Abstract
Who among us hasn’t fantasized about a diet that allows ingestion of a surfeit of calories that are burned off effortlessly by ramping up energy expenditure? In this issue of the JCI, research led by Christopher Morrison suggests that this dream may become a reality; however, a complete understanding of the molecular interface that connects nutrient choices with our cellular metabolism will be required. Laeger et al. show that the expression and secretion of the weight-reducing hormone fibroblast growth factor 21 (FGF21) is regulated by dietary proteins and not, as has been heretofore assumed, simply triggered by reduced caloric intake. This study not only sheds new light on the role of FGF21 in systems metabolism, but also on the ways our bodies cope with the ever-changing availability of different dietary macronutrients.
FGF21 production benefits energy metabolism
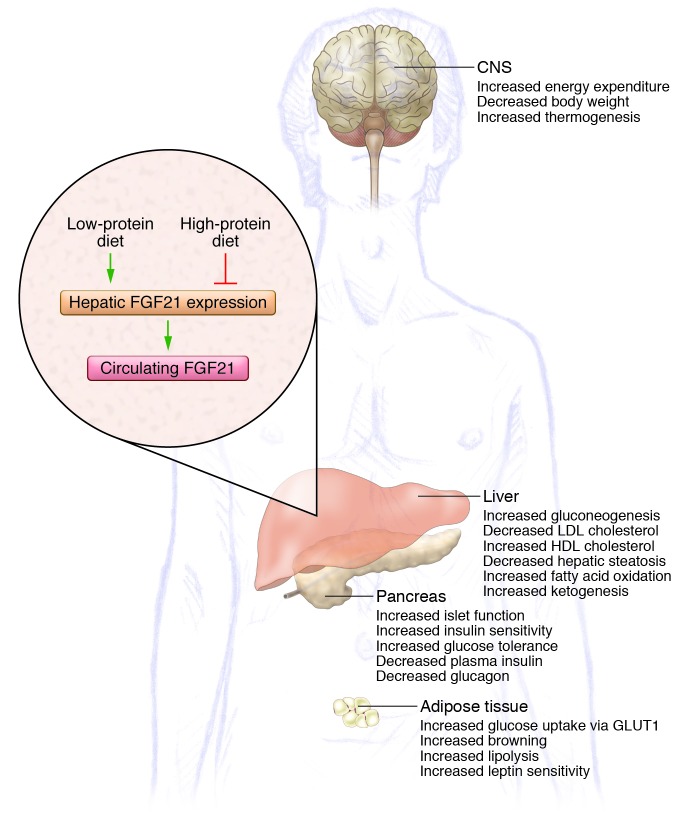
After an exhausting day and when craving dinner, not many of us appreciate that over the course of the day, various signaling molecules have been delivered into the bloodstream in order to cope with continually changing energy demands without a sacrifice in optimal function. One of the key players in fine-tuning the endocrine machinery is FGF21, which has widely been considered a fasting-induced hormone. Primarily expressed in the liver and adipose tissue (1, 2), FGF21 exerts a number of beneficial effects on energy metabolism (Figure 1), making it a popular drug target for the pharmaceutical industry. In adipose tissue, FGF21 has been reported to promote glucose uptake in an insulin-independent fashion, and this FGF21-dependent glucose uptake appears to be mediated through modulation of glucose transporter GLUT1 expression and is additive to the glycemic action of insulin (3). In line with its participation in glycemic regulation, FGF21 enhances hepatic gluconeogenesis and helps maintain normoglycemia under conditions of increased glucose utilization. FGF21 also lowers body weight by increasing energy expenditure, an effect that seemingly involves FGF21 signaling in both the CNS (4) and the periphery (5). Based on its combined effects on both glucose homeostasis and energy expenditure, FGF21 is considered a potential weapon against the negative metabolic consequences associated with the metabolic syndrome.
Figure 1. Metabolic action of FGF21.
Generation of hepatic FGF21 is induced in response to a LP diet. Once FGF21 enters the circulation, it targets different sites throughout the body, resulting in overall beneficial effects on energy metabolism. In contrast, a high-protein diet blocks hepatic FGF21 production.
A low-protein diet induces FGF21 expression
Current thought is that FGF21 is a fasting hormone secreted from the liver into circulation in response to increased fatty acid oxidation, as occurs after fasting or exposure to a ketogenic diet (6–8). While numerous studies have established that levels of circulating FGF21 increase under conditions of nutrient deprivation, the exact physiologic stimulus that regulates FGF21 secretion has been elusive. In this issue, Laeger et al. provide an answer to this important problem and demonstrate that serum levels of FGF21 increase specifically upon exposure to low-protein (LP) diets, regardless of overall caloric intake, in both rodents and humans (9). Laeger et al. assessed hepatic FGF21 expression in rats following exposure to a diet either low in protein, low in energy, or low in both. In this feeding paradigm, rats restricted for dietary protein but not energy intake displayed a robust increase in hepatic FGF21 expression. In contrast, rats that were energy restricted but not protein restricted showed decreased levels of FGF21. Importantly, Laeger et al. corroborated this observation in humans by demonstrating increased FGF21 levels in participants of a clinical study that were fed a LP diet.
Next, Laeger et al. assessed FGF21 levels upon fasting and refeeding and observed that the fasting-induced increase in FGF21 is potentiated by refeeding with an LP diet, but not a high-protein diet. To this end, protein supplementation in mice fed a ketogenic diet lowered FGF21 levels to approximately 50% of the level observed in mice fed a ketogenic diet without protein supplementation. Notably, carbohydrate supplementation in animals on a ketogenic diet had no effect on FGF21 levels, confirming that dietary protein restriction underlies the increased levels of circulating FGF21 typically observed after feeding with a ketogenic diet. These data confirm and extend the findings of a previous study by Bielohuby and colleagues, in which they demonstrated that circulating FGF21 levels are increased in rats fed a ketogenic diet limited to 10% protein, but not in rats fed an isocaloric ketogenic diet comprising 20% or 30% protein (10).
While previous studies have reported that hepatic expression of FGF21 is regulated via activation of PPARα (6, 7), Laeger et al. found no changes in PPARα expression or signaling following protein deprivation. Instead, increased FGF21 levels were associated with increased phosphorylation of hepatic eukaryotic initiation factor 2α (eIF2α), which is phosphorylated by the serine/threonine kinase general control nonderepressible 2 (GCN2) under conditions of protein deprivation. Moreover, Laeger et al. evaluated the effect of protein restriction on circulating FGF21 levels in mice deficient for either GCN2 or PPARα. Strikingly, mice lacking either GCN2 or PPARα did not exhibit an increase in FGF21 expression following exposure to a LP diet, indicating that both GCN2 and PPARα are involved in the regulation FGF21 expression in response to protein restriction. Laeger et al. also assessed whether FGF21 is required for the behavioral and metabolic response to protein restriction by evaluating the metabolic phenotype of WT and Fgf21-KO mice during a 14-day exposure to an LP diet. In line with the authors’ initial observations that dietary proteins regulate FGF21 secretion, WT mice exposed to the LP diet showed a marked increase in both food intake and energy expenditure, assessed by analysis of covariance (ANCOVA), as previously suggested (11). Of note, neither food intake nor energy expenditure was affected in Fgf21-KO mice upon protein restriction, indicating that these metabolic alterations are mediated by FGF21 signaling.
Conclusions and future directions
This elegant series of studies by Laeger and colleagues combines mechanistic studies with translational human trials, offering important insights into the molecular interface that connects macronutrient intake with endogenous control of nutrient partitioning. While the work described by Laeger et al. convincingly shows that FGF21 is regulated specifically by protein restriction rather than energy deprivation, further studies will need to address whether FGF21 expression is regulated by the lack of dietary proteins in general or by a deficiency in specific types of dietary proteins. In this regard, food-derived opioid peptides (nutropioids) were recently shown to regulate gut-brain neural circuits that control intestinal gluconeogenesis and satiety via activation of μ-opioid receptors (MORs) in the portal vein (12, 13). It might be informative not only to assess the role of specific dietary proteins, such as nutropioids, but also the effect of general classes of dietary proteins, such as essential, nonessential, and conditional amino acids, on FGF21 metabolism. Moreover, FGF21 has been recently described in the regulation of transdifferentiation of white adipose tissue into brown/brite adipose tissue (14); therefore, future research will undoubtedly revisit the specific role of dietary proteins in this process.
The findings described by Laeger et al. are encouraging and certainly advance our knowledge on how FGF21 expression is regulated. Importantly, these results challenge the current view that FGF21 is simply a classical fasting-induced hormone. The work by Laeger and colleagues also emphasizes the importance and relevance of well-designed diet-controlled studies in investigating and truly appreciating the physiological underpinnings of the complex neuroendocrine signaling nodes responsible for regulating systems metabolism. These results follow an era of high-protein diets, such as Atkins, South Beach, and the Paleo diet. While these diets all focus on enhanced protein consumption, they vary in their prescribed consumption of fats and carbohydrates. For example, the Atkins diet dramatically limits the intake of carbohydrates but not fat, and many on the diet self restrict overall calorie consumption, resulting in weight loss. Because these high-protein diets have received broad attention and considerable media coverage, it may seem tempting (and lucrative) to usher in yet a new dietary era focused on protein restriction rather than energy deprivation. Undoubtedly, a diet that naturally increases secretion of a candidate factor for the treatment of the metabolic syndrome suggests that protein restriction might offer desirable benefits despite a potential undesired impact on muscle mass.
Acknowledgments
The authors thank Silke Morin for her skillful language editing.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2014;124(9):3691–3693. doi:10.1172/JCI77508.
See the related article beginning on page 3913.
References
- 1.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492(1):203–206. doi: 10.1016/S0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 3.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarruf DA, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59(7):1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc Natl Acad Sci U S A. 2010;107(28):12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5(6):426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Inagaki T, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Foo JP, Aronis KN, Chamberland JP, Paruthi J, Moon HS, Mantzoros CS. Fibroblast growth factor 21 levels in young healthy females display day and night variations and are increased in response to short-term energy deprivation through a leptin-independent pathway. Diabetes Care. 2013;36(4):935–942. doi: 10.2337/dc12-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laeger T, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–3922. doi: 10.1172/JCI77508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bielohuby M, et al. Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein. Am J Physiol Endocrinol Metab. 2011;300(1):E65–E76. doi: 10.1152/ajpendo.00478.2010. [DOI] [PubMed] [Google Scholar]
- 11.Tschop MH, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2012;9(1):57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duraffourd C, et al. Mu-opioid receptors and dietary protein stimulate a gut-brain neural circuitry limiting food intake. Cell. 2012;150(2):377–388. doi: 10.1016/j.cell.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Pfluger PT, Schriever SC, Tschop MH. Nutropioids, hedonism in the gut? Cell Metab. 2012;16(2):137–139. doi: 10.1016/j.cmet.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Fisher FM, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]