Abstract
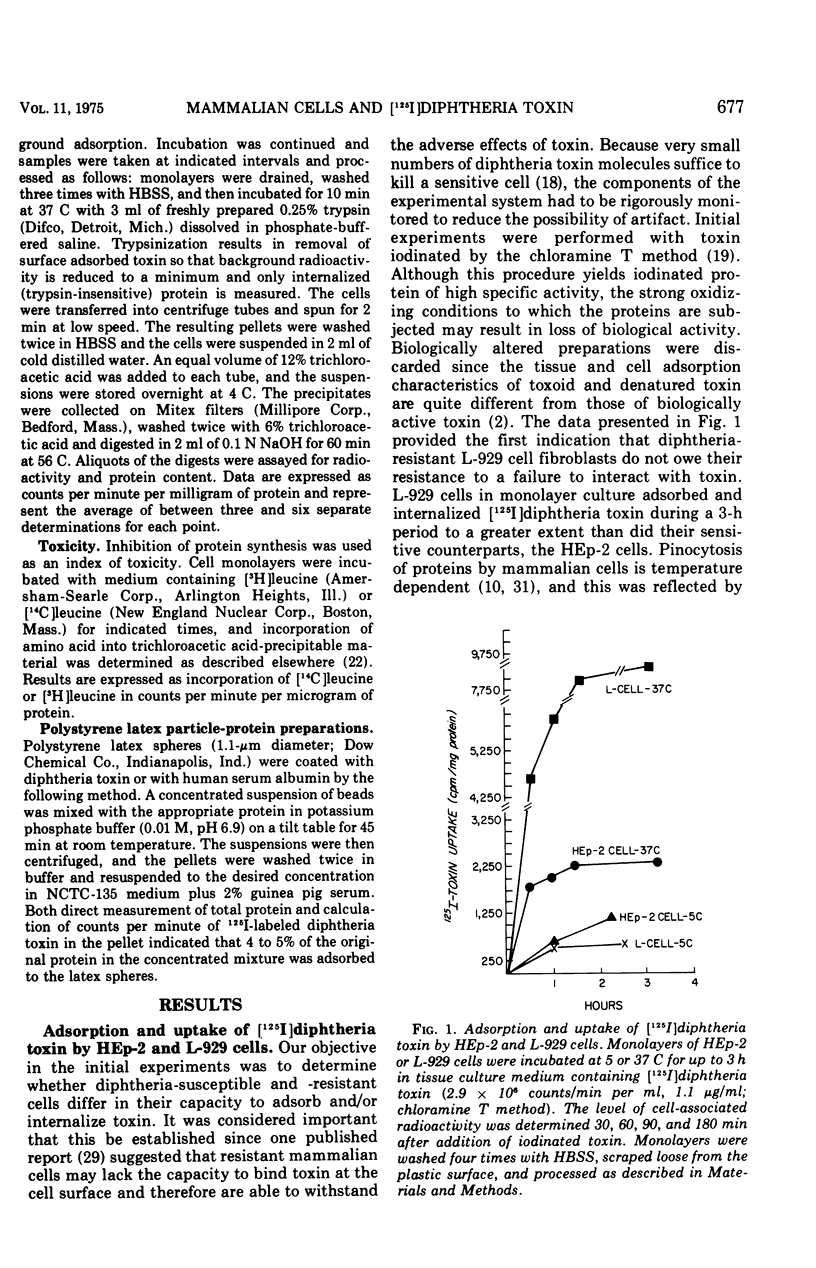
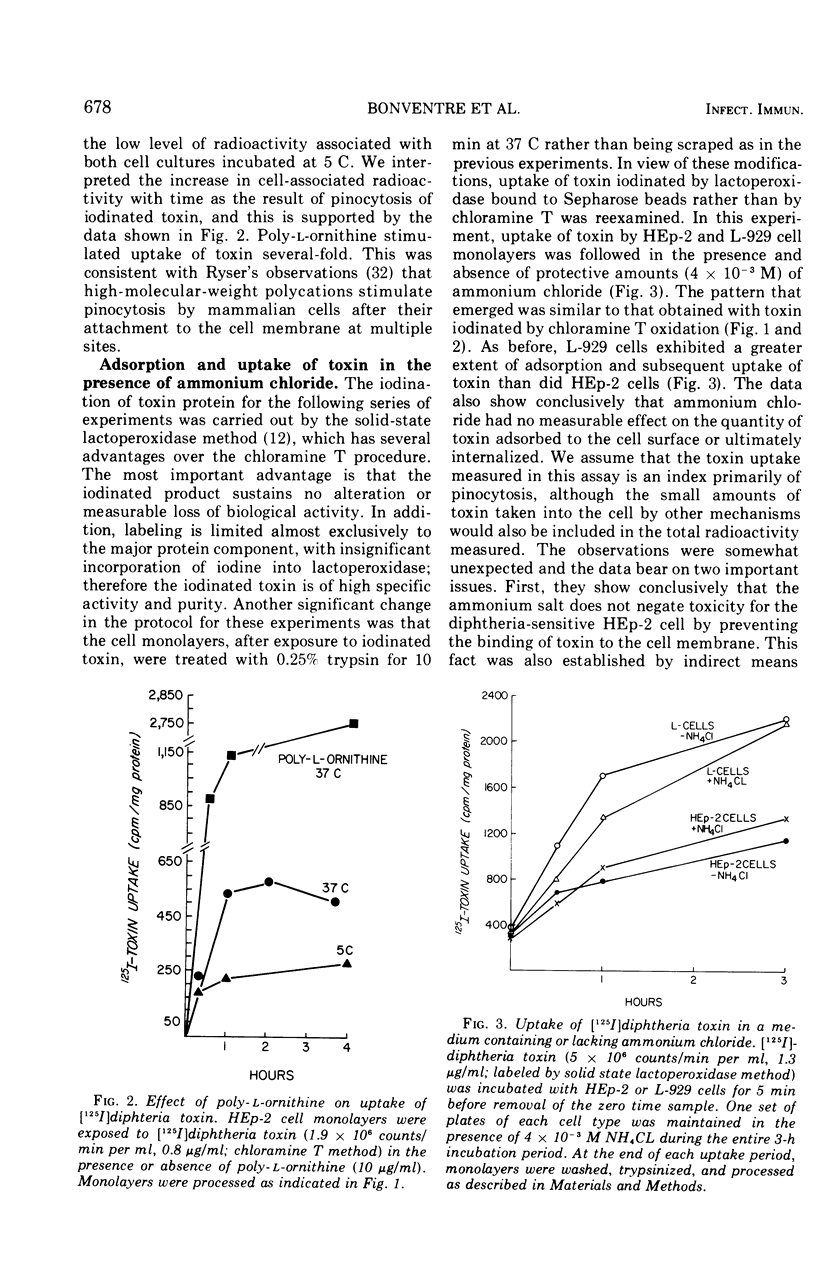
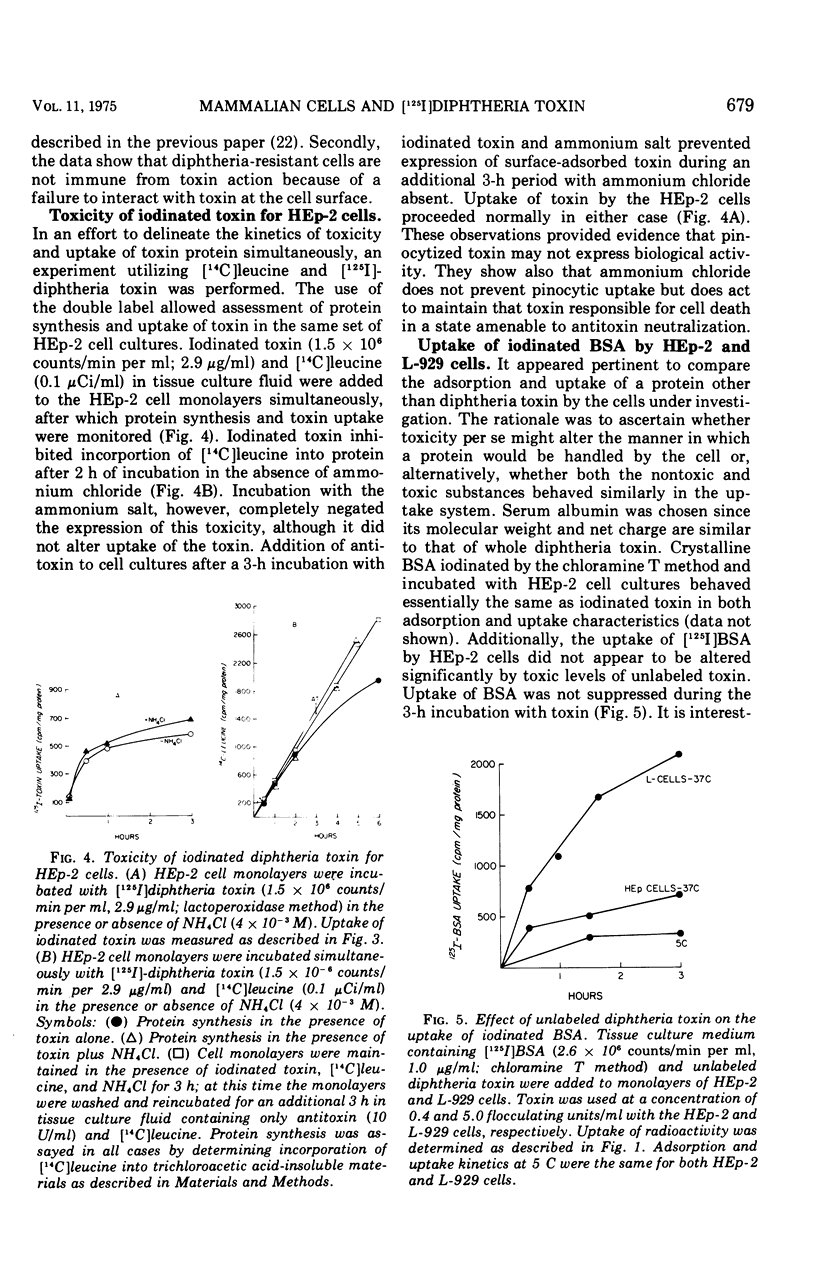
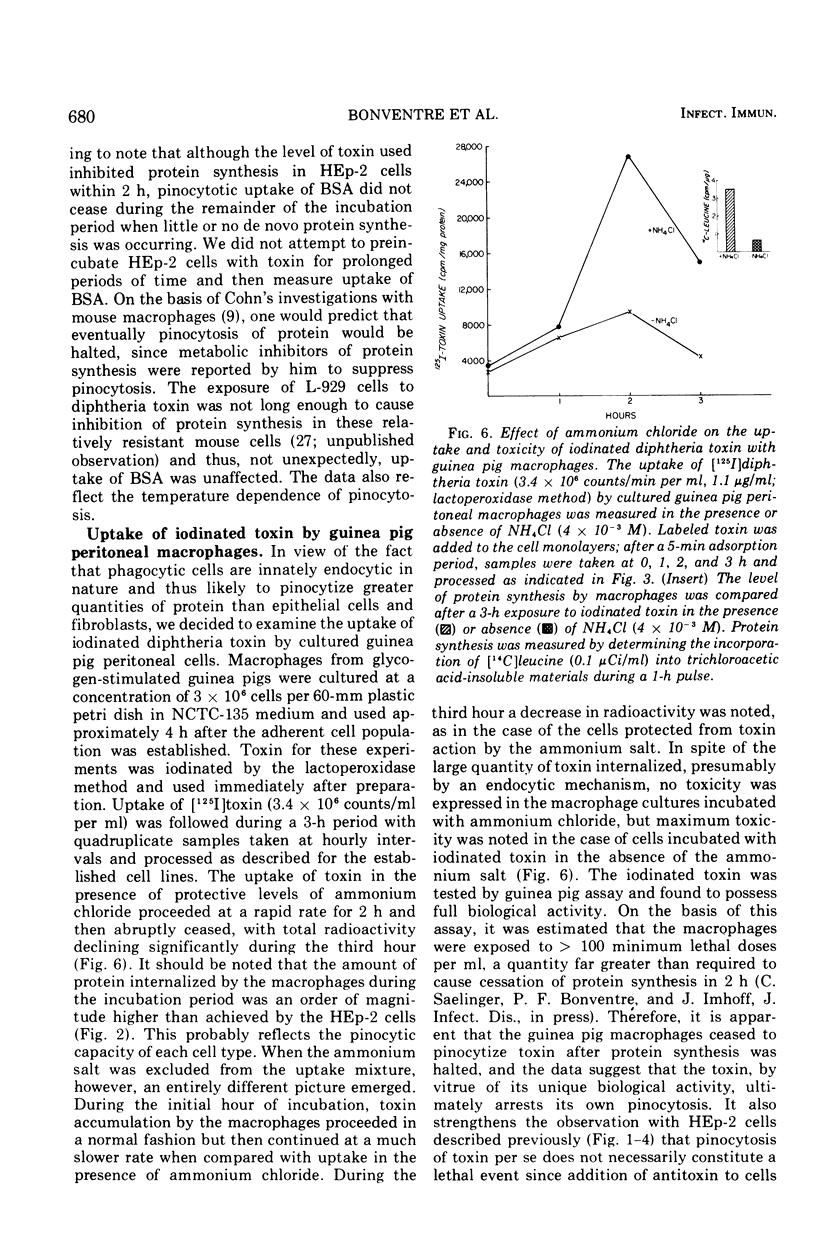
The characteristics of cell adsorption and pinocytotic uptake of diphtheria toxin by several mammalian cell types were studied. Purified toxin iodinated by a solid-state lactoperoxidase method provided preparations of high specific activity and unaltered biological activity. Dephtheria toxin-sensitive HEp-2 cells and guinea pig macrophage cultures were compared with resistant mouse L-929 cells. At 37 C the resistant cells in monolayer adsorbed and internalized [125I] toxin to a greater extent than did the HEp-2 cell cultures; no significant differences were observed at 5 C. Ammonium chloride protection levels did not alter uptake of toxin by either L-929 OR HEp-2 cells. Biological activity of the iodinated toxin, however, was negated provided the presence of ammonium chloride was maintained. The ammonium salt appears to maintain toxin in a state amenable to antitoxin neutralization. Guinea pig macrophages internalized iodinated toxin to a level 10 times greater than the established cell lines. In spite of the increased uptake of toxin by the endocytic cells, ammonium chloride prevented expression of toxicity. In an artificial system, toxin adsorbed to polystyrene latex spheres and internalized by guinea pig macrophages during phagocytosis did express biological activity. Ammonium chloride afforded some but not total protection against toxin present in the phagocytic vacuoles. The data suggest that two mechanisms of toxin uptake by susceptible cells may be operative. Toxin taken into the cell by a pinocytotic process probably is not ordinarily of physiological significance since it is usually degraded by lysosomal enzymes before it can reach cytoplasmic constituents on which it acts. When large quantities of toxin are pinocytized, toxicity may be expressed before enzymatic degradation is complete. A more specific uptake involving direct passage of the toxin through the plasma membrane may be the mechanism leading to cell death in the majority of instances.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRACHET J. Effects of ribonuclease on the metabolism of living root-tip cells. Nature. 1954 Nov 6;174(4436):876–877. doi: 10.1038/174876a0. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Pappenheimer A. M., Jr, Gill D. M., Harper A. A. Action of diphtheria toxin in the guinea pig. J Exp Med. 1970 Dec 1;132(6):1138–1152. doi: 10.1084/jem.132.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Studies on the mode of action of diphtheria toxin. I. Protein synthesis in guinea pig tissues. J Exp Med. 1966 Dec 1;124(6):1107–1122. doi: 10.1084/jem.124.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Studies on the mode of action of diphtheria toxin. II. Protein synthesis in primary heart cell cultures. J Exp Med. 1967 Dec 1;126(6):1079–1086. doi: 10.1084/jem.126.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Saelinger C. B. Inhibition of protein synthesis after intravenous or intramuscular challenge with diphtheria toxin. Infect Immun. 1972 Sep;6(3):418–421. doi: 10.1128/iai.6.3.418-421.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F. Studies on the mode of action of diphtheria toxin. V. Protein metabolism in a guinea pig model simulating chronic diphtheritic toxemia. Infect Immun. 1973 Apr;7(4):556–560. doi: 10.1128/iai.7.4.556-560.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. G., Bonventre P. F. Studies on the mode of action of diphtheria toxin. III. Effect on subcellular components of protein synthesis from the tissues of intoxicated guinea pigs and rats. J Exp Med. 1970 Apr 1;131(4):659–674. doi: 10.1084/jem.131.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967 Feb 1;125(2):213–232. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. The regulation of pinocytosis in mouse macrophages. I. Metabolic requirements as defined by the use of inhibitors. J Exp Med. 1966 Oct 1;124(4):557–571. doi: 10.1084/jem.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- Duncan J. L., Groman N. B. Activity of diphtheria toxin. II. Early events in the intoxication of HeLa cells. J Bacteriol. 1969 Jun;98(3):963–969. doi: 10.1128/jb.98.3.963-969.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich B. A., Cohn Z. A. The fate of peptides pinocytosed by macrophages in vitro. J Exp Med. 1969 Jan 1;129(1):227–245. doi: 10.1084/jem.129.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich B. A., Cohn Z. A. The uptake and digestion of iodinated human serum albumin by macrophages in vitro. J Exp Med. 1967 Nov 1;126(5):941–958. doi: 10.1084/jem.126.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIRKET H., CHEVREMONT-COMHAIRE S., CHEVREMONT M. Action of ribonuclease on living cells in vitro and synthesis of deoxyribonucleic acid. Nature. 1955 Dec 3;176(4492):1075–1076. doi: 10.1038/1761075a0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr, Uchida T. Diphtheria toxin, protein synthesis, and the cell. Fed Proc. 1973 Apr;32(4):1508–1515. [PubMed] [Google Scholar]
- Iglewski B. H., Rittenberg M. B. Selective toxicity of diphtheria toxin for malignant cells. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2707–2710. doi: 10.1073/pnas.71.7.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittelson T. R., Gill D. M. Diphtheria toxin: specific competition for cell receptors. Nature. 1973 Mar 30;242(5396):330–332. doi: 10.1038/242330b0. [DOI] [PubMed] [Google Scholar]
- Johnson W., Kuchler R. J., Solotorovsky M. Site in cell-free protein synthesis sensitive to diphtheria toxin. J Bacteriol. 1968 Oct;96(4):1089–1098. doi: 10.1128/jb.96.4.1089-1098.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDOUX L. Action of ribonuclease on certain ascites tumours. Nature. 1955 Feb 5;175(4449):258–259. doi: 10.1038/175258b0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liebow C., Rothman S. S. Membrane transport of proteins. Nat New Biol. 1972 Dec 6;240(101):176–178. doi: 10.1038/newbio240176a0. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. The response of cultured mammalian cells to diphtheria toxin. II. The resistant cell: enhancement of toxin action by poly-L-ornithine. J Exp Med. 1968 Mar 1;127(3):541–554. doi: 10.1084/jem.127.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Brown R. Studies on the mode of action of diphtheria toxin. VI. Site of the action of toxin in living cells. J Exp Med. 1968 Jun 1;127(6):1073–1086. doi: 10.1084/jem.127.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYSER H. J. THE MEASUREMENT OF I131-SERUM ALBUMIN UPTAKE BY TUMOR CELLS IN TISSUE CULTURE. Lab Invest. 1963 Oct;12:1009–1017. [PubMed] [Google Scholar]
- Ryser H. J. A membrane effect of basic polymers dependent on molecular size. Nature. 1967 Aug 26;215(5104):934–936. doi: 10.1038/215934a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]



