Abstract
Background
Conclusive data regarding cardiovascular (CV) toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) are sparse. We hypothesized that regular NSAID use is associated with increased risk for CV events in post-menopausal women, and that this association is stronger with greater cyclooxygenase (cox)-2 compared with cox-1 inhibition.
Methods and Results
Post-menopausal women enrolled in the Women’s Health Initiative (WHI) were classified as regular users or non-users of non-aspirin NSAIDs. Cox regression examined NSAID use as a time-varying covariate and its association with the primary outcome of total CV disease defined as CV death, nonfatal myocardial infarction, or nonfatal stroke. Secondary analyses considered the association of selective cox-2 inhibitors (e.g., celecoxib), non-selective agents with cox-2>cox-1 inhibition (e.g., naproxen), and non-selective agents with cox-1>cox-2 inhibition (e.g., ibuprofen) with the primary outcome. Overall, 160,801 participants were available for analysis (mean follow-up 11.2 years). Regular NSAID use at some point in time was reported by 53,142 participants. Regular NSAID use was associated with an increased hazard for CV events versus no NSAID use (HR=1.10[95% CI 1.06–1.15], Pitalic>0.001). Selective cox-2 inhibitors were associated with a modest increased hazard for CV events (HR=1.13[1.04–1.23], P=0.004; celecoxib only HR=1.13[1.01–1.27], P=0.031). Among aspirin users, concomitant selective cox-2 inhibitor use was no longer associated with increased hazard for CV events. There was an increased risk for agents with cox-2>cox-1 inhibition (HR=1.17[1.10–1.24], Pbold>0.001; naproxen only HR=1.22[1.12–1.34], P<0.001). This harmful association remained among concomitant aspirin users. We did not observe a risk elevation for agents with cox-1>cox-2 inhibition (HR=1.01[0.95–1.07], P=0.884; ibuprofen only HR=1.00[0.93–1.07], P=0.996).
Conclusions
Regular use of selective cox-2 inhibitors and non-selective NSAIDs with cox-2>cox-1 inhibition showed a modestly increased hazard for CV events. Non-selective agents with cox-1>cox-2 inhibition were not associated with increased CV risk.
Clinical Trial Registration
Keywords: non-steroidal anti-inflammatory drugs, coronary artery disease, myocardial infarction, stroke, cox inhibition
Introduction
Selective inhibition of the cyclooxygenase (cox)-2 isoenzyme has been associated with an increase in adverse cardiovascular (CV) events, which resulted in the removal of rofecoxib from the market.1,2 In contrast, inhibition of cox-1 by aspirin has been associated with a reduction in adverse CV events.3–5
Non-selective non-steroidal anti-inflammatory drugs (NSAIDs) inhibit cox-1 and cox-2 to varying degrees.6 Some studies have demonstrated that non-selective NSAIDs increase the hazard for adverse CV outcomes,7–11 while others have documented a reduced frequency of adverse CV events.7, 11–14 The Safety of Non-Steroidal Anti-Inflammatory Drug Project recently concluded that important knowledge gaps exist, particularly with the traditional NSAIDs.15 While the overall association of NSAIDs and CV risk remains uncertain, even less information is known about the potential CV toxicity of NSAIDs among women.16,17 In the United States, all non-selective NSAIDs carry a black box warning about possible CV toxicity (fatal/nonfatal myocardial infarction and fatal/nonfatal stroke), especially among those with existing CV disease,18 while the European Medicines Agency concluded that a small increased risk of CV toxicity cannot be excluded.19
Existing reports have not studied a suitable number of women to determine the potential CV effects of NSAIDs in women. Accordingly, our first objective was to evaluate the association between regular NSAID use and adverse CV outcomes among a large cohort of post-menopausal women. We hypothesized that regular NSAID use is associated with an increased risk of adverse CV outcomes. Our second objective was to evaluate if the association between regular NSAID use and adverse CV events is different for the preparations that differ by relative cox-2 and cox-1 inhibition. We hypothesized that agents with relatively more cox-2 than cox-1 inhibition would be associated with an increased hazard for adverse CV outcomes.
Methods
Study cohort
The Women’s Health Initiative (WHI) study enrolled post-menopausal women 50 to 79 years of age from 40 clinical centers across the United States into one or more randomized clinical trials (n = 68,132) or an observational study (n = 93,676).20 Enrollment was from October 1, 1993, to December 31, 1998. All participants signed consent forms for participation, which were approved by the institutional review boards of collaborating institutions. The combined cohort of all participants (n = 161,808) was selected for these analyses.
Exposure assessment
Medication use was obtained from direct examination of prescription and non-prescription pill bottles that participants brought to clinic visits. 21 Medication generic/trade names were converted into National Drug Codes from the Master Drug Data Base (Medi-Span, Indianapolis, IN). Regular medication use was defined as at least twice per week for the last 2 weeks. The medication inventory was collected at baseline and updated at years 1, 3, 6, and 9 for randomized trial participants and at year 3 for observational study participants.
Any non-aspirin NSAID use was considered as a single group, including selective cox-2 inhibitors and any of the non-selective NSAIDs. In addition, NSAIDs were then classified according to 3 groups based on their relative selectivity of the cox-2 compared with cox-1 isoenzyme. Group 1 included the selective cox-2 inhibitors (rofecoxib and celecoxib). Group 2 included non-selective NSAIDs with relatively more cox-2 than cox-1 inhibition (naproxen, nabumetone, diclofenac, etodolac, piroxicam, sulidac, salsalate, ketorolac, and non-aspirin salicylates, and group 3 included non-selective NSAIDs with relatively more cox-1 than cox-2 inhibition (ibuprofen, oxaprozin, ketoprofen, indomethacin, flurbiprofen, tolmetin, and fenoprofen).22 The above sub-groups and analyses were pre-planned. In addition, we estimated a Cox regression that classified medication usage by type of medication, plus one group for concurrent usage of several NSAIDs.
Outcome assessment
CV outcomes were ascertained by self-administered medical history updates, which were conducted semi-annually for randomized trial participants and annually for observational study participants.23 Participants who were lost to follow-up (for example, from a disabling disease) or suspected to be dead were matched to the National Death Index to confirm occurrence and cause of death.23 Trained physician adjudicators reviewed medical records and death certificates to identify outcomes. Nonfatal MI was defined as a chest pain syndrome with characteristic electrocardiographic changes or enzymatic evidence of myocardial damage (elevated creatine kinase–MB or troponin values). Nonfatal stroke was defined as a rapid onset of a neurological deficit attributable to an obstruction or rupture of an arterial vessel which lasted more than 24 hours or a compatible lesion was documented on an imaging study (computed tomography or magnetic resonance imaging). All participants were followed for outcomes from enrollment until 2005. A subsample of 115,400 women (71.3% of the initial cohort) consented to participate in WHI extension I, and was followed for events from 2005 to 2010.
Potential confounders and effect modifiers
All models were adjusted for time-varying usage of aspirin, acetaminophen, and statin medications, as well as baseline demographic information (age, ethnicity, education, income, region of residence within the United States, body mass index, systolic blood pressure, physical activity level, menopausal hormone use, and smoking status) and comorbidities (hypertension, hypercholesterolemia, diabetes mellitus, myocardial infarction, stroke or transient ischemic attack, congestive heart failure, coronary artery bypass grafting or percutaneous coronary intervention, peripheral arterial disease, and rheumatoid arthritis). Baseline reported history of stomach or duodenal ulcer, bleeding problem, osteoarthritis, and/or cancer were considered as well, but found to not be statistically significantly associated with the primary outcome and were not included in the final models.
We conducted subgroup analyses based on two perceived levels of risk for CV events: those with a history of myocardial infarction, cerebrovascular accident/transient ischemic attack, congestive heart failure, diabetes, or prior coronary artery bypass grafting/percutaneous coronary intervention, and those without any such history. Since the high-risk patients were also likely to be aspirin users, we separately examined a subgroup with no baseline or subsequent use of aspirin and a subgroup with baseline use of aspirin and subsequent use of aspirin (time-varying).
All our Cox-regression models utilize stratification into six strata, one each for the four hormone therapy arms (estrogen alone active treatment and placebo; estrogen-progestin active treatment and placebo; combined n = 27,241), one for the dietary modification (n = 40,654), and one for the observational cohort participants (n = 92,906). The two hormone therapy trials (estrogen alone and estrogen-progestin) had different enrollment criteria (hysterectomy/intact uterus),24,25 therefore, we wanted to distinguish between those two cohorts. In addition, both hormone therapy trials were stopped early for risk, but at different times, and we therefore found it advisable to control for treatment and control arm in each respective hormone therapy trial. Participants for the calcium-vitamin D trial were recruited from hormone therapy and dietary modification participants during the course of those clinical trials, and we did not use this information for additional stratification. In case a woman was enrolled in both a hormone therapy and the dietary modification trial, she was assigned to the appropriate hormone therapy arm for our stratification.
Statistical analysis
We utilized Cox regression (PROC PHREG, SAS software version 9.3, SAS Institute, Inc.; Cary, North Carolina) where the primary outcome/dependent variable was the time to the first occurrence of CV mortality, nonfatal myocardial infarction, or nonfatal stroke, utilizing six strata as detailed in the potential confounders and effect modifiers section above, thus allowing for different baseline hazard function for each of the difference cohorts. This association was reported as hazard ratio (HR) and 95% confidence interval (CI). We also individually investigated time to CV mortality, nonfatal myocardial infarction, nonfatal stroke, congestive heart failure, and all-cause mortality. If the respective specific outcome was not reported for a participant, that woman was censored at the time of death from any cause or at the point in time of the last documented contact. Confounding was controlled by including statistically significant covariates and interaction terms as suggested by a cut-off value of 0.05 for the respective marginal P values together with the model fit criteria Akaike’s information criterion and Schwarz criterion. We assessed co-linearity and over-fitting by observing changes in parameter estimate and their associated confidence intervals for the remaining covariates when including/excluding individual covariates.
Results
Of the 161,808 women enrolled in WHI, 160,801 (99.4%) were available for analysis (Figure 1). At baseline, the 31,433 NSAID users were more likely to be white, overweight or obese, and with higher blood pressure (Table 1). Complete variables and the number of missing values for each variable are available in Supplemental Table 1. Other differences among NSAID users were a higher prevalence of diabetes, peripheral arterial disease, and rheumatoid arthritis.
Figure 1.
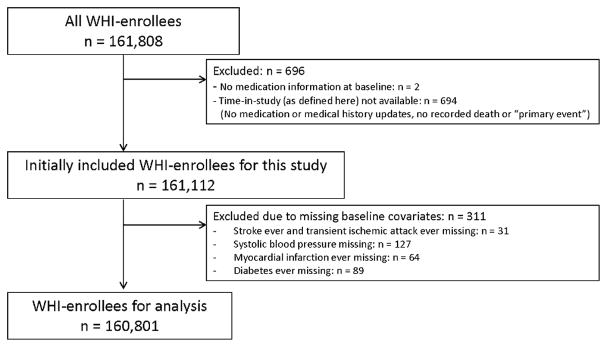
Of all 161,808 Women Health Initiative (WHI) enrollees, 160,801 were utilized in the analysis. Women were only excluded due to missing baseline covariates (n = 311) if the number of women with that missing variable was small. Otherwise, a separate factor level “missing” was maintained in categorical variables.
Table 1.
Baseline Characteristics for Women With Regular Non-Steroidal Anti-Inflammatory Drug (NSAID) Use and Those With No NSAID Use
| Characteristic | NSAID Use at Baseline (n = 31,433) | No NSAID Use at Baseline (n = 129,368) | P Value |
|---|---|---|---|
| Age, mean years (SD) | 63.3 (7.1) | 63.2 (7.2) | 0.689 |
| Age >70 years, % | 21.6 | 22.1 | 0.031 |
| Race/ethnicity, % | |||
| White | 85.2 | 82.1 | <0.001 |
| Black or African American | 8.8 | 9.1 | |
| BMI, mean kg/m2 (SD) | 29.4 (6.4) | 27.6 (5.8) | <0.001 |
| BMI categories, % | |||
| Normal (18.5–24.9) | 26.2 | 35.9 | |
| Overweight (25.0–29.9) | 33.5 | 34.7 | |
| Obesity I (30.0–34.9) | 21.9 | 17.5 | <0.001 |
| Obesity II (35.0– 39.9) | 10.6 | 6.8 | |
| Extreme Obesity III (≥40) | 6.6 | 3.3 | |
| Systolic blood pressure, mm Hg, SD | 128.7 (17.6) | 127.0 (17.8) | <0.001 |
| Systolic blood pressure, % | |||
| ≤120 | 34.7 | 39.4 | |
| 120–140 | 42.6 | 40.4 | <0.001 |
| >140 | 22.8 | 20.2 | |
|
| |||
| History of, % | |||
| Hypertension | 38.1 | 32.4 | <0.001 |
| Hypercholesterolemia | 14.3 | 13.1 | <0.001 |
| Diabetes mellitus | 6.9 | 5.7 | <0.001 |
| Smoking status: | |||
| Never smoked | 48.4 | 50.8 | |
| Past smoker | 43.7 | 41.0 | <0.001 |
| Current smoker | 6.7 | 6.9 | |
| Congestive heart failure | 0.9 | 0.8 | 0.074 |
| Myocardial infarction | 2.3 | 2.3 | 0.479 |
| CABG or PCI | 1.7 | 1.7 | 0.970 |
| Stroke or transient ischemic attack | 3.0 | 3.0 | 0.825 |
| Peripheral arterial disease | 2.6 | 1.9 | <0.001 |
| Gastric or duodenal ulcer | 7.1 | 6.3 | <0.001 |
| Bleeding problem | 2.7 | 2.5 | 0.117 |
| Rheumatoid arthritis | 8.3 | 4.0 | <0.001 |
| Cancer* | 9.6 | 9.1 | 0.010 |
|
| |||
| Medications, % | |||
| Aspirin | 22.2 | 22.7 | 0.036 |
| Acetaminophen | 21.4 | 11.2 | <0.001 |
| Statin | 8.7 | 7.3 | <0.001 |
| Menopausal hormones | 47.4 | 40.8 | <0.001 |
BMI = body mass index, CABG = coronary artery bypass grafting, PCI = percutaneous coronary intervention, SD = standard deviation.
excluding non-melanoma skin cancer
The 160,801 participants contributed a total of 1,793,222 person-years to this study (mean follow-up of 11.2 years). Of this total time, 53,142 women reported regular NSAID use during at least one visit (baseline and post-baseline visits), of which 39,613 women also reported at least some time without regular NSAID usage. Table 2 summarizes the use of specific types of NSAID at baseline and during follow-up. Of 12,720 women reporting use of a group 2 NSAID at baseline, 14% report later use of a group 1 NSAID, 13% use of a group 3 NSAID, and 52% report no later NSAID use. Likewise, of the 19,817 women reporting use of a group 3 NSAID at baseline, 10% later report use of a group 1 NSAID, 13% use of a group 2 NSAID, and 60% report no later NSAID use. Some women reported concurrent use of NSAIDs from more than one group (at baseline, 1,104 women reported NSAID use from both groups 2 and 3).
Table 2.
Number of Women Reporting Non-Steroidal Anti-Inflammatory Drug (NSAID) Use at Baseline and During Any Point in the Study
| NSAID | Baseline | Any time |
|---|---|---|
| Celecoxib | 0 | 5,274 |
| Rofecoxib | 0 | 4,321 |
|
| ||
| Naproxen | 5,625 | 12,347 |
| Nabumetone | 2,081 | 3,615 |
| Diclofenac | 1829 | 3,483 |
| Etodolac | 1027 | 1,720 |
| Piroxicam | 936 | 1,360 |
| Sulindac | 667 | 1,132 |
| Salsalate | 562 | 894 |
| Ketorolac | 176 | 315 |
| non-aspirin salicylates | 22 | 42 |
|
| ||
| Ibuprofen | 17,108 | 28,118 |
| Oxaprozin | 1,055 | 1,730 |
| Ketoprofen | 842 | 1,164 |
| Indomethacin | 535 | 825 |
| Flurbiprofen | 327 | 400 |
| Tolmetin | 117 | 152 |
| Fenoprofen | 53 | 60 |
Multiple concurrent usages are counted in each of the respective categories such that groups are not mutually exclusive.
The primary outcome (CV mortality, nonfatal myocardial infarction, or nonfatal stroke) was observed in 12,733 cases with an overall incidence rate of 71 events per 10,000 person-years. The unadjusted HR associated with any type of NSAID usage was 1.16 (95% CI 1.11–1.21; P<0.001), and the adjusted HR was 1.10 (95% CI 1.06–1.15; P<0.001) (Table 3). We re-estimated our Cox model for the primary outcome based on the subset of women enrolled in one of the clinical trials (with more frequent medication updates); HR for any NSAID usage was 1.11 (95% CI 1.04–1.18). Likewise, when we censored all women 3 years after their last medication update we estimated the HR for any NSAID usage as 1.11 (95% CI 1.05–1.18).
Table 3.
Association of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) for the Primary Outcome (Cardiovascular Death, Nonfatal Myocardial Infarction, or Nonfatal stroke)
| Exposure | Person-time (years) | # events (cases) | Incidence rate (per 100 person-years) | Hazard Rate (covariate-adjusted Cox regression)* | |
|---|---|---|---|---|---|
| HR (95% CI) | P Value | ||||
| Group 1 NSAID (cox-2 selective agents) | 1.13 (1.04–1.23) | 0.004 | |||
|
| |||||
| Celecoxib only | 29,344 | 317 | 1.08 | 1.13 (1.01–1.27) | 0.031 |
| Rofecoxib only | 23,835 | 240 | 1.01 | 1.14 (1.00–1.29) | 0.055 |
|
| |||||
| Group 2 NSAID (non-selective agents with cox-2>cox-1 inhibition) | 1.17 (1.10–1.24) | <0.001 | |||
|
| |||||
| Naproxen only | 58,623 | 530 | 0.90 | 1.22 (1.12–1.34) | <0.001 |
| Nabumetone only | 16,580 | 148 | 0.89 | 1.14 (0.97–1.34) | 0.118 |
| Diclofenac only | 18,226 | 165 | 0.91 | 1.15 (0.99–1.35) | 0.070 |
| Etodolac only | 7,591 | 61 | 0.80 | 1.01 (0.78–1.30) | 0.963 |
| Piroxicam only | 6,536 | 63 | 0.96 | 1.19 (0.93–1.53) | 0.168 |
| Sulindac only | 5,860 | 61 | 1.04 | 1.15 (0.89–1.47) | 0.292 |
| Salsalate only | 4,209 | 44 | 1.05 | 1.29 (0.96–1.74) | 0.090 |
| Ketorolac only | 924 | 16 | 1.73 | 1.86 (1.14–3.04) | 0.013 |
| non-aspirin salicylates only | 137 | 1 | 0.72 | 0.96 (0.14–6.82) | |
|
| |||||
| Group 3 NSAID (non-selective agents with cox-1>cox-2 inhibition) | 1.01 (0.95–1.07) | 0.884 | |||
|
| |||||
| Ibuprofen only | 14,7006 | 914 | 0.62 | 1.00 (0.93–1.07) | 0.996 |
| Oxaprozin only | 7,133 | 35 | 0.49 | 0.64 (0.46–0.89) | 0.008 |
| Ketoprofen only | 4,131 | 42 | 1.02 | 1.47 (1.08–1.99) | 0.013 |
| Indomethacin only | 3,713 | 38 | 1.02 | 1.23 (0.89–1.69) | 0.206 |
| Flurbiprofen only | 1,665 | 17 | 1.02 | 1.66 (1.03–2.67) | 0.038 |
| Tolmetin only | 668 | 11 | 1.65 | 1.79 (0.99–3.24) | 0.053 |
| Fenoprofen only | 250 | 1 | 0.40 | 0.49 (0.07–3.50) | 0.478 |
|
| |||||
| Concurrent use >1 agent | 14,667 | 111 | 0.76 | 1.07 (0.89–1.30) | 0.456 |
| None of the above | 1,442,116 | 9,918 | 0.69 | 1 (comparison) | – |
|
| |||||
| Total | 1,793,222 | 12,733 | 0.71 | ||
Individual agents are mutually exclusive exposure groups. Concurrent use of more than one agent is considered as a separate group
Covariates included in the final model of the primary outcome are (P value): age (<0.001), ethnicity (<0.001), education (0.022), income (<0.001), region of residence within the United States (0.045), body mass index (0.0002), systolic blood pressure (<0.001), physical activity (<0.001), history of rheumatoid arthritis (<0.001), hypertension (<0.001), high cholesterol (0.0009), diabetes (<0.001), myocardial infarction (<0.001), stroke or transient ischemic attack (<0.001), congestive heart failure (<0.001), coronary artery bypass grafting or percutaneous coronary intervention (<0.001), peripheral arterial disease (<0.001), smoking status (<0.001), menopausal hormone use (0.001), statin use (time-varying covariate, 0.025), acetaminophen use (time-varying, 0.015), and aspirin use (time-varying, 0.025). In addition, we included an interaction term between aspirin and menopausal hormones (0.005) because of improved model fit as indicated by Akaike’s information criterion.
We replaced usage of any NSAID in the above model by three time-varying indicators signifying usage according to our pre-defined NSAID groups (Figure 2, which also shows results for secondary outcomes). Among the selective cox-2 inhibitors, HR=1.13 (95% CI 1.04–1.23; P=0.004), non-selective agents with cox-2>cox-1 inhibition group, HR=1.17 (95% CI 1.10–1.24; P<0.001), and non-selective agents with cox-1>cox-2 inhibition, HR=1.01 (95% CI 0.95–1.07; P=0.884). In addition, we estimated HRs associated with specific NSAID type (time-varying), as detailed in Table 3. Celecoxib-only HR was 1.13 (1.01–1.27), P=0.031; naproxen-only HR was 1.22 (1.12–1.34), P<0.001; and the ibuprofen-only HR was 1.00 (0.93–1.07), P=0.996.
Figure 2.
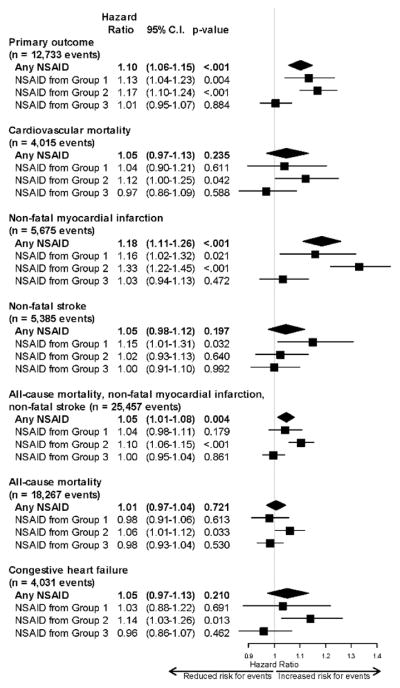
Adjusted hazard ratios for the primary outcome (cardiovascular mortality, nonfatal myocardial infarction, or nonfatal stroke) and secondary outcomes for regular NSAID use versus no NSAID use. Group 1=cox-2 selective agents, group 2=non-selective agents with cox-2>cox-1 inhibition, group 3=non-selective agents with cox-1>cox-2 inhibition.
The group with a history of myocardial infarction, cerebrovascular accident/transient ischemic attack, congestive heart failure, diabetes, or prior coronary artery bypass grafting/percutaneous coronary intervention experienced 3,241 events and did not show an increased hazard with regular NSAID usage (HR=1.00; 95% CI 0.92–1.09; P=0.961). However, the group without any of these prior diagnoses and conditions experienced 9,312 events and maintained a statistically significantly increased hazard with regular NSAID usage (HR=1.14; 95% CI 1.08–1.20; P<0.001).
Among aspirin users who also took NSAIDs, we observed the following associations with NSAID use: group 1 agents (HR=0.90; 95% CI 0.76–1.07; P=0.22), group 2 agents (HR=1.17; 95% CI 1.04–1.31; P=0.008), and group 3 agents (HR=0.97; 95% CI 0.87–1.08; P=0.58). Among non-aspirin users, we observed the following associations with NSAID use: group 1 agents (HR=1.24; 95% CI 1.12–1.36; P<0.0001), group 2 agents (HR=1.18; 95% CI 1.10–1.27; P<0.0010), and group 3 agents (HR=1.02; 95% CI 0.95–1.10; P=0.59).
Discussion
In a large cohort of post-menopausal women, regular NSAID use was associated with a modestly increased hazard for CV mortality, nonfatal myocardial infarction, or nonfatal stroke. Such an increased risk was observed among selective cox-2 inhibitors (e.g., rofecoxib and celecoxib) and non-selective NSAIDs with relatively more cox-2 than cox-1 inhibition (e.g., naproxen). Non-selective NSAIDs with relatively more cox-1 than cox-2 inhibition (e.g., ibuprofen) were not associated with increased risk. While the hazard for the primary outcome among non-selective NSAIDs with relatively more cox-2 than cox-1 inhibition was slightly larger than the hazard for cox-2 selective agents, we interpret the magnitude of risk with these 2 classes of agents to be similar. However, cox-2 selective agents appeared to be associated with a greater hazard for stroke, while non-selective NSAIDs with relatively more cox-2 than cox-1 inhibition were associated with a greater hazard for myocardial infarction. The risk for CV mortality and all-cause mortality was marginally increased only among agents with relatively more cox-2 than cox-1 inhibition.
Subgroup analysis was performed according to aspirin use. The results largely mirrored the main study findings, except for concomitant users of selective cox-2 inhibitors. Among aspirin users, concomitant selective cox-2 inhibitors were no longer associated with increased hazard for CV events, although they were still associated with increased hazard for CV events among non-aspirin users. This observation is consistent with the findings from a platelet function study which found that rofecoxib did not affect the pharmacodynamics of aspirin.26
Our approach to examine medication use as a time-varying covariate is a distinct advantage over analyses that only examined baseline medication use. In our approach, participants could ‘migrate’ to other risk sets during the course of follow-up. For example, a given participant could first contribute time in the risk set of ‘regular NSAID use’, then contribute time in the risk set of ‘no NSAID use’, then possibly contribute time again in the risk set of ‘regular NSAID use’. In fact, we observed significant migration to and from NSAID use and also between types of NSAIDs, which supports our time-varying methodology. Likewise, we also included other important medications such as aspirin as a time-varying covariate. At the time women were enrolled in WHI (i.e. between 1983–88), selective cox-2 inhibitors were not in clinical use; however, over time participants reported use of both celecoxib and rofecoxib. Since we performed a time-varying analysis, these women were still able to contribute person-time data to the model. Both of these agents were associated with the same magnitude of hazard for increased CV events. This is consistent with other reports that have documented a class effect among the selective cox-2 inhibitors.27
Counter to previous reports,2,10,28–32 naproxen was associated with increased risk, while ibuprofen was not. This was consistent with our a priori study hypothesis. This finding is also consistent with a clinical trial designed to prevent Alzheimer’s dementia with the use of NSAIDs. This study was terminated early due to possibly increased risk for adverse CV events among naproxen versus placebo users.33 Other agents within group 2 were also directionally associated with risk increase; however, only ketorolac reached statistical significance. Associations within group 3 ranged from statistically significant decreased risk (e.g., oxaprozin) to increased risk (e.g., ketoprofen and flurbiprofen). However, these were based upon very few events and these findings have to be interpreted with caution in this observational study.
A recent meta-analysis of randomized trials documented an increased hazard for major vascular events with high-dose diclofenac and coxib medications, but not with high-dose naproxen.34 The reason for the differences in these study findings is not known. One potential explanation why naproxen was not observed to be associated with increased hazard for CV events in the meta-analysis is that high dose naproxen (e.g., 500 mg twice daily) is able to produce an aspirin-like effect through near-complete inhibition of cox-1.35 Although we did not have data on medication dosage in the current analysis, naproxen use in WHI was likely closer to 220 mg twice daily. With a lower dosage (and less frequent use) of naproxen, inhibition of cox-2 compared with cox-1 might become more pronounced and allow for more platelet aggregation to occur.7,35 There may also be variability in the hazardous effects of these medications according to duration of use (median follow-up >10 years in WHI) and gender which was exclusively women in WHI.
We recognize that elevations in blood pressure could be caused by regular NSAID use and thus could be viewed as a biological intermediate in the causal pathway of NSAID usage to the primary event. To investigate potential “over-control” for this risk factor, we estimated models with and without history of hypertension and systolic blood pressure, and the resulting differences on the NSAID related estimates were very small. Accordingly, we decided to keep these variables in the overall model. Clearly, high blood pressure can have causes other than NSAID usage, and harmful effects of NSAIDs could be influenced by blood pressure independent pathways (e.g., platelet aggregation).
Limitations
The classification of NSAIDs according to relative inhibition of cox-2 compared with cox-1 was motivated by an ex-vivo study performed in healthy volunteers.22 It is unknown if similar findings would be observed among individuals with established atherosclerosis; however, this constituted a minority of the WHI cohort.
We did not have information on when aspirin or non-aspirin NSAIDs were taken in relation to each other. This could be important since ibuprofen has been shown to block the anti-platelet effect of aspirin when taken before this agent.26 We did not have information of dosage of NSAIDs; therefore, our findings apply to NSAID use in a general sense. Women who participated in the different components of WHI may systematically differ in their respective risk for CV events, due to different exclusion criteria in the WHI clinical trials. However, we allowed for that possibility by stratifying our Cox regression on WHI trial membership. Our statistical model only required that all women share the same relative effect of a covariate (including NSAID usage) across strata, with possibly different baseline hazard functions for CV events.
The available medication information is detailed, but sparsely collected, especially for the women in the observational component of WHI who were only sampled at baseline and year 3. We implicitly assumed that a woman’s medication did not change between the actually recorded medication updates. This was an assumption that was increasingly questionable the less frequently the medication information was updated. This motivated the sensitivity analyses to restrict to randomized trial participants with more frequent medication history updates as well as censor all women 3 years after their last medication update. We found that NSAID medication was not stable over a long period of time with migration to nonuse and to other types of NSAIDs. This finding supports our assertion that NSAID use was best modeled as a time-varying covariate.
We did not have outcome information on acute renal failure, renal function, or gastrointestinal hemorrhage which are all possible mechanisms by which NSAIDs could exert harmful effects. Congestive heart failure was recorded; however, this was not as rigorously adjudicated as other WHI outcomes. Selection bias was mitigated by including a “missing” group in categorical covariates whenever feasible that allowed us to keep women with partially missing data in this study, a strategy that allowed us to utilize 160,801 out of the 161,808 WHI enrollees (99.4%). This, of course, does not control for any bias that might be inherent in the group of women enrolled in WHI.
Conclusions
The regular use of selective cox-2 inhibitors and non-selective NSAIDs with cox-2>cox-1 inhibition showed a modestly increased hazard for CV events. Concomitant aspirin attenuated the increased hazard for CV events associated with selective cox-2 inhibitors, but not non-selective NSAIDs with cox-2>cox-1 inhibition. We did not identify greater CV risk for medications with more cox-1>cox-2 inhibition. As such, these agents may be safer from a CV perspective for long-term use among post-menopausal women.
Supplementary Material
Acknowledgments
Funding Sources
The WHI Extension 2010–15 is funded by the National Heart Lung and Blood Institute (NHLBI), National Institutes of Health (NIH), United States Department of Health and Human Services (HHSN268201100046C, HSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C).
Footnotes
Disclosures
Dr Bavry is a contractor for the American College of Cardiology's Cardiosource. The other authors report no conflicts.
References
- 1.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. New Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 2.Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:2021–2029. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- 3.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randomised trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 5.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 6.Van Hecken A, Schwartz JI, Depre M, De Lepeleire I, Dallob A, Tanaka W, Wynants K, Buntinx A, Arnout J, Wong PH, Ebel DL, Gertz BJ, De Schepper PJ. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol. 2000;40:1109–1120. [PubMed] [Google Scholar]
- 7.Fosbol EL, Folke F, Jacobsen S, Rasmussen JN, Sorensen R, Schramm TK, Andersen SS, Rasmussen S, Poulsen HE, Kober L, Torp-Pedersen C, Gislason GH. Cause-specific cardiovascular risk associated with nonsteroidal antiinflammatory drugs among healthy individuals. Circ Cardiovasc Qual Outcomes. 2010;3:395–405. doi: 10.1161/CIRCOUTCOMES.109.861104. [DOI] [PubMed] [Google Scholar]
- 8.Garcia Rodriguez LA, Gonzalez-Perez A, Bueno H, Hwa J. NSAID use selectively increases the risk of non-fatal myocardial infarction: a systematic review of randomised trials and observational studies. PLoS One. 2011;6:e16780. doi: 10.1371/journal.pone.0016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bavry AA, Khaliq A, Gong Y, Handberg EM, Cooper-Dehoff RM, Pepine CJ. Harmful effects of NSAIDs among patients with hypertension and coronary artery disease. Am J Med. 2011;124:614–620. doi: 10.1016/j.amjmed.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh G, Wu O, Langhorne P, Madhok R. Risk of acute myocardial infarction with nonselective non-steroidal anti-inflammatory drugs: a meta-analysis. Arthritis Res Ther. 2006;8:R153. doi: 10.1186/ar2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 12.Kimmel SE, Berlin JA, Reilly M, Jaskowiak J, Kishel L, Chittams J, Strom BL. The effects of nonselective non-aspirin non-steroidal anti-inflammatory medications on the risk of nonfatal myocardial infarction and their interaction with aspirin. J Am Coll Cardiol. 2004;43:985–990. doi: 10.1016/j.jacc.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 13.Ko D, Wang Y, Berger AK, Radford MJ, Krumholz HM. Nonsteroidal antiinflammatory drugs after acute myocardial infarction. Am Heart J. 2002;143:475–481. doi: 10.1067/mhj.2002.121270. [DOI] [PubMed] [Google Scholar]
- 14.Fischer LM, Schlienger RG, Matter CM, Jick H, Meier CR. Discontinuation of nonsteroidal anti-inflammatory drug therapy and risk of acute myocardial infarction. Arch Intern Med. 2004;164:2472–2476. doi: 10.1001/archinte.164.22.2472. [DOI] [PubMed] [Google Scholar]
- 15.Salvo F, Fourrier-Reglat A, Bazin F, Robinson P, Riera-Guardia N, Haag M, Caputi AP, Moore N, Sturkenboom MC, Pariente A. Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89:855–866. doi: 10.1038/clpt.2011.45. [DOI] [PubMed] [Google Scholar]
- 16.Chan AT, Manson JE, Albert CM, Chae CU, Rexrode KM, Curhan GC, Rimm EB, Willett WC, Fuchs CS. Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation. 2006;113:1578–1587. doi: 10.1161/CIRCULATIONAHA.105.595793. [DOI] [PubMed] [Google Scholar]
- 17.Garcia Rodriguez LA, Varas C, Patrono C. Differential effects of aspirin and non-aspirin nonsteroidal antiinflammatory drugs in the primary prevention of myocardial infarction in postmenopausal women. Epidemiology. 2000;11:382–387. doi: 10.1097/00001648-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Post-authorisation evaluation of medicines for human use. London: Nov 6, 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/01/WC500054344.pdf. [Google Scholar]
- 20.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 22.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 23.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 24.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 26.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, Vyas SN, FitzGerald GA. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 27.Andersohn F, Suissa S, Garbe E. Use of first- and second-generation cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs and risk of acute myocardial infarction. Circulation. 2006;113:1950–1957. doi: 10.1161/CIRCULATIONAHA.105.602425. [DOI] [PubMed] [Google Scholar]
- 28.Schjerning Olsen AM, Fosbol EL, Lindhardsen J, Folke F, Charlot M, Selmer C, Lamberts M, Bjerring Olesen J, Kober L, Hansen PR, Torp-Pedersen C, Gislason GH. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation. 2011;123:2226–2235. doi: 10.1161/CIRCULATIONAHA.110.004671. [DOI] [PubMed] [Google Scholar]
- 29.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Juni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson DJ, Rhodes T, Cai B, Guess HA. Lower risk of thromboembolic cardiovascular events with naproxen among patients with rheumatoid arthritis. Arch Intern Med. 2002;162:1105–1110. doi: 10.1001/archinte.162.10.1105. [DOI] [PubMed] [Google Scholar]
- 31.Rahme E, Pilote L, LeLorier J. Association between naproxen use and protection against acute myocardial infarction. Arch Intern Med. 2002;162:1111–1115. doi: 10.1001/archinte.162.10.1111. [DOI] [PubMed] [Google Scholar]
- 32.Solomon DH, Glynn RJ, Levin R, Avorn J. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med. 2002;162:1099–1104. doi: 10.1001/archinte.162.10.1099. [DOI] [PubMed] [Google Scholar]
- 33.ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer’s Disease Anti-Inflammatory Prevention Trial (ADAPT) PLoS Clin Trials. 2006;1:e33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhala N, Emberson J, Merhi A, et al. Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capone ML, Tacconelli S, Sciulli MG, Grana M, Ricciotti E, Minuz P, Di Gregorio P, Merciaro G, Patrono C, Patrignani P. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation. 2004;109:1468–1471. doi: 10.1161/01.CIR.0000124715.27937.78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


