Abstract
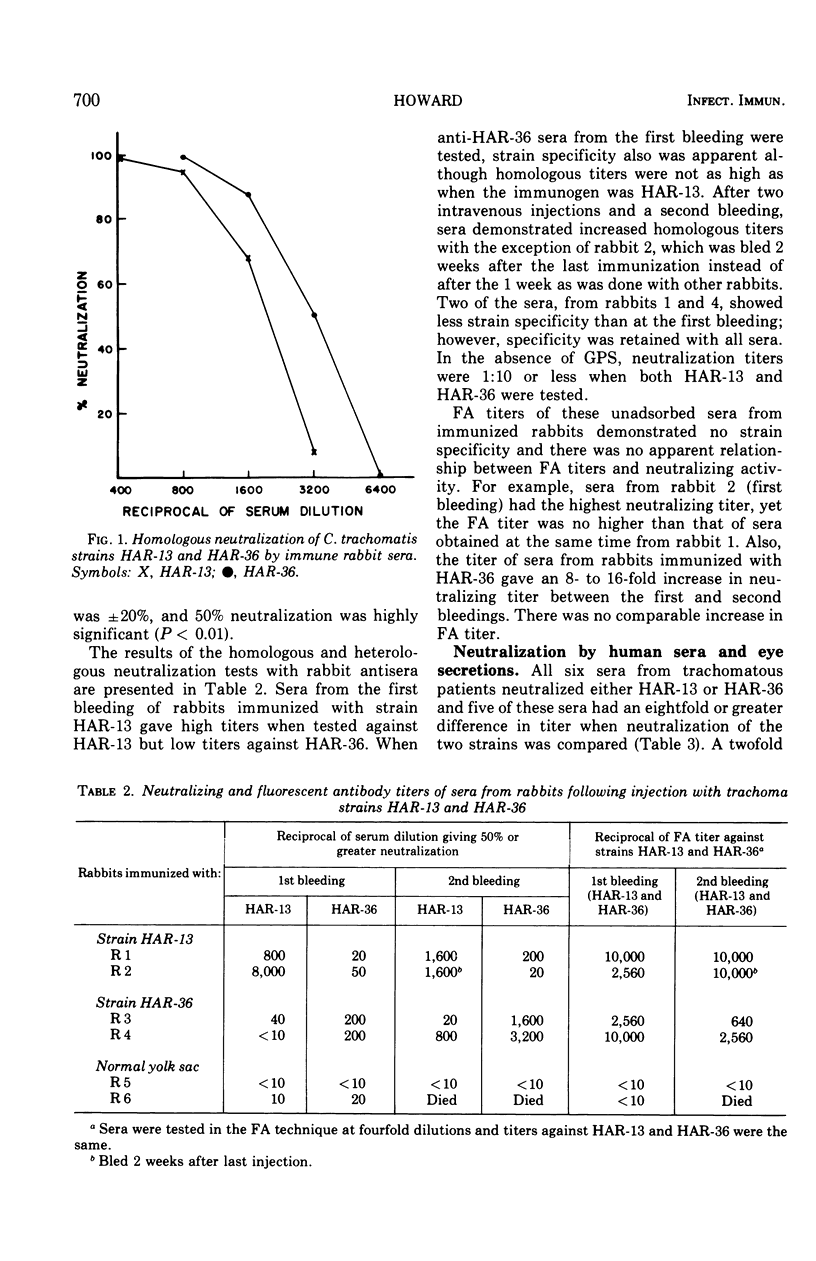
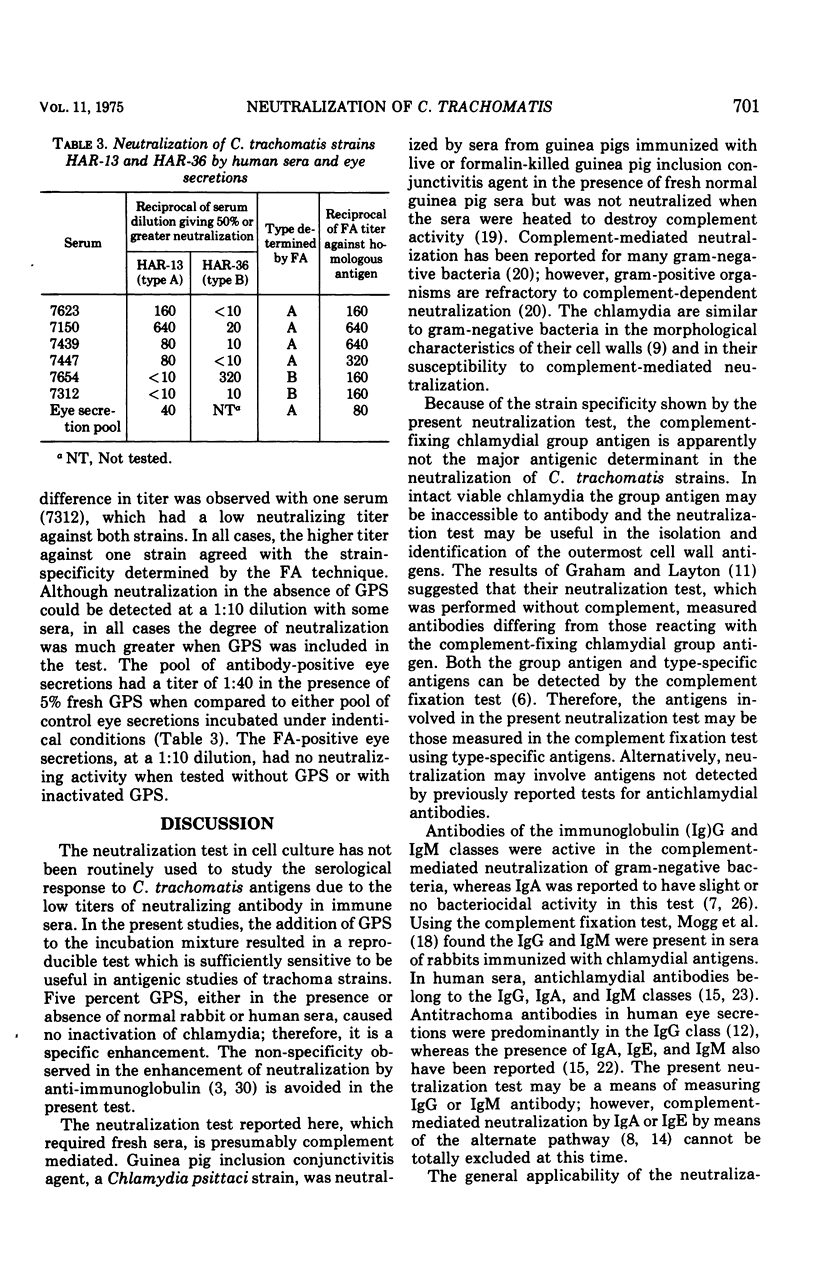
Neutralization of Chlamydia trachomatis was assayed by the decrease in inclusion-forming units in baby hamster kidney cells grown in culture. Five percent fresh guinea pig sera increased neutralization titers of rabbit antisera 100- to 1,000-fold but had no effect when normal rabbit sera were tested. Neutralization of a type A or B trachoma isolate was strain specific. Neutralization by human eye secretions and sera also was demonstrated when guinea pig sera were included in the test. All of the six human sera tested showed strain specificity against types A or B, in agreement with typing by the fluorescent antibody technique.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNKOPF H. The susceptibility of white mice to a strain of trachoma virus and their use in neutralization tests. Bull Res Counc Isr Sect E Exp Med. 1959 Oct;8E:25–29. [PubMed] [Google Scholar]
- BLYTH W. A., REEVE P., GRAHAM D. M., TAVERNE J. The production of antisera that neutralize inclusion blennorrhoea virus. Br J Exp Pathol. 1962 Jun;43:340–343. [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth W. A., Taverne J. Neutralization of TRIC organisms by antibody: enhancement by antisera prepared against immunoglobulins. J Hyg (Lond) 1974 Feb;72(1):129–134. doi: 10.1017/s0022172400023299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D. S., Schulkind M. L., Robbins J. B. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium "O" antibodies from rabbit intestinal fluid and colostrum. J Immunol. 1971 Jan;106(1):181–190. [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T., Alexander E. R. TRIC type K, a new immunologic type of Chlamydia trachomatis. J Immunol. 1974 Aug;113(2):591–596. [PubMed] [Google Scholar]
- McComb D. E., Nichols R. L. Antibody type specificity to trachoma in eye secretions of saudi arab children. Infect Immun. 1970 Jul;2(1):65–68. doi: 10.1128/iai.2.1.65-68.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E. S., Charbonnet L. T., MacDonald A. B. Immunity to chlamydial infections of the eye. I. The role of circulatory and secretory antibodies in resistance to reinfection with guinea pig inclusion conjunctivitis. J Immunol. 1973 Jun;110(6):1518–1525. [PubMed] [Google Scholar]
- Nichols R. L., Oertley R. E., Fraser E. C., MacDonald A. B., McComb D. E. Immunity to chlamydial infections of the eye. VI. Homologous neutralization of trachoma infectivity for the owl monkey conjuctivae by eye secretions from humans with trachoma. J Infect Dis. 1973 Apr;127(4):429–432. doi: 10.1093/infdis/127.4.429. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Gordon F. B., Quan A. L. Fluorescent antibody responses to chlamydial infection in patients with lymphogranuloma venereum and urethritis. J Immunol. 1974 Jun;112(6):2126–2134. [PubMed] [Google Scholar]
- REEVE P., GRAHAM D. M. A neutralization test for trachoma and inclusion blennorrhoea viruses grown in HeLa cell cultures. J Gen Microbiol. 1962 Jan;27:177–180. doi: 10.1099/00221287-27-1-177. [DOI] [PubMed] [Google Scholar]
- REEVE P., TAVERNE J. A simple method for total particle counts of trachoma and inclusion blennorrhoea viruses. Nature. 1962 Sep 1;195:923–924. doi: 10.1038/195923a0. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia. I. Isolates of ovine origin. Infect Immun. 1974 Jan;9(1):92–94. doi: 10.1128/iai.9.1.92-94.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



