Abstract
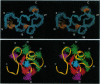
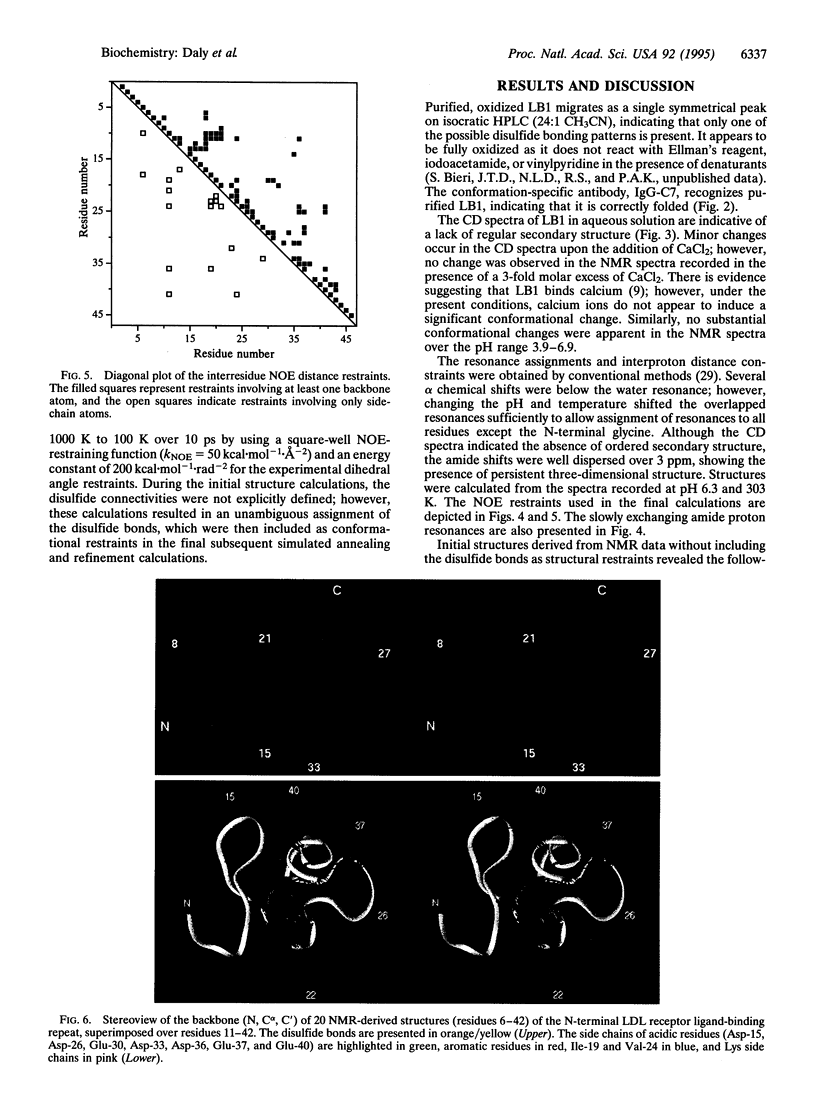
The low-density lipoprotein (LDL) receptor plays a central role in mammalian cholesterol metabolism, clearing lipoproteins which bear apolipoproteins E and B-100 from plasma. Mutations in this molecule are associated with familial hypercholesterolemia, a condition which leads to an elevated plasma cholesterol concentration and accelerated atherosclerosis. The N-terminal segment of the LDL receptor contains a heptad of cysteine-rich repeats that bind the lipoproteins. Similar repeats are present in related receptors, including the very low-density lipoprotein receptor and the LDL receptor-related protein/alpha 2-macroglobulin receptor, and in proteins which are functionally unrelated, such as the C9 component of complement. The first repeat of the human LDL receptor has been expressed in Escherichia coli as a glutathione S-transferase fusion protein, and the cleaved and purified receptor module has been shown to fold to a single, fully oxidized form that is recognized by the monoclonal antibody IgG-C7 in the presence of calcium ions. The three-dimensional structure of this module has been determined by two-dimensional NMR spectroscopy and shown to consist of a beta-hairpin structure, followed by a series of beta turns. Many of the side chains of the acidic residues, including the highly conserved Ser-Asp-Glu triad, are clustered on one face of the module. To our knowledge, this structure has not previously been described in any other protein and may represent a structural paradigm both for the other modules in the LDL receptor and for the homologous domains of several other proteins. Calcium ions had only minor effects on the CD spectrum and no effect on the 1H NMR spectrum of the repeat, suggesting that they induce no significant conformational change.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Daniel T. O., Schneider W. J., Goldstein J. L., Brown M. S. Visualization of lipoprotein receptors by ligand blotting. J Biol Chem. 1983 Apr 10;258(7):4606–4611. [PubMed] [Google Scholar]
- DiScipio R. G., Gehring M. R., Podack E. R., Kan C. C., Hugli T. E., Fey G. H. Nucleotide sequence of cDNA and derived amino acid sequence of human complement component C9. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7298–7302. doi: 10.1073/pnas.81.23.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles C., Güntert P., Billeter M., Wüthrich K. Efficient analysis of protein 2D NMR spectra using the software package EASY. J Biomol NMR. 1991 Jul;1(2):111–130. doi: 10.1007/BF01877224. [DOI] [PubMed] [Google Scholar]
- Esser V., Limbird L. E., Brown M. S., Goldstein J. L., Russell D. W. Mutational analysis of the ligand binding domain of the low density lipoprotein receptor. J Biol Chem. 1988 Sep 15;263(26):13282–13290. [PubMed] [Google Scholar]
- Esser V., Limbird L. E., Brown M. S., Goldstein J. L., Russell D. W. Mutational analysis of the ligand binding domain of the low density lipoprotein receptor. J Biol Chem. 1988 Sep 15;263(26):13282–13290. [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K. K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988 Dec 20;7(13):4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs H. H., Brown M. S., Goldstein J. L., Russell D. W. Deletion of exon encoding cysteine-rich repeat of low density lipoprotein receptor alters its binding specificity in a subject with familial hypercholesterolemia. J Biol Chem. 1986 Oct 5;261(28):13114–13120. [PubMed] [Google Scholar]
- Holm L., Sander C. Protein structure comparison by alignment of distance matrices. J Mol Biol. 1993 Sep 5;233(1):123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Rall S. C., Jr, Innerarity T. L., Jacobson S. F., Urdea M. S., Levy-Wilson B., Powell L. M., Pease R. J., Eddy R., Nakai H. Human apolipoprotein B: structure of carboxyl-terminal domains, sites of gene expression, and chromosomal localization. Science. 1985 Oct 4;230(4721):37–43. doi: 10.1126/science.2994225. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Mehta K. D., Chen W. J., Goldstein J. L., Brown M. S. The low density lipoprotein receptor in Xenopus laevis. I. Five domains that resemble the human receptor. J Biol Chem. 1991 Jun 5;266(16):10406–10414. [PubMed] [Google Scholar]
- Raychowdhury R., Niles J. L., McCluskey R. T., Smith J. A. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989 Jun 9;244(4909):1163–1165. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Sakai J., Hoshino A., Takahashi S., Miura Y., Ishii H., Suzuki H., Kawarabayasi Y., Yamamoto T. Structure, chromosome location, and expression of the human very low density lipoprotein receptor gene. J Biol Chem. 1994 Jan 21;269(3):2173–2182. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Beta-turns and their distortions: a proposed new nomenclature. Protein Eng. 1990 May;3(6):479–493. doi: 10.1093/protein/3.6.479. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Billeter M., Braun W. Pseudo-structures for the 20 common amino acids for use in studies of protein conformations by measurements of intramolecular proton-proton distance constraints with nuclear magnetic resonance. J Mol Biol. 1983 Oct 5;169(4):949–961. doi: 10.1016/s0022-2836(83)80144-2. [DOI] [PubMed] [Google Scholar]
- van Driel I. R., Goldstein J. L., Südhof T. C., Brown M. S. First cysteine-rich repeat in ligand-binding domain of low density lipoprotein receptor binds Ca2+ and monoclonal antibodies, but not lipoproteins. J Biol Chem. 1987 Dec 25;262(36):17443–17449. [PubMed] [Google Scholar]