Abstract
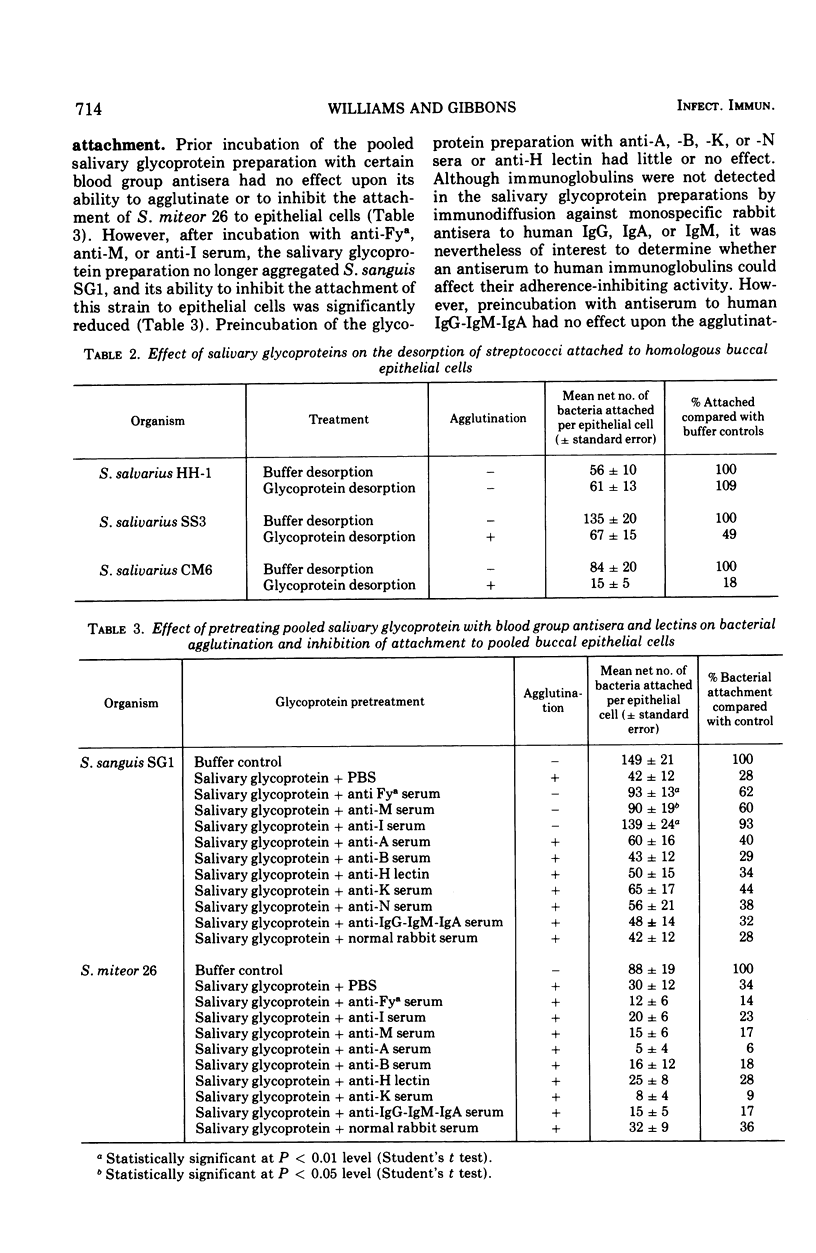
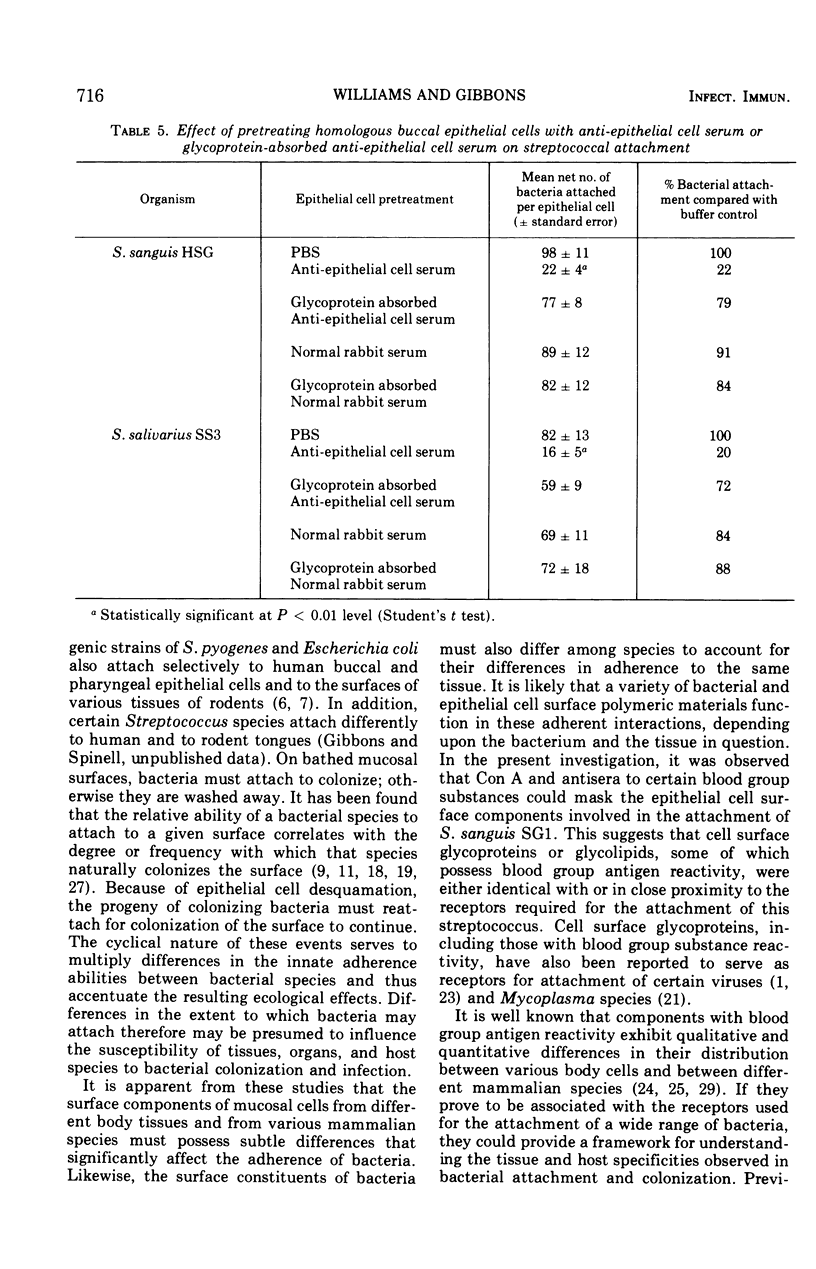
Preparations of salivary glycoproteins inhibited the attachment of certain indigenous oral streptococci to human buccal epithelial cells and fostered the desorption of previously attached bacteria. The adherence-inhibiting and desorptive activities of the glycoproteins correlated with their ability to aggregate these organisms. Pretreatment of glycoprotein preparations with certain blood group antisera impaired their adherence-inhibiting effect, suggesting that components with blood group substance reactivity were involved. Pretreatment of buccal epithelial cells with certain blood group antisera or concanavalin A masked the receptors associated with the attachment of Streptococcus sanguis SG1. The association of blood-group-reactive substances with the receptors involved in bacterial attachment may provide a basis for understanding the distinct specificities that bacteria exhibit for attaching to different tissues, organs, and hosts. Antiserum raised in rabbits to human epithelial cells also exhibited receptor-masking activity, and absorption of this serum with homologous salivary glycoproteins removed the antibodies responsible. These observations indicate that some salivary glycoproteins are antigenically similar to components on the epithelial cell surfaces they bathe. It is suggested that by mimicking the receptors present on epithelial cells, the mucinous glycoproteins of secretions may competitively inhibit the sorption of infectious agents and facilitate their removal after they are attached. These activities help to explain how mucinous glycoproteins augment the cleansing action of secretions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., STONE J. D. Electrophoretic studies of virus-red cell interaction: mobility gradient of cells treated with viruses of the influenza group and the receptor-destroying enzyme of V. cholerae. Br J Exp Pathol. 1950 Jun;31(3):263–274. [PMC free article] [PubMed] [Google Scholar]
- BUCKWALTER J. A., NAIFEH G. S., AUER J. E. Rheumatic fever and the blood groups. Br Med J. 1962 Oct 20;2(5311):1023–1027. doi: 10.1136/bmj.2.5311.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Gibbons R. J., Spinell D. M. Characteristics of some high molecular weight constituents with bacterial aggregating activity from whole saliva and dental plaque. Caries Res. 1971;5(2):111–123. doi: 10.1159/000259739. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Ability of Veillonella and Neisseria species to attach to oral surfaces and their proportions present indigenously. Infect Immun. 1971 Sep;4(3):264–268. doi: 10.1128/iai.4.3.264-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZULMAN A. E. The histological distribution of blood group substances A and B in man. J Exp Med. 1960 Jun 1;111:785–800. doi: 10.1084/jem.111.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer G. F. Importance of blood-group substances in interactions between man and microbes. Ann N Y Acad Sci. 1970 Feb 13;169(1):134–152. doi: 10.1111/j.1749-6632.1970.tb55979.x. [DOI] [PubMed] [Google Scholar]
- Sönju T., Christensen T. B., Kornstad L., Rölla G. Electron microscopy, carbohydrate analyses and biological activities of the proteins adsorbed in two hours to tooth surfaces in vivo. Caries Res. 1974;8(2):113–122. doi: 10.1159/000260099. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Determination of fructose and fructose-yielding carbohydrates with cold anthrone. Anal Biochem. 1967 Apr;19(1):193–194. doi: 10.1016/0003-2697(67)90152-2. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]



