Abstract
Background
Racial disparities in lung cancer outcomes have been observed in the general population. However, it is unclear whether survival differences persist when patients have equal access to healthcare. Our objective was to determine if lung cancer survival differed among black and white patients in the U.S. Military Health System (MHS), an equal access healthcare system.
Methods
The study subjects were 10,181 black and white patients identified through the Department of Defense’s Automated Central Tumor Registry, who were ≥20 years old and diagnosed with lung cancer between 1990 and 2003. Racial differences in all-cause survival were examined using the Kaplan–Meier method and Cox proportional hazards regression models stratified by histology. For comparison, survival rates in the general population were calculated using Surveillance, Epidemiology and End Results (SEER)-9 data.
Results
Analyses included 9,154 white and 1,027 black patients: 1,834 small cell lung cancers, 3,876 adenocarcinomas, 2,741 squamous cell carcinomas, and 1,730 large cell carcinomas. Although more favorable crude survival was observed among black patients than white patients with small cell lung cancer (p=0.04), survival was similar between the two groups after covariate adjustment. Racial differences in survival were non-significant for adenocarcinomas, squamous cell carcinomas and large cell carcinomas. Survival rates appeared to be better in the MHS than in the general population.
Conclusions and Impact
All-cause survival was similar among black and white lung cancer patients in the MHS. Providing equal access to healthcare may eliminate racial disparities in lung cancer survival while improving the outcome of all cases.
Keywords: equal access to healthcare, histologic subtypes, lung cancer, racial disparity, survival
Introduction
Lung cancer is the leading cause of cancer-related death among men and women in the United States. An estimated 226,160 new cases of lung cancer were expected in 2012, with 160,340 deaths due to this disease, accounting for roughly 28% of all cancer deaths (1). Despite the declining or leveling trends in lung cancer mortality among men and women, respectively, racial differences in lung cancer mortality persist, especially among men. The most recent statistics indicate that the lung cancer mortality rate (per 100,000) was 85.4 among black men, which was 28% higher than that among white men (66.9) (1).
Mortality and survival are related to timely and effective cancer diagnosis and treatment. In the general population, racial groups differ in their accessibility to medical care, and thus diagnosis and treatment. Black Americans are more likely than white Americans to have no health insurance coverage or inadequate coverage (2), which may limit their access to cancer prevention, early detection and high-quality treatment. Even though there is no specific recommendations for lung cancer screening for the general population and only about 5% of small cell lung cancer (SCLC) and 15% of non-small cell lung cancer (NSCLC) are diagnosed at local stages (3), individuals with health insurance still tend to be diagnosed earlier than those without (4, 5). As a likely result, black patients tend to be diagnosed with more advanced lung cancers and have worse survival than white patients (4, 6–9). However, even within the same tumor stage, black patients tend to have worse survival than white patients (3, 6, 10, 11). These within-stage survival differences may be at least partially due to variations in cancer treatment. Analyses of population-based cancer registry data have shown that black lung cancer patients, in comparison to white patients, are less likely to receive appropriate cancer treatment (10, 12–14). Racial disparities in lung cancer survival have often been found to be non-significant after adjustment for the receipt of cancer treatments and other prognostic factors (6, 9, 11, 15, 16). Furthermore, no survival differences have been observed between black and non-black patients who received similar treatment during clinical trials (17, 18). Therefore, variations in access to healthcare may be largely responsible for the observed racial disparities in lung cancer survival.
The Department of Defense’s (DoD’s) Military Health System (MHS) provides universal health care to military service members, retirees, and their dependents regardless of race. Therefore, the MHS offers a unique environment to evaluate whether racial differences in lung cancer survival remain when access to medical care is equal. In a previous study of NSCLC survival at one military hospital, no crude or covariate adjusted differences were observed between blacks and whites (19). To determine if these findings were unique to this hospital and/or to NSCLC, we conducted DoD-wide comparisons of lung cancer survival between whites and blacks diagnosed with NSCLC as well as SCLC. Histologic subtypes of NSCLC include squamous cell carcinoma (SCC), adenocarcinoma, and large cell carcinoma (LCC) (3). Recent studies indicate that histologic subtype may influence treatment regimen and survival outcome for NSCLC (20, 21); therefore, NSCLC subtype-stratified analyses were conducted. Age at diagnosis, sex, tumor stage, receipt of surgery and recurrence affect survival (20, 22), so, these variables were assessed as possible effect modifiers.
Materials and Methods
Sources of data
Data on patients diagnosed with lung cancer between 1990 and 2003 were collected from the DoD’s Automated Central Tumor Registry (ACTUR), a database and clinical tracking system for all cancer patients who were diagnosed and/or received cancer treatment at military treatment facilities. Military medical treatment facilities are required to report cancer data on all DoD beneficiaries, including active-duty military personnel, retired military personnel, Reserve and National Guard personnel who are temporarily activated, and their dependents. Upon receipt by ACTUR, the data are reviewed by registrars who verify that the correct diagnoses are reported and then follow all identified cancer cases until death.
This study was based on non-identifiable data and was approved by the institutional review boards of the U.S. Military Cancer Institute, the Armed Forces Institute of Pathology, and the National Institute of Health. The following items from the ACTUR database were used in the data analysis: race, age at diagnosis, sex, active duty status at diagnosis, primary cancer site, histology, tumor stage, tumor grade, cancer treatments, recurrence, date of last follow-up, and vital status.
Study Subjects
Eligible study subjects were black and white patients aged 20 years or older, who had a histologically confirmed first primary SCLC or NSCLC, with known NSCLC subtypes diagnosed between January 1, 1990 and December 31, 2003 (n=11,092). Cancer site and histology were classified using the tumor site (C34.0–C34.9) and morphology codes of the International Classification of Diseases for Oncology, third edition (ICD-O-3) (23). Histologic codes were grouped as SCLC (ICDO-3 codes 8040–8045, 8246) and NSCLC, which included (1) squamous cell carcinoma (SCC; 8050–8078, 8083–8084), (2) adenocarcinoma (8140, 8211, 8230–8231, 8250–8260, 8323, 8480–8490, 8550–8551, 8570–8574, 18576), and (3) large cell carcinoma (LCC; 8010–8012, 8014–8031, 8035, 8310). We excluded 835 patients who had a previous or concurrent diagnosis of another cancer type, which would likely affect their survival, and 76 patients with incomplete data on active duty status, sex, date of last contact, or no follow up. The total number of study subjects included in data analysis was 10,181.
Statistical Analysis
Survival time was compared between black and white patients. The analytic outcome, which was used to determine the survival time, was all-cause death. The observed survival time was calculated from the date of cancer diagnosis to the date of death among those who died. If an individual did not die during the study period then survival time was censored at the date of last contact. Follow-up was conducted through December 31, 2007. The length of follow-up ranged from 1 day to 208 months, with 60 months of follow-up available on 1,667 patients and 120 months of follow-up available on 565 patients.
We took the following steps in data analysis. First, differences in the distribution of demographic and tumor characteristics between whites and blacks by histology were evaluated with chi-square heterogeneity tests. Second, Kaplan-Meier survival curves were constructed and compared between whites and blacks stratified by histology using log-rank test for homogeneity. Third, Cox proportional-hazards modeling was utilized to investigate the association between race and survival stratified by histology after adjusting for demographic and tumor characteristics. Stratified analyses were further conducted by age (<65; ≥65), sex, tumor stage, receipt of surgery, and recurrence status to assess whether racial differences in survival varied by these variables. Finally, using the same exclusion criteria as with the ACTUR data, 5-year survival rates by race and histology were calculated based on Surveillance, Epidemiology and End Results (SEER)-9 data in SEER*Stat version 7.0.9.(24). The SEER-9 registries cover approximately 10% of the general US population (25). In order to determine if within-race survival rates differed between the MHS and the general populations, observed 5-year survival rates by histology among whites and blacks were compared between the two populations using chi-square tests. Data management and statistical analyses were performed using SAS software version 9.3.0 (SAS Institute, Inc., Cary, NC). All reported p-values are two-sides and the significance level was set at p<0.05.
Results
The study cohort consisted of 1,834 SCLCs and 8,347 NSCLCs, which included 3,876 adenocarcinomas, 2,741 SCCs and 1,730 LCCs. Regardless of histology at diagnosis, black patients were more likely to be active-duty and younger than white patients (p<0.01; Table 1). Among patients with adenocarcinoma or LCC, the proportion of men was higher among black patients than white patients (p≤0.01). Additionally among adenocarcinoma patients, black patients were more likely to receive chemotherapy or have unknown status on chemotherapy (p<0.01) and to experience a recurrence than were white patients (p=0.05). Among SCLC patients, black patients were more likely to receive radiation therapy than were white patients (p<0.01). The distribution of tumor grade and the receipt of surgery were not shown to vary significantly by race for any of the histologic types.
Table 1.
Demographic and tumor characteristics of lung cancer patients diagnosed during 1990–2003, ACTUR.
| Characteristic | Small Cell Lung Cancer | Adenocarcinoma | Squamous Cell Carcinoma | Large Cell Carcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White n=1,710 | Black n=124 | P-value^ | White n=3,443 | Black n=433 | P-value^ | White n=2,467 | Black n=274 | P-value^ | White n=1,534 | Black n=196 | P-value^ | |
| Age (years)* | No. of patients (%) | No. of patients (%) | No. of patients (%) | 43 | ||||||||
| 20–44 | 30 (1.7) | 7 (5.7) | 142 (4.1) | 35 (8.1) | 34 (1.4) | 7 (2.5) | 55 (3.6) | 17 (8.7) | ||||
| 45–54 | 205 (12.0) | 18 (14.5) | 399 (11.6) | 95 (21.9) | 198 (8.0) | 35 (12.8) | 194 (12.6) | 38 (19.4) | ||||
| 55–64 | 673 (39.4) | 53 (42.7) | 1,346 (39.1) | 180 (41.6) | 896 (36.3) | 135 (49.3) | 560 (36.5) | 76 (38.8) | ||||
| 65–74 | 573 (33.5) | 42(33.9) | 1,153 (33.5) | 94 (21.7) | 965 (39.1) | 80 (29.2) | 531 (34.6) | 51 (26.0) | ||||
| ≥75 | 229 (13.4) | 4 (3.2) | <0.01 | 403 (11.7) | 29 (6.7) | <0.01 | 374 (15.2) | 17 (6.2) | <0.01 | 194 (12.7) | 14 (7.1) | <0.01 |
| Sex* | ||||||||||||
| Men | 1,058 (61.9) | 73 (58.9) | 2,103 (61.1) | 297 (68.6) | 1,880 (76.2) | 203 (74.1) | 1,055 (68.8) | 155 (79.1) | ||||
| Women | 652 (38.1) | 51 (41.1) | 0.51 | 1,340 (38.9) | 136 (31.4) | <0.01 | 587 (23.8) | 71 (25.9) | 0.44 | 479 (31.2) | 41 (20.9) | <0.01 |
| Active Duty* | ||||||||||||
| Yes | 26 (1.5) | 7 (5.6) | 92 (2.7) | 27 (6.2) | 26 (1.1) | 8 (2.9) | 43 (2.8) | 13 (6.6) | ||||
| No | 1,684 (98.5) | 117 (94.4) | <0.01 | 3,351 (97.3) | 406 (93.8) | <0.01 | 2,441 (98.9) | 266 (97.1) | 0.01 | 1,491 (97.2) | 183 (93.4) | <0.01 |
| Tumor Stage* | ||||||||||||
| Localized | 242 (14.2) | 15 (12.1) | 1,191 (34.6) | 141 (32.6) | 840 (34.1) | 84 (30.7) | 338 (22.0) | 35 (17.9) | ||||
| Regional | 455 (26.6) | 41 (33.1) | 957 (27.8) | 119 (27.5) | 931 (37.7) | 105 (38.3) | 449 (29.3) | 62 (31.6) | ||||
| Distant | 893 (52.2) | 60 (48.4) | 1,151 (33.4) | 159 (36.7) | 573 (23.2) | 76 (27.7) | 629 (41.0) | 85 (43.4) | ||||
| Unknown | 120 (7.0) | 8 (6.4) | 0.47 | 144 (4.2) | 14 (3.2) | 0.47 | 123 (5.0) | 9 (3.3) | 0.22 | 118 (7.7) | 14 (7.1) | 0.56 |
| Tumor Grade* | ||||||||||||
| I | 6 (0.3) | 0 (0.0) | 384 (11.1) | 48 (11.1) | 114 (4.6) | 18 (6.6) | 11 (0.7) | 1 (0.5) | ||||
| II | 22 (1.3) | 2 (1.6) | 9.5 (26.3) | 97 (22.4) | 838 (34.0) | 85 (31.0) | 38 (2.5) | 5 (2.5) | ||||
| III | 172 (10.1) | 18 (14.5) | 1,310 (38.1) | 177 (40.9) | 931 (37.7) | 91 (33.2) | 694 (45.2) | 89 (45.4) | ||||
| IV | 586 (34.3) | 40 (32.3) | 23 (0.7) | 4 (0.9) | 12 (0.5) | 1 (0.4) | 228 (14.9) | 15 (7.7) | ||||
| Unknown | 924 (54.0) | 64 (51.6) | 0.56 | 821 (23.8) | 107 (24.7) | 0.47 | 572 (23.2) | 79 (28.8) | 0.12 | 563 (36.7) | 86 (43.9) | 0.06 |
| Surgery | ||||||||||||
| Yes | 171 (10.0) | 8 (6.5) | 1,832 (53.2) | 216 (49.6) | 1,057 (42.8) | 106 (38.7) | 449 (29.3) | 55 (28.1) | ||||
| No | 1,531 (89.5) | 115 (92.7) | 1,601 (46.5) | 215 (49.9) | 1,405 (57.0) | 168 (61.3) | 1,077 (70.2) | 141 (71.9) | ||||
| Unknown | 8 (0.5) | 1 (0.8) | 0.39 | 10 (0.3) | 2 (0.5) | 0.33 | 5 (0.2) | 0 (0.0) | 0.30 | 8 (0.5) | 0 (0.0) | 0.55 |
| Radiation | ||||||||||||
| Yes | 755 (44.2) | 77 (62.1) | 1,333 (38.7) | 223 (39.5) | 1,241 (50.3) | 145 (52.9) | 829 (54.0) | 112 (57.2) | ||||
| No | 883 (51.6) | 44 (35.5) | 1,984 (57.6) | 304 (55.0) | 1,121 (45.4) | 118 (43.1) | 602 (39.3) | 71 (36.2) | ||||
| Unknown | 72 (4.2) | 3 (2.4) | <0.01 | 126 (3.7) | 25 (5.5) | 0.13 | 105 (4.3) | 11 (4.0) | 0.71 | 103 (6.7) | 13 (6.6) | 0.70 |
| Chemotherapy | ||||||||||||
| Yes | 1,362 (79.7) | 100 (80.7) | 805 (23.4) | 116 (26.8) | 555 (22.5) | 66 (24.1) | 425 (27.7) | 68 (34.7) | ||||
| No | 293 (17.1) | 19 (15.3) | 2,524 (73.3) | 290 (67.0) | 1,825 (74.0) | 199 (72.6) | 1,028 (67.0) | 120 (61.2) | ||||
| Unknown | 55 (3.2) | 5 (4.0) | 0.79 | 114 (3.3) | 27 (6.2) | <0.01 | 87 (3.5) | 9 (3.3) | 0.66 | 81 (5.3) | 8 (4.1) | 0.11 |
| Recurrence | ||||||||||||
| Yes | 338 (19.8) | 32 (25.8) | 600 (17.4) | 92 (21.3) | 385 (15.6) | 40 (14.6) | 219 (14.3) | 33 (16.8) | ||||
| No | 1,372 (80.2) | 136 (74.2) | 0.11 | 2,843 (82.6) | 341 (78.7) | 0.05 | 2,082 (84.4) | 234 (85.4) | 0.66 | 1,315 (85.7) | 163 (83.2) | 0.34 |
At the time of diagnosis;
Chi-square test.
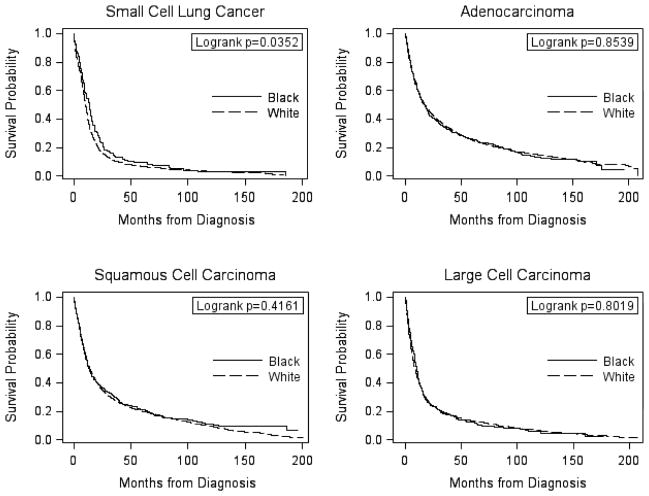
Kaplan-Meier survival curves for each histologic type by race are provided in Figure 1. Survival curves were not significantly different between black and white patients for adenocarcinoma, SCC or LCC. Among SCLC patients, black patients appeared to have a better survival than white patients (p=0.04); however, after covariate adjustment, this association was no longer significant (HR=0.90; 95% CI=0.75–1.09; Table 2). No racial differences in survival were observed for the other histologic types in the multivariate analyses. No evidence of effect modification by age at diagnosis, sex, tumor stage, receipt of surgery or recurrence status was observed for any of the histologic types (Table 2).
Figure 1.
Kaplan-Meier survival curves comparing black and white lung cancer patients diagnosed 1990–2003, ACTUR.
Table 2.
Multivariate survival analysis comparing black and white patients diagnosed during 1990–2003, ACTUR.
| Strata | Race | Small Cell Lung Cancer | Adenocarcinoma | Squamous Cell Carcinoma | Large Cell Carcinoma |
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI)* | HR (95% CI)* | HR (95% CI)* | HR (95% CI)* | ||
| Overall | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.90 (0.75–1.09) | 1.04 (0.93–1.16) | 1.01 (0.89–1.16) | 0.98 (0.84–1.14) | |
| Age(years) | |||||
| 20–64 | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.87 (0.68–1.11) | 1.08 (0.95–1.24) | 1.03 (0.86–1.22) | 1.00 (0.82–1.22) | |
| ≥65 | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.96 (0.70–1.31) | 0.93 (0.76–1.14) | 1.04 (0.83–1.29) | 0.98 (0.75–1.27) | |
| Sex | |||||
| Men | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.90 (0.70–1.14) | 1.04 (0.91–1.18) | 1.00 (0.85–1.16) | 1.01 (0.85–1.20) | |
| Women | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.93 (0.69–1.26) | 1.02 (0.83–1.25) | 1.08 (0.82–1.43) | 0.85 (0.60–1.21) | |
| Tumor Stage | |||||
| Local | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.75 (0.42–1.37) | 1.03 (0.82–1.28) | 1.01 (0.78–1.32) | 1.07 (0.70–1.63) | |
| Regional | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.90 (0.64–1.27) | 1.02 (0.83–1.26) | 0.95 (0.76–1.18) | 1.04 (0.79–1.37) | |
| Distant | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.90 (0.69–1.17) | 0.95 (0.80–1.13) | 1.10 (0.86–1.41) | 0.97 (0.77–1.22) | |
| Surgery | |||||
| Yes | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.60 (0.69–3.71) | 0.95 (0.80–1.13) | 0.86 (0.67–1.10) | 1.16 (0.83–1.60) | |
| No | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.89 (0.73–1.08) | 1.03 (0.89–1.20) | 1.12 (0.95–1.32) | 0.98 (0.82–1.17) | |
| Recurrence | |||||
| Yes | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.82 (0.56–1.19) | 1.04 (0.82–1.32) | 0.88 (0.62–1.24) | 0.99 (0.66–1.48) | |
| No | White | 1.00 | 1.00 | 1.00 | |
| Black | 0.91 (0.73–1.13) | 1.03 (0.91–1.17) | 1.04 (0.90–1.21) | 0.98 (0.82–1.16) | |
ACTUR: Automated Tumor Registry; HR: Hazard Ratio; CI: Confidence Interval
Unless stratified by the variable, all models were adjusted for age, sex, active duty status, tumor stage, tumor grade, receipt off surgery, radiotherapy, chemotherapy and recurrence.
Better 5-year survival rates were observed within ACTUR than in SEER-9 among both whites and blacks (Table 3). This finding was statistically significant (p<0.01) for all histologic comparisons, except among white SCLC patients (p=0.09). In addition, unlike in ACTUR, white patients tended to have higher 5-year survival rates than blacks for all histologic types in SEER.
Table 3.
Comparison of within-race 5-year survival rates by histology between ACTUR and SEER-9 among black and white lung cancer patients diagnosed during 1990–2003.
| Histology | White | Black | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ACTUR % ± SE | SEER-9 % ± SE | p* | ACTUR % ± SE | SEER-9 % ± SE | p* | |
| Small Cell Lung Cancer | 6.6 ± 0.6 | 5.7 ± 0.1 | 0.09 | 9.4 ± 2.7 | 4.6 ± 0.4 | <0.01 |
| Adenocarcinoma | 24.9 ± 0.7 | 18.5 ± 0.1 | <0.01 | 25.6 ± 2.1 | 14.2 ± 0.4 | <0.01 |
| Squamous Cell Carcinoma | 19.9 ± 0.8 | 14.8 ± 0.2 | <0.01 | 21.2 ± 2.5 | 10.9 ± 0.4 | <0.01 |
| Large Cell Carcinoma | 13.1 ± 0.8 | 7.9 ± 0.1 | <0.01 | 12.3 ± 2.4 | 7.5 ± 0.4 | 0.01 |
ACTUR: Automated Tumor Registry; SEER: Surveillance, Epidemiology End Results.
Chi-square test p-value (alive/not alive at 5 years after diagnosis)
Discussion
In the current study, no black-white disparities were observed for any of the NSCLC histologic subtypes in either crude or covariate adjusted comparisons within the MHS. Covariate adjusted comparisons also revealed similar SCLC survival between the two groups, although crude comparisons showed that black patients had better SCLC survival than white patients. Comparatively, 5-year all-cause survival rates appeared to be better in the MHS than in the general population, regardless of race or histology.
This DoD-wide study confirms the findings of no differences in NSCLC survival between black and white patients that were observed in a previous study based on one military treatment facility’s data (19). The current study further indicates that black and white patients within the DoD health care system experience similar lung cancer survival regardless of histology. Without a reliable screening tool for early detection, lung cancer survival depends largely on the histological features of cancer, stage of disease, and timely access to high quality cancer treatment. All patients in the study were beneficiaries of the DoD health care system and were entitled to equal access to medical care. There were no significant differences in tumor grade or stage distribution between black and white patients. No racial differences were observed with regard to the receipt of specific cancer treatments after controlling for potential confounders, except for a higher receipt of radiation for SCLC among black patients compared to white patients (data not shown). These findings indicate that for lung cancer patients in the MHS, there were no racial differences in tumor stage at diagnosis, and black patients received appropriate cancer treatments as frequently as their white counterparts.
Studies in the general population have demonstrated that lack of adequate health insurance coverage is associated with poorer access to cancer prevention, diagnoses at later stages and poorer outcomes among cancer patients (7, 26, 27). In comparison to white lung cancer patients, black lung cancer patients are more likely to be uninsured, which may limit their access to high-quality cancer care (27, 28). There is evidence that lack of a regular source of care is associated with lower surgical rates among black lung cancer patients (29) and that lower surgical rates among black patients with NSCLC can largely account for their poorer survival (12). Our finding that black and white lung cancer patients with equal access to care had similar survival provides further support for the notion that racial disparities in lung cancer survival mainly result from inequalities in access to and receipt of quality health care, especially high-quality cancer treatments. These results are significant, suggesting that equivalent survival can be achieved for each of the four major lung cancer histologic types by providing equal access to care. The comparisons of our results with those from SEER demonstrated such a possibility; lung cancer patients, especially black patients, in the MHS had significantly better 5-year survival rates than their counterparts in the general population in which white patients tended to have better survival than blacks. The greater improvement in survival among blacks in the equal access system may have helped eliminate the racial disparities.
While our study had important strengths, it also had several limitations. Only all-cause death data, not cancer-specific death, are included in the ACTUR database; therefore, the potential effects of death from other causes cannot be excluded. However, unless deaths from other causes were less common among black persons than white persons, which is unlikely, comparing all-cause survival between the groups should not have concealed more lung cancer deaths and shorter lung cancer survival among black persons. Missing information on tumor characteristics and treatment might have affected our results especially if it was differential by race. While we do not exclude this possibility, such effects might be limited because the proportion of patients with unknown information was generally low and adjustment accounted for unknown categories in the multivariate analysis. We also do not exclude the possibility that the study power to detect racial differences was limited due to relatively small sample sizes of black patients, particularly when stratified by histology. However, when not stratified by histology, analyses also showed no racial differences (all lung cancer: HR=0.99; 95% CI=0.92–1.06; all NSCLC: HR=0.99; 95% CI=0.92–1.06; data not shown), suggesting that limited power does not appear to explain our findings. Finally, other variables, in addition to access to care, may be related to the observed differences in survival between ACTUR and SEER-9. While our further analyses stratified by age and sex (data not shown) confirmed those in table 3 and thus minimized the potential effects by these variables, caution should be taken in the interpretation of the results due to potential differences in other data features between the two data sources.
In conclusion, no difference in all-cause survival among lung cancer patients was observed by race in the MHS, which is an equal access healthcare system. All-cause survival appeared to be better within the MHS than in the general population. These results, therefore, indicate that race is not an independent prognostic factor for lung cancer survival and better healthcare access can result in improved lung cancer outcomes for all cases.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.DeNavas-Walt C, Proctor BD, Smith JC. Current Population Reports, 60-239. U.S. Government Printing Office; Washington, DC: 2011. Income, Poverty, and Health Insurance Coverage in the United States: 2010. [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- 4.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14(8):761–6. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- 5.Ward EM, Fedewa SA, Cokkinides V, Virgo K. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J. 2010;16(6):614–21. doi: 10.1097/PPO.0b013e3181ff2aec. [DOI] [PubMed] [Google Scholar]
- 6.Hardy D, Xia R, Liu CC, Cormier JN, Nurgalieva Z, Du XL. Racial disparities and survival for nonsmall-cell lung cancer in a large cohort of black and white elderly patients. Cancer. 2009;115(20):4807–18. doi: 10.1002/cncr.24521. [DOI] [PubMed] [Google Scholar]
- 7.Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979–2003. Cancer. 2011;117(14):3242–51. doi: 10.1002/cncr.25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flenaugh EL, Henriques-Forsythe MN. Lung cancer disparities in African Americans: health versus health care. Clin Chest Med. 2006;27(3):431–9. vi. doi: 10.1016/j.ccm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Yang R, Cheung MC, Byrne MM, Huang Y, Nguyen D, Lally BE, et al. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010;116(10):2437–47. doi: 10.1002/cncr.24986. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald HP, Polissar NL, Borgatta EF, McCorkle R, Goodman G. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health. 1998;88(11):1681–4. doi: 10.2105/ajph.88.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant AS, Cerfolio RJ. Impact of race on outcomes of patients with non-small cell lung cancer. J Thorac Oncol. 2008;3(7):711–5. doi: 10.1097/JTO.0b013e31817c60c7. [DOI] [PubMed] [Google Scholar]
- 12.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 13.Esnaola NF, Gebregziabher M, Knott K, Finney C, Silvestri GA, Reed CE, et al. Underuse of surgical resection for localized, non-small cell lung cancer among whites and African Americans in South Carolina. Ann Thorac Surg. 2008;86(1):220–6. doi: 10.1016/j.athoracsur.2008.02.072. discussion 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy D, Liu CC, Xia R, Cormier JN, Chan W, White A, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115(10):2199–211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 15.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Low socioeconomic status is a poor prognostic factor for survival in stage I nonsmall cell lung cancer and is independent of surgical treatment, race, and marital status. Cancer. 2008;112(9):2011–20. doi: 10.1002/cncr.23397. [DOI] [PubMed] [Google Scholar]
- 16.Blackstock AW, Herndon JE, 2nd, Paskett ED, Perry MC, Graziano SL, Muscato JJ, et al. Outcomes among African-American/non-African-American patients with advanced non-small-cell lung carcinoma: report from the Cancer and Leukemia Group B. J Natl Cancer Inst. 2002;94(4):284–90. doi: 10.1093/jnci/94.4.284. [DOI] [PubMed] [Google Scholar]
- 17.Blackstock AW, Herndon JE, 2nd, Paskett ED, Miller AA, Lathan C, Niell HB, et al. Similar outcomes between African American and non-African American patients with extensive-stage small-cell lung carcinoma: report from the Cancer and Leukemia Group B. J Clin Oncol. 2006;24(3):407–12. doi: 10.1200/JCO.2005.02.1436. [DOI] [PubMed] [Google Scholar]
- 18.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrogi AJ. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(1):25–31. doi: 10.1158/1055-9965.EPI-05-0537. [DOI] [PubMed] [Google Scholar]
- 20.Ost D, Goldberg J, Rolnitzky L, Rom WN. Survival after surgery in stage IA and IB non-small cell lung cancer. Am J Respir Crit Care Med. 2008;177(5):516–23. doi: 10.1164/rccm.200706-815OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams SA, Smith ER, Hardin J, Prabhu-Das I, Fulton J, Hebert JR. Racial differences in follow-up of abnormal mammography findings among economically disadvantaged women. Cancer. 2009;115(24):5788–97. doi: 10.1002/cncr.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lantz PM, Mujahid M, Schwartz K, Janz NK, Fagerlin A, Salem B, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96(12):2173–8. doi: 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–62. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 25.Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SS, Eley JW. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109(3):545–57. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 26.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–31. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 27.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 28.Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296(16):1973–80. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 29.Cykert S, Dilworth-Anderson P, Monroe MH, Walker P, McGuire FR, Corbie-Smith G, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303(23):2368–76. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]