Abstract
Organic anion transporter 3 (OAT3, SLC22A8), a transporter expressed on the basolateral membrane of the proximal tubule, plays a critical role in the renal excretion of organic anions including many therapeutic drugs. The goal of this study was to evaluate the in vivo effects of the OAT3-Ile305Phe variant (rs11568482), present at 3.5% allele frequency in Asians, on drug disposition with a focus on cefotaxime, a cephalosporin antibiotic. In HEK293- Flp-In cells, the OAT3-Ile305Phe variant had a lower maximum cefotaxime transport activity, Vmax, [159 ± 3 nmol*(mg protein)−1/min (mean ± SD)] compared with the reference OAT3 [305 ± 28 nmol*(mg protein)−1/min, (mean ± SD), p < 0.01], whereas the Michaelis-Menten constant values (Km) did not differ. In healthy volunteers, we found volunteers that were heterozygous for the Ile305Phe variant and had a significantly lower cefotaxime renal clearance (CLR; mean ± SD: 84.8 ± 32.1 mL/min, n = 5) compared with volunteers that were homozygous for the reference allele (158 ± 44.1 mL/min, n = 10; p = 0.006). Furthermore, the net secretory component of cefotaxime renal clearance (CLsec) was reduced in volunteers heterozygous for the variant allele [33.3 ± 31.8 mL/min (mean ± SD)] compared with volunteers homozygous for the OAT3 reference allele [97.0 ± 42.2 mL/min (mean ± SD), p = 0.01]. In summary, our study suggests that a low-frequency reduced-function polymorphism of OAT3 associates with reduced cefotaxime CLR and CLsec.
Keywords: Organic anion transporter, cefotaxime, tubular secretion, OAT3, polymorphisms, pharmacogenomics, pharmacokinetics, renal clearance
INTRODUCTION
OAT1 (SLC22A6) and OAT3 (SLC22A8) are well-studied organic anion transporters known to reabsorb and secrete endogenous organic anions (e.g., pyruvate, α-ketoglutarate, oxalate, urate, and estrone sulfate) and exogenous anions (e.g., methotrexate, ciprofloxacin, acyclovir, captopril, and thiazides) across the basolateral membrane of the proximal tubule.1–4 In vivo studies in Oat3 knockout mice demonstrate the importance of OAT3 in the renal elimination of drugs. The area under the plasma concentration time curve (AUC) is increased, and the renal clearance (CLR) of drugs such as ciprofloxacin,5 methotrexate,6 and penicillin7 is reduced in Oat3 knockout mice compared with wild-type mice. Importantly, organic-anion transporter-mediated drug-drug interactions in the kidney have been described in several clinical studies. In particular, administration of inhibitors of OATs, such as probenecid, may result in increased plasma levels and reduced clearances of drugs including furosemide and ciprofloxacin.8,9 Despite the importance of OAT3 in the pharmacokinetics of many drugs, limited data on the functional impact of genetic polymorphisms in human SLC22A8 (OAT3) exist. Nonsynonymous variants in SLC22A8 are uncommon,10, 11 whereas variants are more common in the promoter regions of the gene encoding OAT3.12, 13 To date, pharmacogenomic studies have examined the effect of SLC22A8 variants on drug disposition and response, such as the pharmacokinetics of pravastatin,14 CLR of torsemide,15 and blood pressure response to hydrochlorothiazide.16 However, none of these studies have demonstrated significant associations of synonymous or intronic variants in SLC22A8 with drug response phenotypes. Studies conducted previously in our laboratory have revealed five less common SLC22A8 variants with allele frequencies of less than 5% in a particular ethnic group, which result in loss of uptake transport.10 Four of them were singletons for which only one individual in the cohort carries the variant. A non- synonymous variant, Ile305Phe (3.5% frequency in Asian-Americans), exhibited functional changes in in vitro assays.10 This variant was also reported in the 1000 Genome Project, exclusively in individuals of Asian ancestry (e.g., allele frequencies 3.6%–6.0% among Japanese and Chinese).17 Based on the 1000 Genome Project and our discovery cohort (http://pharmacogenetics.ucsf.edu/), other reduced-function variants with allele frequencies of greater than 3% have not been described. Hence, the current study focused on the effect of the OAT3 Ile305Phe variant on drug disposition.
Cefotaxime is a third-generation cephalosporin anti-bacterial drug commonly used to treat upper respiratory tract infections and bacterial meningitis.18 Approximately 40%–60% of cefotaxime is excreted unchanged in the urine.19, 20 Cefotaxime belongs to class 3 of the Biopharmaceutics Drug Disposition Classification System (BDDCS), which are characterized by high water solubility, poor passive permeability, and poor metabolism (<70% metabolism).21 For example, these drugs generally require transporters to permeate plasma membranes. The CLR of cefotaxime exceeds creatinine clearance (CLCR), suggesting active tubular secretion.22 Cefotaxime is known to inhibit the uptake of estrone sulfate (Ki = 290 µM) in OAT3 overexpressing cells, and the inhibition is more selective for OAT3 compared with OAT1 and OAT4.23 The goals of this study were twofold: to determine the effect of OAT3 and the variant Ile305Phe on the uptake of cefotaxime in HEK293-Flp-in stable cells and to determine the effect of the OAT3 variant on the pharmacokinetics of cefotaxime in healthy Asian-Americans.
RESULTS AND DISCUSSION
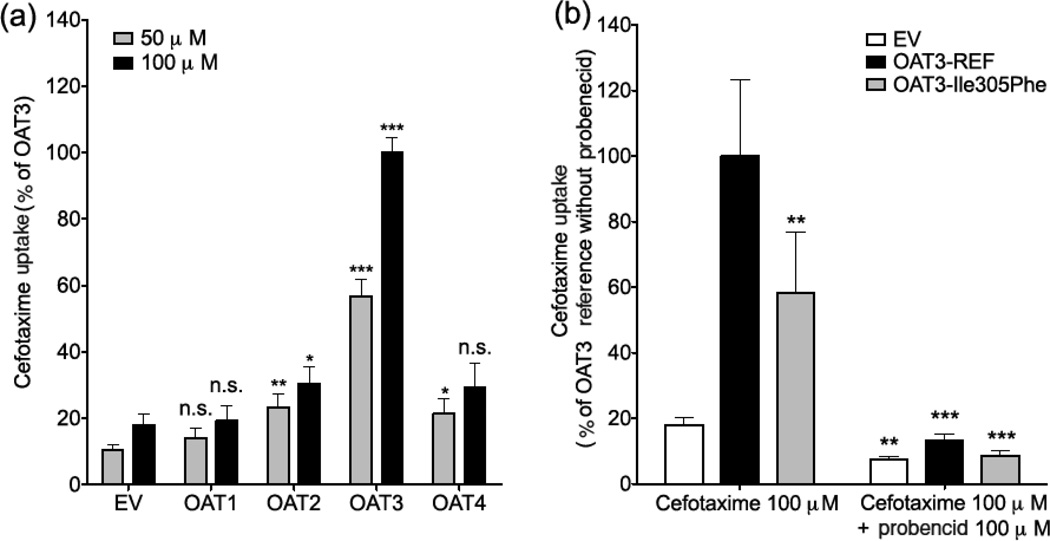
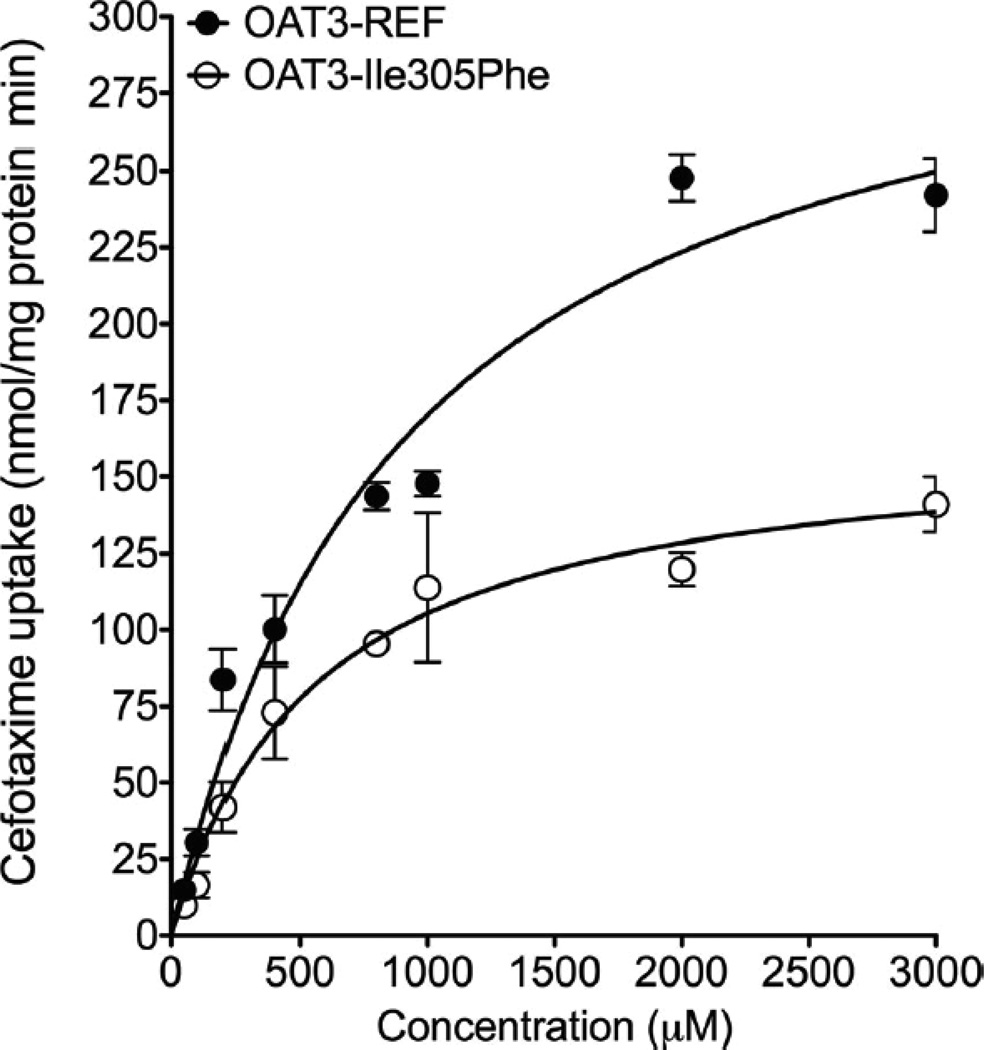
In vitro cefotaxime transport was measured using HEK293-Flp-in cells stably transfected with empty vector (EV), reference SLC22A8 cDNA or with OAT3-Ile305Phe variant cDNA, reference SLC22A6 (OAT1), SLC22A7 (OAT2), and SLC22A11 (OAT4) cDNA subcloned into the mammalian expression vector pcDNA5/FRT. These cell lines, which were previously generated in our laboratory,10, 24–26 took up radiolabeled model substrates of organic anion transporters (Fig. S1). Cefotaxime uptake studies were performed at 37°C for 5 min, which was in the linear portion of the uptake versus time curve (data not shown). Results showed that cefotaxime had a greater uptake (5–10-fold) in HEK293-Flp-in cells transfected with OAT3 compared with EV (Fig. 1). Interestingly, HEK293-Flp-in cells transfected with OAT2 and OAT4 showed weak fold uptake (approx. twofold) of cefotaxime compared with EV-transfected cells, whereas OAT1 expressing cells did not take up cefotaxime significantly (Fig. 1a). OAT3-Ile305Phe expressing cells had lower fold uptake of cefotaxime compared with OAT3 reference cells (Fig. 1b, p < 0.01), and probenecid (100 µM), an inhibitor of OAT3 and other organic anion transporters, significantly inhibited uptake. Kinetic studies revealed that (1) the rate of cefotaxime uptake by both reference and variant of OAT3 was saturable; and (2) the mean ± SD of the Km and maximum cefotaxime transport activity (Vmax) for OAT3 reference were 717 ± 141 µM and 305 ± 28 nmol/mg protein/min, respectively. For OAT3-Ile305Phe, the Km and Vmax were 549 ± 21 µM and 159 ± 3 nmol/mg protein/min, respectively (Fig. 2). The Vmax, but not the Km, of the variant was significantly lower than that of the reference (p < 0.01). Previous studies of chimeric GFP-tagged transporters suggested that both OAT3 and OAT3-Ile305Phe had similar localization patterns.10 However, because of limitations in our previous studies, which used confocal microscopy in transiently transfected cells, we conducted cell surface biotinylation studies in stably transfected cells (see Supplementary Information). The studies showed that HEK293-Flp-In cells stably expressing the OAT3 variant Ile305Phe had lower cell surface protein levels (36% lower) compared with cells expressing OAT3 reference (Fig. S2a). The SLC22A8 mRNA levels in these two cell lines were similar (Fig. S2b). Consistent with the lower surface expression, the cells with OAT3-Ile305Phe variant showed lower uptake of four OAT3 substrates (Figs. S1 and S3). The methods used in the in vitro uptake studies and analytical method to measure cefotaxime levels in HEK293-Flp-in cells using liquid chromatography–tandem mass spectrometry (LC–MS/MS) are available in the Supplementary Information.
Figure 1.
Uptake of cefotaxime in HEK293 Flp-In cells expressing organic anion transporters (OATs). (a) Uptake of cefotaxime (50 µM and 100 µM) in HEK293 Flp-In cells expressing human OAT1, OAT2, OAT3 and OAT4. The cells were exposed to cefotaxime for 5 minutes. Significantly greater cefotaxime (50 µM and 100 µM) uptake was observed in HEK293-Flp-In cells transfected with OAT3 compared to cells transfected with empty vector (EV), OAT1, OAT2 and OAT4 (p< 0.005). Asterisks indicate significantly different uptake values from EV controls. Note: * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.005, n.s. not-significant. Multiple comparisons were analyzed using one-way analysis of variance followed by Dunnett’s two-tailed test. Data are shown as mean ± SD from 2 repeated experiments, and each experiment was performed in triplicate. Results are expressed as the percent of activity of the OAT3-reference. (b) Uptake of cefotaxime (100 µM) with and without the OAT inhibitor, probenecid (100 µM), in HEK293 Flp-In cells expressing OAT3 reference (OAT3-REF) and OAT3 variant, Ile305Phe. The cells were exposed to cefotaxime with or without probenecid for 5 minutes. The result showed significantly reduced cefotaxime uptake in cells transfected with OAT3 Ile305Phe compared to OAT3 reference, ** p-value < 0.01. In the presence of probenecid, cefotaxime uptake in cells transfected with empty vector (EV), OAT3 reference (OAT3-REF) or OAT3 variant (OAT3-Ile305Phe), were significantly reduced compared to cells without probenecid, ** p-value < 0.01, *** p-value < 0.005. Data are shown as mean ± SD from 2 repeated experiments and each experiment was performed in triplicate. Results are expressed as the percent of activity of the OAT3-reference (OAT3-REF).
Figure 2.
Cefotaxime kinetics in HEK293 Flp-In cells expressing OAT3 reference (OAT3-REF) and its variant (OAT3-Ile305Phe). In the kinetic studies, uptake rates were determined at 5 minutes. The mean ± SEM Km and Vmax of the OAT3-reference were 717 ± 141 µM and Vmax = 305 ± 28 nmol/mg protein/min respectively; the Km and Vmax of the OAT3-Ile305Phe were 549 ± 21 µM and 159 ± 3 nmol/mg protein/min respectively. The Vmax for the variant was significantly lower than that of reference (p < 0.01), while the Km value was not significantly different between the reference and the variant. Data points in the figure represent the mean ± SD from triplicate wells in a representative experiment. Two separate experiments were performed and the overall results showed that the Vmax for the variant was significantly lower than that of reference (p<0.05) and no significant difference between the Km values.
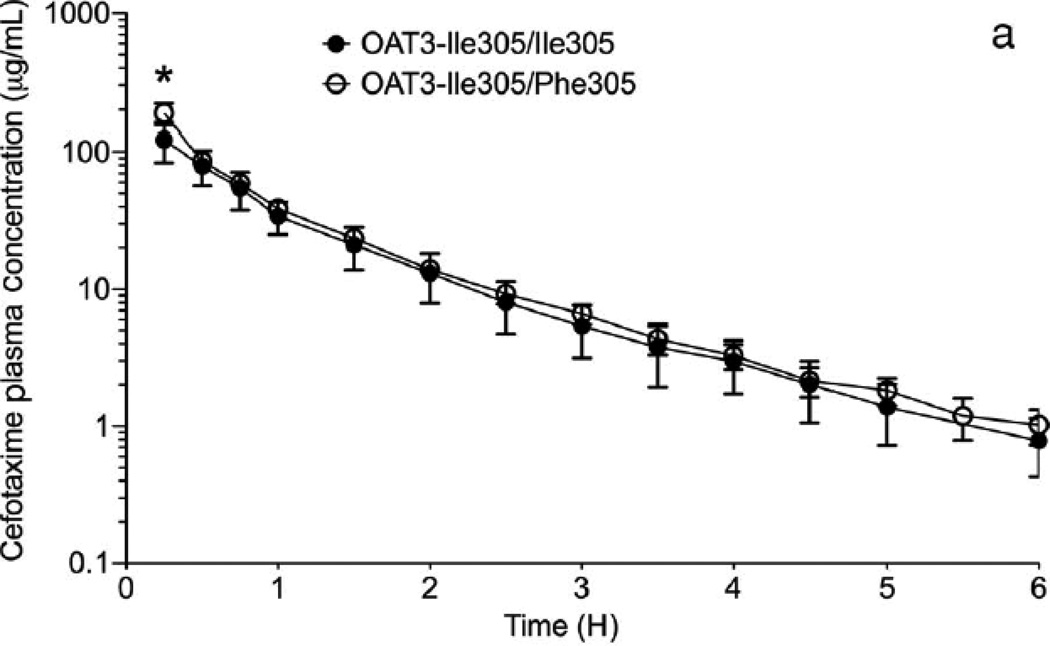
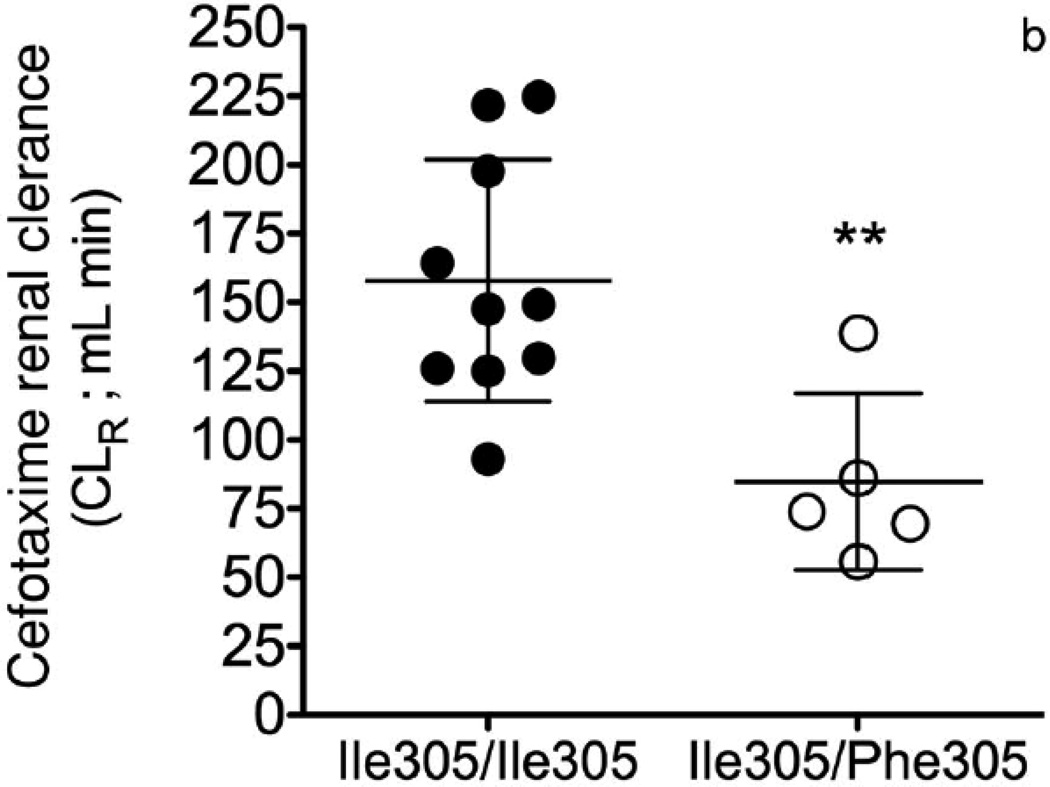
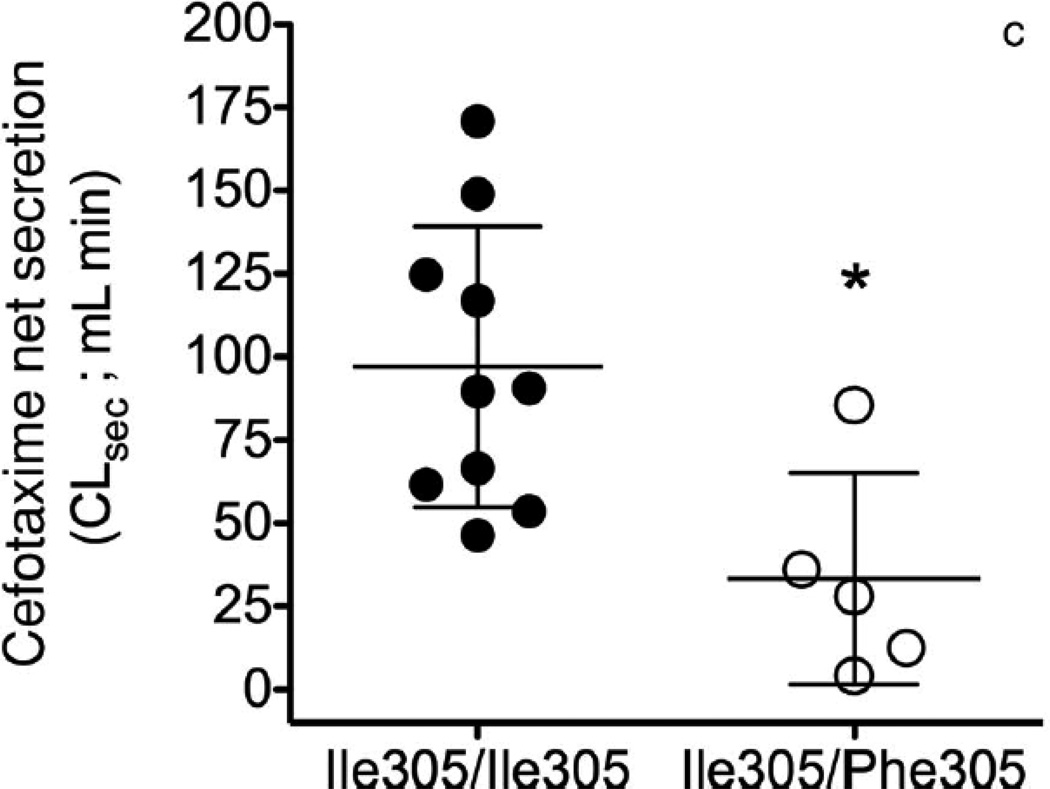
The collective information from the in vitro studies suggested that OAT3-Ile305Phe might affect the disposition of cefotaxime in humans. Our Pharmacogenomics of Membrane Transporters (PMT) group has led several genotype-to-phenotype clinical studies focused on transporter variants.27–31 The goal of the clinical studies is to test hypotheses about the effects of genetic variants in transporters on clinical pharmacokinetics and pharmacodynamics. In this clinical study, participants carrying the OAT3 variant Ile305Phe were identified by direct sequencing of exon 7 (NM 004254.2) and 100 bp of flanking intronic region of this exon. After informed consent was obtained, healthy females (age 18–40 years old) with known OAT3 genotypes were recruited into this open label study (NCT00187655). Subjects were admitted to the Clinical and Translational Science Institute (CTSI) Clinical Research Center at San Francisco General Hospital in a fasting state and remained at this center for the duration of the study. Blood samples were collected at multiple time points up to 10 h and urine samples were collected before cefotaxime administration and starting 2 h after administration. The methods for the sequencing, clinical study design, clinical sample collection, and analytical procedures for measuring cefotaxime in human plasma and urine by LC–MS/MS method are available in the Supplementary Information. The pharmacokinetics of cefotaxime was evaluated in 10 volunteers homozygous for the common reference allele, Ile305, of OAT3, and five volunteers heterozygous for the uncommon variant Phe305. Demographic and baseline characteristics (height, weight, age, and serum creatinine) of volunteers in the two genotype groups were similar (p > 0.2, Student’s unpaired t-test; Table S1). The cefotaxime plasma concentration–time profile was evaluated by noncompartmental analysis (WinNonlin 4.1, Pharsight Corporation, Mountain View, California). Two grams of cefotaxime was given as a single intravenous (i.v.) bolus over 5min (mean cefotaxime plasma concentration–time curves, Fig. 3a). Overall, healthy volunteers heterozygous for the OAT3 variant allele, Ile305Phe, had significantly higher AUCs and initial plasma concentrations (C0) of cefotaxime (p < 0.05, see Table 1). The CLR of 134 ± 53.0 mL/min in the 15 subjects was higher than CLCR (96.3 ± 17.5 mL/min). A significant difference between the genotype groups was found with respect to CLR of cefotaxime and net secretory clearance of cefotaxime (CLsec; Table 1). Specifically, volunteers with Ile305/Ile305 exhibited a greater cefotaxime CLR (158 ± 44.1 mL/min) compared with volunteers who were heterozygous for the Ile305/Phe305 (84.8 ± 32.1 mL/min, p = 0.006). The CLCR did not differ between the genotype groups (Table 1). The net CLsec was calculated by subtracting filtration clearance of cefotaxime from the total cefotaxime CLR. The filtration clearance of cefotaxime is CLCR fraction of the cefotaxime unbound to the plasma protein (fu), that is, CLR = CLsec + (fuCLCR). The reported fu of cefotaxime ranges from 0.4 to 0.8.20, 32, 33 In this clinical study, we used fu = 0.6. The CLsec differed significantly different between the two groups: 97.0 ± 42.2 mL/min in volunteers with Ile305/Ile305 versus 33.3 ± 31.8 mL/min in the volunteers with the Ile305/Phe305 (p = 0.01; pharmacokinetic parameters, Table 1, Figs. 3b and 3c). Similar to results in Oat3 knockout mice and in drug–drug interaction studies of probenecid and OAT3 drug substrates (e.g., cephalosporin antibiotics34–36), we observed an increased AUC in the volunteers who were heterozygous for the variant allele (p < 0.05; Table 1). The apparent volume of distribution at steady state was reduced in individuals harboring the variant OAT3 allele (p = 0.053, Table 1), suggesting that the drug distributes less to body tissues. Because OAT3 is predominantly expressed in the kidney, it is possible that individuals with the variant transporter will have lower kidney levels of the drug. Cefotaxime CLR and CLsec were significantly reduced in Ile305Phe group (Table 1) because of a primary effect of the variant allele on tubular secretion, which was likely because of reduced expression of the variant transporter on the cell surface. In addition to our noncompartmental analysis, we analyzed the data using a nonlinear mixed effects approach available in NONMEM R 7 (ICON plc, Dublin, Ireland).37 The First Order Conditional Estimation with interaction (FOCEI) method was employed throughout the analysis. As has been previously reported, we observed that the pharmacokinetic data were best fit by a two-compartment model.20, 38, 39 Cefotaxime clearance was parameterized using the Eqs. 1a and 1b (Fig. S4). Overall, the results from the two-compartmental model showing that the mean cefotaxime CLR in 15 healthy volunteers was 7.3 ± 1.6 L/h (mean ± SD) were similar to a previous study in 52 healthy subjects (8.5 ± 1.4 L/h).20 Similarly to the analysis using noncompartmental analysis, cefotaxime filtration (CLfiltration) was calculated using fuCLCR (see Table S2 footnote). Separate parameters defining the transport component of the CLR were estimated for the patients carrying OAT3 reference and variant allele. The model structure is visualized in Fig. S4. The individual parameters were assumed to be log-normally distributed, and proportional error was employed for description of residual variability. The likelihood ratio test, diagnostic plots, and internal model validation techniques, including visual and numerical predictive checks, guided the model-building procedure.37 The estimates for the hepatic and renal part of total clearance were similar, suggesting that both routes of elimination are equally represented. In this model, CLR of cefotaxime also differed significantly between the two genotype groups. Specifically, the active transport component of the CLR was 45% lower in the volunteers who carried the variant allele compared with those who were homozygous for the reference allele (see Table S2). The net CLsec was significantly lower in volunteers heterozygous for the variant allele [n = 5, 3.2 ± 0.28 L/h (mean ± SD); RSE 26%] compared with the volunteers that were homozygous for the OAT3 reference allele [n = 10, 5.9 ± 0.69 L/h (mean ± SD); RSE 13%, p < 0.01; Table S2]. Cefotaxime is generally considered a safe medication; however, rare side effects include allergic reactions, hepatotoxicity, and nephrotoxicity. Although speculative, our data would imply that individuals with the Phe305 allele would be more susceptible to non-renal side effects related to drug levels and less susceptible to nephrotoxicity as the transporter plays a role in renal uptake of the drug. The variant would, therefore, result in lower levels of cefotaxime in the kidney.
Figure 3.
Effect of OAT3 genotype on (a) plasma concentrations (b) renal clearance (CLR) and (c) net secretory clearance (CLsec) of cefotaxime in healthy volunteers. A single 2 gram dose of cefotaxime was administered as an intravenous bolus dose over 5 min to volunteers homozygous for the reference (Ile305/Ile305, n=10) or heterozygous for the variant (Ile305/Phe305, n=5). CLR parameters were determined using non-compartmental analyses. Data shown represent mean ± SEM (Figure 3(a)) or mean ± SD (Figure 3(b) and (c)). Significant differences between genotype groups as assessed by unpaired two-tailed t-test) are indicated as, * p<0.05, ** p<0.01 versus volunteers homozygous for the OAT3 reference allele. The pharmacokinetic parameters are shown in Table 1.
Table 1.
Effects of OAT3 genotype on cefotaxime pharmacokinetic parameters in healthy volunteers after a single intravenous bolus dose (2 grams over 5 min).
| Pharmacokinetic Parameters |
Total sample | Genotype | Unpaired t-test | |
|---|---|---|---|---|
| 305Ile/305Ile (n=10) | 305Ile/305Phe (n=5) | p-values | ||
| C0 (µg/mL) | 278 ± 215 | 190 ± 68.4 | 453 ± 305 | 0.018# |
| t1/2 (h) | 1.00 ± 0.200 | 1.10 ± 0.200 | 1.00 ± 0.200 | 0.338 |
| AUC0–6 hrs (µg*h/mL) | 149 ± 50.3 | 129 ± 31.4 | 188 ± 61.6 | 0.027# |
| Vss (mL) | 13400 ± 5620 | 15300 ± 4990 | 9450 ± 5100 | 0.053 |
| Total CL (mL/min) | 247 ± 74.9 | 273 ± 68.9 | 193 ± 60.0 | 0.046 |
| CLR (mL/min) | 134 ± 53.0 | 158 ± 44.1 | 84.8 ± 32.1 | 0.006# |
| CLCR (mL/min) | 96.3 ± 17.5 | 102 ± 17.9 | 85.8 ± 11.8 | 0.101 |
| CLsec (mL/min) | 75.8 ± 49.0 | 97.0 ± 42.2 | 33.3 ± 31.8 | 0.011# |
The cefotaxime pharmacokinetic parameters (mean ± SD) were determined using noncompartmental analysis (WinNonLin 4.1, Pharsight Corporation). The pharmacokinetics parameters are consistent with previously reported values.20,22
C0, is the plasma concentration at first collection time point at 15 min; t1/2, elimination half-life; AUC0–6 h, area under the plasma concentration–time curve (AUC) from the initial time to the last time point at 6 h. The plasma concentrations of cefotaxime are negligible (<% of the initial concentrations) after 6 h.
CLCR, measured creatinine clearance; Vss, apparent volume of distribution at steady state. The renal clearance of cefotaxime (CLR) is the sum of the net secretory clearance (CLsec) and filtration clearance of cefotaxime. Filtration clearance of cefotaxime is creatinine clearance (CLCR) fraction of the cefotaxime unbound to plasma proteins (fu), that is, CLR = CLsec + (fuCLCR). We used 0.6 as the cefotaxime fraction unbound (fu) based on published results.20,32 In one individual who is heterozygous for variant allele, the estimated GFR was used as CLCR instead of using the measured CLCR above. This individual had an abnormal measured CLCR (2.6-fold higher than estimated GFR), whereas other individual had similar values for estimated GFR and measured CLCR.
p value is significant (p < 0.05) when the individual who is heterozygous for variant allele, with an abnormal measured CLCR was removed from the analysis. Overall, the C0, CLR, CLsec, and AUC remained significant when the analysis was performed in 14 healthy volunteers, excluding the individual with the extremely high creatinine clearance.
CONCLUSIONS
Cefotaxime is a third-generation cephalosporin with broad-spectrum antibacterial activity. In this study, we show that cefotaxime is a substrate of OAT3 and that the low-frequency OAT3 variant, Ile305Phe, found in Asians (3.5% allele frequency), is associated with significant reduced cefotaxime uptake and reduced Vmax in in vitro kinetic studies. Our cell surface biotinylation study suggests that the mechanism of the reduced cefotaxime Vmax in OAT3-Ile305Phe cells is because of lower surface protein expression levels of the transporter. Despite the small sample size, our in vitro clinical studies in healthy volunteers indicated that individuals with the OAT3 variant Ile305Phe had a substantially lower CLR and net CLsec and a higher AUC compared with those homozygous for the reference allele. To our knowledge, this is the first study to show a clinical effect of the OAT3-Ile305Phe on CLR of a drug. However, because of the limited sample size, it will be necessary to confirm our findings in other clinical studies and to extend our findings beyond pharmacokinetics of the drug. In particular, the effect of the variant allele on drug safety should be evaluated. Together with previous studies in Oat3 knockout mice and OAT3-mediated drug-drug interaction studies, this genotype-to-phenotype study further supports the importance of OAT3 in as a determinant of drug disposition.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institutes of Health (NIH; GM61390). C.B. was supported by National Research Service Award T32 GM07546 from the NI H. This work was also made possible in part by core services provided by the Clinical and Translational Science Institute (CTSI), Clinical Research Center at San Francisco General Hospital, funded by the National Center for Research Resources (NIH MO1-RR00083-44). We thank all staff at UCSF Drug Studies Unit for developing the analytical procedure for determining cefotaxime in clinical samples. We thank Howard Horng and Kari Morrissey for their guidance to develop the analytical method for determining cefotaxime in cell lines.
REFERENCES
- 1.Sato M, et al. Renal secretion of uric acid by organic anion transporter 2 (OAT2/SLC22A7) in human. Biol Pharm Bull. 2010;33:498–503. doi: 10.1248/bpb.33.498. [DOI] [PubMed] [Google Scholar]
- 2.Cha SH, et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- 3.Nagle MA, et al. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem. 2011;286:243–251. doi: 10.1074/jbc.M110.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal Transporters in Drug Development. Annu Rev Pharmacol Toxicol. 2012 doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 5.Vanwert AL, Srimaroeng C, Sweet DH. Organic anion transporter 3 (oat3/slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin. Mol Pharmacol. 2008;74:122–131. doi: 10.1124/mol.107.042853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.VanWert AL, Sweet DH. Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharm Res. 2008;25:453–462. doi: 10.1007/s11095-007-9407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanwert AL, Bailey RM, Sweet DH. Organic anion transporter 3 (Oat3/Slc22a8) knockout mice exhibit altered clearance and distribution of penicillin G. Am J Physiol Renal Physiol. 2007;293:F1332–F1341. doi: 10.1152/ajprenal.00319.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaehde U, et al. Effect of probenecid on the distribution and elimination of ciprofloxacin in humans. Clin Pharmacol Ther. 1995;58:532–541. doi: 10.1016/0009-9236(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 9.Vree TB, van den Biggelaar-Martea M, Verwey-van Wissen CP. Probenecid inhibits the renal clearance of frusemide and its acyl glucuronide. Br J Clin Pharmacol. 1995;39:692–695. [PMC free article] [PubMed] [Google Scholar]
- 10.Erdman AR, et al. The human organic anion transporter 3 (OAT3; SLC22A8): genetic variation and functional genomics. Am J Physiol Renal Physiol. 2006;290:F905–F912. doi: 10.1152/ajprenal.00272.2005. [DOI] [PubMed] [Google Scholar]
- 11.Xu G, et al. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)] Kidney Int. 2005;68:1491–1499. doi: 10.1111/j.1523-1755.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 12.Ogasawara K, et al. Analysis of regulatory polymorphisms in organic ion transporter genes (SLC22A) in the kidney. J Hum Genet. 2008;53:607–614. doi: 10.1007/s10038-008-0288-9. [DOI] [PubMed] [Google Scholar]
- 13.Bhatnagar V, et al. Analyses of 5' regulatory region polymorphisms in human SLC22A6 (OAT1) and SLC22A8 (OAT3) J Hum Genet. 2006;51:575–580. doi: 10.1007/s10038-006-0398-1. [DOI] [PubMed] [Google Scholar]
- 14.Nishizato Y, et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73:554–565. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 15.Vormfelde SV, et al. Torsemide renal clearance and genetic variation in luminal and basolateral organic anion transporters. Br J Clin Pharmacol. 2006;62:323–335. doi: 10.1111/j.1365-2125.2006.02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han YF, et al. Association of intergenic polymorphism of organic anion transporter 1 and 3 genes with hypertension and blood pressure response to hydrochlorothiazide. Am J Hypertens. 2011;24:340–346. doi: 10.1038/ajh.2010.191. [DOI] [PubMed] [Google Scholar]
- 17.Abecasis GR, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad K, Kumar A, Gupta PK, Singhal T. Third generation cephalosporins versus conventional antibiotics for treating acute bacterial meningitis. Cochrane Database Syst Rev. 2007:CD001832. doi: 10.1002/14651858.CD001832.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ings RM, Fillastre JP, Godin M, Leroy A, Humbert G. The pharmacokinetics of cefotaxime and its metabolites in subjects with normal and impaired renal function. Rev Infect Dis. 1982;4(Suppl):S379–S391. doi: 10.1093/clinids/4.supplement_2.s379. [DOI] [PubMed] [Google Scholar]
- 20.Esmieu F, Guibert J, Rosenkilde HC, Ho I, Le Go A. Pharmacokinetics of cefotaxime in normal human volunteers. J Antimicrob Chemother. 1980;6(Suppl A):83–92. doi: 10.1093/jac/6.suppl_a.83. [DOI] [PubMed] [Google Scholar]
- 21.Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13:519–547. doi: 10.1208/s12248-011-9290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ings RM, et al. The human pharmacokinetics of cefotaxime and its metabolites and the role of renal tubular secretion on their elimination. J Pharmacokinet Biopharm. 1985;13:121–142. doi: 10.1007/BF01059394. [DOI] [PubMed] [Google Scholar]
- 23.Takeda M, Babu E, Narikawa S, Endou H. Interaction of human organic anion transporters with various cephalosporin antibiotics. Eur J Pharmacol. 2002;438:137–142. doi: 10.1016/s0014-2999(02)01306-7. [DOI] [PubMed] [Google Scholar]
- 24.Fujita T, et al. Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1) Pharmacogenet Genomics. 2005;15:201–209. doi: 10.1097/01213011-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Cropp CD, et al. Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol. 2008;73:1151–1158. doi: 10.1124/mol.107.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shima JE, et al. Genetic variants of human organic anion transporter 4 demonstrate altered transport of endogenous substrates. Am J Physiol Renal Physiol. 2010;299:F767–F775. doi: 10.1152/ajprenal.00312.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, et al. Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet Genomics. 2009;19:497–504. doi: 10.1097/FPC.0b013e32832cc7e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu Y, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shu Y, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tahara H, et al. Functional genetic variation in the basal promoter of the organic cation/carnitine transporters OCTN1 (SLC22A4) and OCTN2 (SLC22A5) J Pharmacol Exp Ther. 2009;329:262–271. doi: 10.1124/jpet.108.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urban TJ, et al. Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin Pharmacol Ther. 2008;83:416–421. doi: 10.1038/sj.clpt.6100271. [DOI] [PubMed] [Google Scholar]
- 32.Seguin P, et al. Plasma and peritoneal concentration following continuous infusion of cefotaxime in patients with secondary peritonitis. J Antimicrob Chemother. 2009;63:564–567. doi: 10.1093/jac/dkn522. [DOI] [PubMed] [Google Scholar]
- 33.Sanofi-Aventis Canada Inc. Product Monograph: Claforan(R) sterile cefotaxime sodium, 500 mg, 1 g, 2 g. 2010 [Google Scholar]
- 34.Verhagen CA, Mattie H, Van Strijen E. The renal clearance of cefuroxime and ceftazidime and the effect of probenecid on their tubular excretion. Br J Clin Pharmacol. 1994;37:193–197. doi: 10.1111/j.1365-2125.1994.tb04260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown GR. Cephalosporin-probenecid drug interactions. Clin Pharmacokinet. 1993;24:289–300. doi: 10.2165/00003088-199324040-00003. [DOI] [PubMed] [Google Scholar]
- 36.Guerrini VH, Filippich LJ, English PB, Cao GR, Bourne DW. Effect of probenecid on the pharmacokinetics of cefotaxime in sheep. J Vet Pharmacol Ther. 1985;8:38–46. doi: 10.1111/j.1365-2885.1985.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 37.Savic RM, Kjellsson MC, Karlsson MO. Evaluation of the nonparametric estimation method in NONMEM VI. Eur J Pharm Sci. 2009;37:27–35. doi: 10.1016/j.ejps.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Urien S, Laurent N, Barre J, Druguet M, Bouvier D’yvoire M, Maire P. Pharmacokinetic modelling of cefotaxime and desacetylcefotaxime—A population study in 25 elderly patients. Eur J Clin Pharmacol. 2004;60:11–16. doi: 10.1007/s00228-003-0725-9. [DOI] [PubMed] [Google Scholar]
- 39.Kemmerich B, Lode H, Belmega G, Jendroschek T, Borner K, Koeppe P. Comparative pharmacokinetics of cefoperazone, cefotaxime, and moxalactam. Antimicrob Agents Chemother. 1983;23:429–434. doi: 10.1128/aac.23.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.