, but only within the multicellular clusters (Fig. 4L). Staining with peanut agglutinin (PNA) as the acrosomal marker [28] revealed the presence of spermatids and spermatozoa in WT (Fig. 4M). PNA positive cells were also present in bs2 tubules within the multiceIIular clusters (Fig. 4N). A significantly greater number of TUNEL positive cells were present in bs2 when compared to WT tubules (Fig. 4O), primarily present within the multicellular clusters (not shown).
Fig. 4.
Testicular abnormalities in bs2 mice. (A) Testes from bs2 mice (n = 6) weigh significantly less than testes from age-matched WT (n = 6) siblings between 4 and 8 weeks of age, P < 0.05. (B) The number of cells per seminiferous tubule in bs2 was significantly less than the number of cells observed in WT tubules (n = 15). Statistical significance was determined by two tailed t-test and error bars represent ± SEM. An asterisk indicates statistical significance with P < 0.05. Histological analysis of adult bs2 seminiferous tubules (D) identified significant germ cell depletion and the presence of large multicellular clusters (arrowhead) that were absent in WT tubules (C). Immunostaining for DAZL, a marker for spermatogonia and Sertoli cells, revealed disorganized DAZL-positive cells in bs2 tubules (F) in contrast to highly organized DAZL-positive cells in WT tubules (E) even though the number of DAZL-positive cells did not significantly differ between the two genotypes (n = 15) shown in (G). The number of EdU-positive cells in WT (H) and bs2 (I) tubules did not differ between the two genotypes (n = 15) shown in (J). In bs2 tubules, TRA54 positive cells were identified within the multicellular clusters (L), whereas in WT tubules characteristic TRA54 crescent shaped staining of spermatids was evident (K). In bs2 tubules, PNA-positive cells were identified in the multicellular clusters (N), whereas in WT tubules PNA stained spermatids and spermatozoa (M). A significantly greater number of TUNEL-positive cells was evident in bs2 tubules compared to WT tubules (n = 15) (O). Scale bar = 25 μm.
3.5. Generating Agps knockout mice
Given that bs2 mice are hypomorphic for AGPS function, we set out to generate Agps-knockout mice and determine phenotypical consequences of complete Agps deficiency. Two Agps-KOMP/+heterozygote mice were recovered and were phenotypically indistinguishable from WT mice, which is consistent with an autosomal recessive inheritance. Agps-KOMP/+mice were generated on the C57BL/6N background [22]; however, it was recently determined that C57BL/6N mice carry a mutation in the Crb1 gene termed rd8 causing retinal degeneration [29]. Genotyping of Agps-KOMP/+mice confirmed the presence of the rd8 allele. It was previously shown that bs2 mice and RCDP3 patients exhibit ocular defects [15]{Liegel in 2011 #4}, we wanted to eliminate any contribution of the rd8 allele to the ocular phenotypes associated with the Agps deficiency. Thus, we set up selective breedings with C57BL/6J mice and subsequently eliminated the rd8 allele from the Agps-KOMP genetic background.
Het to het breeding of Agps-KOMP/+mice recovered 8% (4/51) of homozygote Agps-KOMP mice, deviating from the expected Mendelian ratio of 25%. This finding suggested ~ 70% prenatal lethality for homozygote Agps-KOMP mice. In addition to the presence of the gene-trapped cassette designed to disrupt Agps splicing following exon 1, loxP sites flank both sides of Agps exon 2 [30]; therefore, crossing of Agps-KOMP mice to EIIa-Cre mice shown to efficiently remove transcripts from all tissues during early embryonic development [23], would assure that we generated the full global Agps knockouts. Breeding of homozygote Agps-KOMP females to Agps-KOMP/+EIIa-Cre males recovered 3.7% (2/53) of Agps-KOMP EIIa-Cre homozygote mice deviating from the 25% expected Mendelian ratio thereby suggesting ~ 85% prenatal lethality of Agps-KOMP EIIa-Cre mice.
3.6. Analysis of Agps-KOMP and Agps-KOMP EIIa-Cre mice
Only a few Agps-KOMP (n = 4) and Agps-KOMP EIIa-Cre mice (n = 2) were recovered at birth and these mice survived into adulthood. Semi-quantitative RT-PCR of RNA from Agps-KOMP and Agps-KOMP EIIa-Cre tissues identified residual levels of full-length Agps at about ~ 11% and < 5% respectively of the levels observed in WT (Fig. 5A). Throughout the postnatal development, Agps-KOMP mice did not appear to differ in size from the WT mice whereas Agps-KOMP EIIa-Cre mice appeared smaller in size at P21 and by six weeks Agps-KOMP EIIa-Cre mice appeared only slightly smaller when compared to the WT littermates (Fig. 5B). Slit-lamp evaluation of Agps-KOMP and Agps-KOMP EIIa-Cre eyes prior to P21 did not identify any lens opacities (not shown). At P21, both Agps-KOMP and Agps-KOMP EIIa-Cre mice exhibited mild bilateral nuclear cataracts (not shown) that rapidly progressed to severe cataracts observed at P28 (not shown) that did not progress further by 4 months of age (Fig. 5C). Histological evaluation of the 4-month old Agps-KOMP and Agps-KOMP EIIa-Cre lenses confirmed severely disrupted lens fiber cells (Fig. 5C). The X-ray analysis of Agps-KOMP and Agps-KOMP EIIa-Cre mice did not identify any obvious shortening of either humerus:radius length ratios or femur:tibia length ratios (not shown). Agps-KOMP and Agps-KOMP EIIa-Cre females were able to produce litters whereas Agps-KOMP and Agps-KOMP EIIa-Cre males were infertile. Agps-KOMP and Agps-KOMP EIIa-Cre testes sizes appeared smaller than WT (not shown) and immunohistochemical analysis determined Agps-KOMP and Agps-KOMP EIIa-Cre seminiferous tubules did not differ from WT in the number of DAZL positive cells and in the number of proliferating spermatogonia (not shown). Additionally, the lumen of Agps-KOMP and Agps-KOMP EIIa-Cre tubules contained multicellular clusters of TRA54 and PNA positive cells (Fig. 5D) that were phenotypically indistinguishable from those observed in bs2 testes (Fig. 4L and N).
Fig. 5.
Characterization of Agps-KOMP and Agps-KOMP Ella-Cre mice. Semi-quantitative RT-PCR (A) shows ratios of full-length Agps and Gapdh transcripts in WT, Agps-KOMP, and Agps-KOMP Ella-Cre testes (n = 3; error bars represent ± SEM). (B) At P21, Agps-KOMP mice did not appear to differ in size and Agps-KOMP Ella-Cre appeared smaller when compared to age-matched WT littermates; however by six weeks Agps-KOMP Ella-Cre only appeared slightly smaller than age-matched Agps-KOMP or WT. Both Agps-KOMP and Agps-KOMP Ella-Cre mice exhibited severe cataracts (C) identified following clinical evaluation (top panel) as well as histological evaluation (bottom panel). Both Agps-KOMP and Agps-KOMP Ella-Cre mice exhibited the presence of multicellular clusters (D) containing TRA54 and PNA positive cells. Scale bar = 25 μm.
3.7. Discussion
As a part of this study, we focused on further defining the mouse phenotypes associated with Agps deficiency. Our previous study determined that a hypomorphic Agps mutation expressing approximately 15% of the full-length Agps transcript levels causes cataracts and male infertility in bs2 mice [21]. As a part of this study, we generated Agps knock-out mice utilizing KOMP resources. The Agps knockout construct was designed as a “Knockout First Mutation (Reporter Tagged Insertion With Conditional Potential)” predicted to generate a null allele through splicing of exon 1 to a lacZ trapping element contained in the targeting cassette that additionally contains the mouse En2 splice acceptor and the SV40 polyadenylation sequences [30], [31]. This strategy was previously shown to be highly effective in creating null alleles in mice [32]. Our results showed that Agps-KOMP is an Agps hypomorphic mutation exhibiting ~ 11% of full length Agps transcript levels when compared to WT mice. Our findings indicate that Agps-KOMP construct is “leaky” allowing for some residual full-length Agps transcript expression. The “Knockout First Mutation” construct also contains loxP sites flanking Agps exon 2 [30], [31]. Therefore, we crossed Agps-KOMP to EIIa-Cre mice in order to remove Agps exon 2 and generate a knock-out. In EIIa-Cre mice, the Cre transgene is under the control of the adenovirus EIIa promoter that targets expression of CRE recombinase to the early mouse embryo prior to implantation in the uterine wall [23]. The presence of less than 5% of full-length Agps transcripts in Agps-KOMP EIIa-Cre mice is most likely associated with the previously reported mosaic expression of the EIIa-Cre transgene where efficiency of the EIIa-Cre allele was determined to be dependent on the target gene and on parental transmission of the transgene [33].
Our results showed that Agps-KOMP and Agps-KOMP EIIa-Cre mice exhibited ~ 70% and ~ 85% embryonic lethality respectively. We previously reported that bs2 mice exhibited ~ 50% prenatal lethality [21]. These findings indicate that levels of full-length Agps expression strongly correlate to the embryonic lethality in the Agps deficient mice. The embryonic lethality observed in all Agps-deficient mice presented an obstacle for detailed evaluation of Agps deficiency in a mouse model. However, Agps-KOMP mice or ES cells can be further modified with FLP recombinase to remove gene-trap cassette mediating “Knockout First Mutation” thus generating a WT allele with loxP sites on either side of Agps exon 2 [30]. This would allow for a subsequent cross with a transgenic Cre strain of choice, thereby providing an opportunity to study Agps deficiency in a specific tissue or desired developmental time points without Agps deficiency-mediated embryonic lethality.
The cause of embryonic lethality in Agps-deficient mice is unknown. Embryonic lethality was also reported for Gnpat−/− mice [19], although Pex7−/− only exhibited postnatal lethality [17], [34]. The surviving bs2 mice were smaller throughout development when compared to WT littermates, a phenotype that bs2 were able to overcome later on in the development. Although we recovered a very small number of Agps-KOMP and Agps-KOMP EIIa-Cre suitable for a statistical analysis, our observation suggested that adult Agps-KOMP mice did not differ in size from age-matched WT littermates whereas the surviving Agps-KOMP EIIa-Cre mice similar to bs2 mice exhibited growth delay that they overcame by adulthood. The cause of the growth delay in bs2 and Agps-KOMP EIIa-Cre mice also remains unknown. Most RCDP children die during the first decade of life due to the complications associated with pulmonary hypoplasia [3], [35]. It is possible that prenatal death of the Agps deficient mice, as well as growth delays observed in the bs2 and Agps-KOMP EIIa-Cre mice are associated with a defect and/or delay in the lung development. However, these studies would require further evaluation.
Proximal shortening of the humerus, and to a lesser extent of the femur, is one of the distinctive characteristics in all RCDP patients [3], [36]. Our results identified a shortening of the humerus in the bs2 mice. Although a very few Agps-KOMP and Agps-KOMP EIIa-Cre mice were evaluated for humeral/femoral phenotypes, our analysis did not identify any obvious rhizomelic bone shortening in these mice. This difference in the humeral phenotype between bs2, Agps-KOMP and Agps-KOMP EIIa-Cre mice may be associated with a different genetic background of bs2 and Agps-KOMP mice. This is further supported by the observation that shortening of the proximal limbs was reported in the Gnpat−/− mice [19], but not in Pex7−/− mice, although Pex7−/− mice exhibited a defect in ossification in the distal bone elements of the limbs, skull and vertebrae [18]. These findings suggest that in mice plasmalogen deficiency adversely affects skeletal development, where the severity of skeletal abnormalities may also be influenced by genetic modifiers resulting in a range of skeletal phenotypic manifestations.
In all Agps-deficient mice from this study, the lenses underwent normal development up to P21. The initial lens abnormality evident at P21 was characterized by the swelling of epithelial cells in the equatorial region ultimately leading to severe lens fiber cell degeneration. In children with RCDP, bilateral congenital cataracts present at birth or during early infancy [3], [37]. Gnpat−/− and Pex−/− 7 mice also exhibit cataracts [18], [19], [34] although the molecular etiology of cataract formation in these mice is unknown. Histological lens analyses from a single RCDP patient with unknown genetic etiology revealed swelling of the equatorial epithelial cells and severe abnormalities of the fiber cells [38], [39]. It was concluded that plasmalogen deficiency in RCDP patients leads to aberrant formation of secondary lens fiber cells and that lens fiber cell abnormalities are not due to a degeneration of already formed lens fibers [38], [39]. Our findings show that in mice plasmalogens are not required for lens development, but they play a critical role in lens homeostasis. This discrepancy between mouse and human cataracts phenotypes associated with plasmalogen deficiency may be because there are significant differences in the lipid composition between human and mouse lenses [40]. Human lenses contain especially high levels of plasmalogens [40], [41], therefore, a plasmalogen deficiency may more adversely affect the human lenses than mouse lenses thus resulting in developmental lens fiber cell abnormalities.
We reported previously that bs2 mice exhibit male infertility [21]. Consistent with these findings, Agps-KOMP and Agps-KOMP EIIa-Cre male mice are also infertile and exhibit testicular abnormalities phenotypically indistinguishable from bs2 mice. It was shown previously in Gnpat−/− mice that azoospermia is due to the arrest of spermatogenesis at the level of round spermatids [19]. Additionally, it was shown that plasmalogen deficiency in Gnpat−/− mice has adverse effects on the establishment and remodeling of the blood–testis barrier, thereby providing evidence that plasmalogens regulate blood–testis permeability and the endocytosis of junctional complexes [42]. Our results support the previously described role of plasmalogens in the development and/or remodeling of the blood–testis barrier [42]. Agps deficient mice don't exhibit abnormalities in germ cell proliferation and maintenance and our results also provide evidence that spermatogenesis in Agps deficient mice progresses beyond spermatids as indicated by the presence of TRA54 and PNA positive cells in Agps deficient tubules. However, they remain “trapped” in aberrantly formed luminal multicellular clusters and undergo apoptosis ultimately resulting in azoospermia and infertility.
3.8. Conclusions
Our results show that in mice proper AGPS function is important for embryonic survival, as well as for the normal development of the lens, testes, and humerus. The molecular mechanisms governing the sensitivity of these tissues to Agps deficiency remain unclear and require further investigation. Embryonic lethality of Agps-deficient mice presents an obstacle for generating a sufficient number of Agps-deficient mice suitable for further studies. However, Agps-KOMP mice or ES cells can be further modified with FLP recombinase to generate mice suitable for conditional Agps ablation with Cre strain of choice allowing generation of a sufficient number of conditionally Agps ablated mice suitable for detailed morphological and functional analyses.
Acknowledgments
This work was supported by the National Institutes of Health grants EY018872 (D.J.S.) and Research Training Program in Vision ScienceEY014537 (R.P.L.).
References
- 1.Wanders R.J., Waterham H.R. Peroxisomal disorders I: biochemistry and genetics of peroxisome biogenesis disorders. Clin. Genet. 2005;67:107–133. doi: 10.1111/j.1399-0004.2004.00329.x. [DOI] [PubMed] [Google Scholar]
- 2.Waterham H.R., Ebberink M.S. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim. Biophys. Acta. 1822;2012:1430–1441. doi: 10.1016/j.bbadis.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 3.White A.L., Modaff P., Holland-Morris F., Pauli R.M. Natural history of rhizomelic chondrodysplasia punctata. Am. J. Med. Genet. A. 2003;118A:332–342. doi: 10.1002/ajmg.a.20009. [DOI] [PubMed] [Google Scholar]
- 4.Gould S.J., Valle D. Peroxisome biogenesis disorders: genetics and cell biology. Trends Genet. 2000;16:340–345. doi: 10.1016/s0168-9525(00)02056-4. [DOI] [PubMed] [Google Scholar]
- 5.Wanders R.J., Waterham H.R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 6.Braverman N., Steel G., Obie C. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat. Genet. 1997;15:369–376. doi: 10.1038/ng0497-369. [DOI] [PubMed] [Google Scholar]
- 7.Motley A.M., Hettema E.H., Hogenhout E.M. Rhizomelic chondrodysplasia punctata is a peroxisomal protein targeting disease caused by a non-functional PTS2 receptor. Nat. Genet. 1997;15:377–380. doi: 10.1038/ng0497-377. [DOI] [PubMed] [Google Scholar]
- 8.Purdue P.E., Zhang J.W., Skoneczny M., Lazarow P.B. Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat. Genet. 1997;15:381–384. doi: 10.1038/ng0497-381. [DOI] [PubMed] [Google Scholar]
- 9.Thai T.P., Heid H., Rackwitz H.R., Hunziker A., Gorgas K., Just W.W. Ether lipid biosynthesis: isolation and molecular characterization of human dihydroxyacetonephosphate acyltransferase. FEBS Lett. 1997;420:205–211. doi: 10.1016/s0014-5793(97)01495-6. [DOI] [PubMed] [Google Scholar]
- 10.Ofman R., Hettema E.H., Hogenhout E.M., Caruso U., Muijsers A.O., Wanders R.J. Acyl-CoA:dihydroxyacetonephosphate acyltransferase: cloning of the human cDNA and resolution of the molecular basis in rhizomelic chondrodysplasia punctata type 2. Hum. Mol. Genet. 1998;7:847–853. doi: 10.1093/hmg/7.5.847. [DOI] [PubMed] [Google Scholar]
- 11.Wanders R.J., Dekker C., Hovarth V.A. Human alkyldihydroxyacetonephosphate synthase deficiency: a new peroxisomal disorder. J. Inherit. Metab. Dis. 1994;17:315–318. doi: 10.1007/BF00711817. [DOI] [PubMed] [Google Scholar]
- 12.de Vet E.C., Ijlst L., Oostheim W., Wanders R.J., van den Bosch H. Alkyl-dihydroxyacetonephosphate synthase. Fate in peroxisome biogenesis disorders and identification of the point mutation underlying a single enzyme deficiency. J. Biol. Chem. 1998;273:10296–10301. doi: 10.1074/jbc.273.17.10296. [DOI] [PubMed] [Google Scholar]
- 13.Braverman N.E., Moser A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta. 1822;2012:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Brites P., Waterham H.R., Wanders R.J. Functions and biosynthesis of plasmalogens in health and disease. Biochim. Biophys. Acta. 2004;1636:219–231. doi: 10.1016/j.bbalip.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Itzkovitz B., Jiralerspong S., Nimmo G. Functional characterization of novel mutations in GNPAT and AGPS, causing rhizomelic chondrodysplasia punctata (RCDP) types 2 and 3. Hum. Mutat. 2012;33:189–197. doi: 10.1002/humu.21623. [DOI] [PubMed] [Google Scholar]
- 16.Purdue P.E., Lazarow P.B. Peroxisomal biogenesis: multiple pathways of protein import. J. Biol. Chem. 1994;269:30065–30068. [PubMed] [Google Scholar]
- 17.Braverman N., Chen L., Lin P. Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum. Mutat. 2002;20:284–297. doi: 10.1002/humu.10124. [DOI] [PubMed] [Google Scholar]
- 18.Brites P., Motley A.M., Gressens P. Impaired neuronal migration and endochondral ossification in Pex7 knockout mice: a model for rhizomelic chondrodysplasia punctata. Hum. Mol. Genet. 2003;12:2255–2267. doi: 10.1093/hmg/ddg236. [DOI] [PubMed] [Google Scholar]
- 19.Rodemer C., Thai T.P., Brugger B. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 2003;12:1881–1895. doi: 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- 20.Chang B., Hawes N.L., Hurd R.E. Mouse models of ocular diseases. Vis. Neurosci. 2005;22:587–593. doi: 10.1017/S0952523805225075. [DOI] [PubMed] [Google Scholar]
- 21.Liegel R., Chang B., Dubielzig R., Sidjanin D.J. Blind sterile 2 (bs2), a hypomorphic mutation in Agps, results in cataracts and male sterility in mice. Mol. Genet. Metab. 2011;103:51–59. doi: 10.1016/j.ymgme.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knockout Mouse Project Repository.
- 23.Lakso M., Pichel J.G., Gorman J.R. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehalow A.K., Kameya S., Smith R.S. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum. Mol. Genet. 2003;12:2179–2189. doi: 10.1093/hmg/ddg232. [DOI] [PubMed] [Google Scholar]
- 25.Liegel R., Handley M., Ronchetti A. Loss-of-function mutations in TBC1D20 cause cataracts and male infertility in blind sterile mice and Warburg micro syndrome in humans. Am. J. Hum. Genet. 2013;93:1–14. doi: 10.1016/j.ajhg.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haston K.M., Tung J.Y., Reijo Pera R.A. Dazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitro. PLoS ONE. 2009;4:e5654. doi: 10.1371/journal.pone.0005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira L.A., Tanaka H., Nagata Y. Characterization and expression of a stage specific antigen by monoclonal antibody TRA 54 in testicular germ cells. Int. J. Androl. 1998;21:34–40. doi: 10.1046/j.1365-2605.1998.00086.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheng F.P., Fazeli A., Voorhout W.F., Marks A., Bevers M.M., Colenbrander B. Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J. Androl. 1996;17:674–682. [PubMed] [Google Scholar]
- 29.Mattapallil M.J., Wawrousek E.F., Chan C.C. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6 N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest. Ophthalmol. Vis. Sci. 2012;53:2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skarnes W.C., Rosen B., West A.P. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Testa G., Schaft J., van der Hoeven F. A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis. 2004;38:151–158. doi: 10.1002/gene.20012. [DOI] [PubMed] [Google Scholar]
- 32.Skarnes W.C., Auerbach B.A., Joyner A.L. A gene trap approach in mouse embryonic stem cells: the lacZ reported is activated by splicing, reflects endogenous gene expression, and is mutagenic in mice. Genes Dev. 1992;6:903–918. doi: 10.1101/gad.6.6.903. [DOI] [PubMed] [Google Scholar]
- 33.Holzenberger M., Lenzner C., Leneuve P. Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a target gene. Nucleic Acids Res. 2000;28:E92. doi: 10.1093/nar/28.21.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braverman N., Zhang R., Chen L. A Pex7 hypomorphic mouse model for plasmalogen deficiency affecting the lens and skeleton. Mol. Genet. Metab. 2010;99:408–416. doi: 10.1016/j.ymgme.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinberg S.J., Dodt G., Raymond G.V., Braverman N.E., Moser A.B., Moser H.W. Peroxisome biogenesis disorders. Biochim. Biophys. Acta. 2006;1763:1733–1748. doi: 10.1016/j.bbamcr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Poulos A., Sheffield L., Sharp P. Rhizomelic chondrodysplasia punctata: clinical, pathologic, and biochemical findings in two patients. J. Pediatr. 1988;113:685–690. doi: 10.1016/s0022-3476(88)80378-0. [DOI] [PubMed] [Google Scholar]
- 37.Happle R. Cataracts as a marker of genetic heterogeneity in chondrodysplasia punctata. Clin. Genet. 1981;19:64–66. doi: 10.1111/j.1399-0004.1981.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 38.Ryan H. Cataracts of dysplasia epiphysialis punctata. Br. J. Ophthalmol. 1970;54:197–199. doi: 10.1136/bjo.54.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugarman G.I. Chondrodysplasia punctata (rhizomelic type): case report and pathologic findings. Birth Defects Orig. Article Ser. 1974;10:334–340. [PubMed] [Google Scholar]
- 40.Deeley J.M., Mitchell T.W., Wei X. Human lens lipids differ markedly from those of commonly used experimental animals. Biochim. Biophys. Acta. 2008;1781:288–298. doi: 10.1016/j.bbalip.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Deeley J.M., Thomas M.C., Truscott R.J., Mitchell T.W., Blanksby S.J. Identification of abundant alkyl ether glycerophospholipids in the human lens by tandem mass spectrometry techniques. Anal. Chem. 2009;81:1920–1930. doi: 10.1021/ac802395d. [DOI] [PubMed] [Google Scholar]
- 42.Komljenovic D., Sandhoff R., Teigler A., Heid H., Just W.W., Gorgas K. Disruption of blood–testis barrier dynamics in ether-lipid-deficient mice. Cell Tissue Res. 2009;337:281–299. doi: 10.1007/s00441-009-0809-7. [DOI] [PubMed] [Google Scholar]