Abstract
Activation of a patient’s immune system offers an attractive approach to prevent and treat hepatocellular carcinoma (HCC). However, the antitumor efficacy of current HCC vaccines was weak owing to insufficient immune activation of targeting self/ tumor antigens. We recently found that epitope-optimized α-fetoprotein effectively activated CD8 T cells and generated potent antitumor effects in the carcinogen-induced autochthonous HCC mouse model. We predict that the same antigen engineering approach of epitope-optimization will enable us to develop effective human vaccines to prevent HCC recurrence after liver resection. The engineered human HCC vaccines may also allow us to identify high-affinity T-cell receptors and antibodies that can be used to reprogram T cells to treat HCC tumors via adoptive transfer.
Keywords: antigen engineering, cancer vaccine, epitope optimization, genetic immunization, hepatocellular carcinoma, immunotherapy, recombinant lentivector
Background
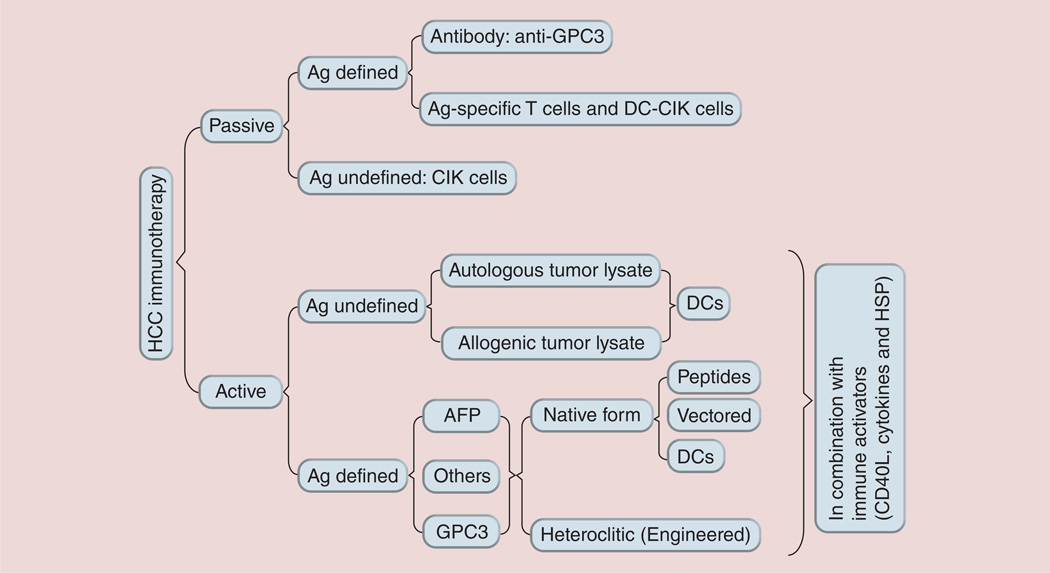
Liver cancer, with approximately 750,000 new cases and approximately 695,000 deaths annually, is the fifth most common cancer and the third most common cause of cancer death in the world [1]. According to the CDC, the incidence rate of hepatocellular carcinoma (HCC), the most common type of liver cancer, is increasing significantly in the USA, while many other major cancers are declining [2]. The number of new HCC cases in the USA has almost tripled over the past two decades to reach an estimated 24,500 in 2013 (American Cancer Society). The hepatitis B virus (HBV) vaccines have markedly reduced new HBV infections and should decrease the number of HBV-associated HCC cases in the long run [3]. However, the large pool of existing chronic HBV (350 million) [4] and hepatitis C virus (HCV; 170 million) [5] -infected patients, and the recent recognition that obesity/diabetes has become a major contributing factor of HCC in the USA [6, 7], suggests that HCC incidence in the USA and worldwide may continue increasing for the next few decades. Unfortunately, the treatment options for HCC are limited and ineffective [8], which contribute to the dismal 3-year survival rate of 17% [1]. Although liver resection and transplantation are potentially curative for small, early-stage HCC tumors, the lack of postsurgery adjuvant therapies is a critical barrier to the success of HCC surgical treatment. For example, the 5-year recurrence rate of HCC following liver resection is approximately 70% [9]. Thus, there is an urgent demand to develop novel approaches to HCC treatments that can prevent relapse after surgery and HCC de novo development in high-risk populations such as HBV and HCV chronically infected patients. One of the approaches is to enlist a patient’s own immune system to prevent HCC de novo development and to guard against HCC recurrence [10, 11]. Multiple immunological approaches involving both cellular and humoral arms of the immune system have been explored as potential immunotherapy approaches for treating HCC in mouse models and human clinical trials (see Figure 1) [12–14]. In this review, we first focus our efforts on reviewing previous literature reports of HCC immunotherapy based on the cellular immune responses; secondarily, we propose and discuss antigen engineering strategies to create more effective HCC vaccines. For antibody-based HCC immunotherapy, readers are encouraged to examine an excellent recent review article by Feng and Ho [15].
Figure 1. Immunological approaches used in hepatocellular carcinoma immunotherapy at the stage of in vitro study in animal models or in clinical trials.
AFP: α-fetoprotein; Ag: Antigen; CIK: Cytokine-induced killer cell; DC: Dendritic cell; GPC3: Glypican-3; HCC: Hepatocellular carinoma; HSP: Heat shock protein.
Current cellular immune approaches for HCC immunotherapy
A number of approaches have been exploited to activate the cellular arm of the host immune system to treat liver cancer in both animal models and human trials. These approaches are either antigen-specific or -non specific and can be grouped into passive immunotherapy (adoptive immunotherapy) and active immunotherapy (cancer vaccines).
Passive immunotherapy
The passive immunotherapy approach involves the transfer of in vitro-activated (antigen-specific or -non-specific) autologous immune effector cells to cancer patients in order to mediate tumor killing in vivo. Thus, it is also referred to as adoptive immunotherapy or adoptive cell transfer. While the tumor-infiltrating lymphocytes or even antigen-specific T cells are frequently used in the immunotherapy of melanoma, the most widely used immune effector cell in the field of HCC immunotherapy is the cytokine-induced killer (CIK) cells, which include NK, T and NKT cells. The CIK cells are generated in vitro from peripheral blood cells by stimulation with a cocktail of cytokines consisting of IFN-γ, IL-2 and anti-CD3 antibodies. The antigen specificity of CIK cells is undefined (or may be antigen nonspecific). The function of CIK cells may not be restricted by MHC molecules [16,17].
In 2000, Takayama et al. reported the results of the first randomized trial of using CIK cells to prevent recurrence of HCC following liver resection [18]. In total, 150 postoperative HCC patients were divided into the CIK cell-treated and control groups. After a median of 4.4 years follow-up, investigators found that CIK cell immunotherapy decreased overall recurrence by 18% (59 vs 77%). The time to recurrence in the CIK cell-treated group was significantly longer than that of the control group (p = 0.01). However, the overall survival was not significantly different. In another clinical trial, postoperative HCC patients were followed up for 5–7 years after CIK cell treatment [19]. The authors corroborated Takayama et al.’s findings by showing that adoptive transfer of CIK cells could significantly prolong the disease-free survival. Once again, the overall survival rate was not improved by CIK cell treatment. Consistent with these reports, two other clinical trials also demonstrated that adoptive transfer of CIK cells did in fact significantly delay the 1- and 3-year recurrence after surgical treatment of HCC, but did not significantly affect the overall 3-year survival [20,21]. However, in a recent study with a considerably larger group of patients (>400 patients), Pan et al. demonstrated that the adoptive transfer of CIK cells after surgery, especially with eight cycles of CIK cell infusions, significantly improved the overall survival of the patients with HCC tumor size >5 cm [22].
For patients with larger HCC tumors that did not qualify for liver resection, CIK cells have been used in combination with nonsurgical approaches of transcatheter arterial chemoembolization and radiofrequency ablation. In a nonrandomized trial, Huang et al. reported that CIK cells increased the overall survival and progression-free survival of HCC patients following transcatheter arterial chemoembolization and radiofrequency ablation treatment [23].
In summary, clinical trials demonstrated that CIK cell adoptive transfer has the potential to delay HCC recurrence following liver resection and to improve the quality of life in advanced HCC patients. However, a major drawback of the CIK cell-mediated immunotherapy is that the antigen-specificity of CIK cells is undefined and the therapy may be nonspecific with a potential for autoimmune disease induction although no side effects have been documented thus far. In addition, the mechanism underpinning the antitumor effect of CIK is not well understood. However, because CIK cells are easily prepared and encompass a wide variety of immune effector cells, CIK cells will continue to be a choice of adjuvant therapy for HCC patients. A greater understanding of the mechanism of how CIK cells works may help improve the efficacy of CIK cells in treating HCC. Furthermore, additional stimulation of the CIK cells with autologous dendritic cells (DCs; DC-CIKs) vaccines may generate stronger and antigen-specific cytolytic immune effectors against HCC tumor cells [24].
Active immunotherapy
Active tumor immunotherapy is a means to administer cancer vaccines to activate the host immune system in vivo to generate antigen-specific immune responses to achieve an antitumor effect. Based on the antigen spectrum and specificity, HCC cancer vaccines can be divided into two groups: antigen undefined and antigen defined.
Antigen-undefined HCC cancer vaccines
This type of vaccine is based on the same principle as most infectious disease vaccines: the usage of the entire tumor cells or tumor cell lysate as tumor antigens that encompass all potential antigens in the HCC tumor cells. To increase immune responses, autologous DCs are frequently used in the vaccine preparation. In the murine HCC model, immunization with bone marrow-derived DCs, pulsed with mouse Hepa1–6 tumor lysate, could generate a partial therapeutic effect [25]. In a human trial, autologous DCs pulsed with self-HCC tumor cell lysate were used as vaccines [26]. Among 31 patients, four (12.9%) exhibited partial response, 17 patients’ (54.8%) diseases remained stable and ten patients’ (32.3%) diseases progressed. The overall 1-year survival rate of all 31 patients was 40.1 ± 9.1%. The patients treated with an additional boosting immunization generated even better 1-year survival rates than those treated with one single immunization (63.3 ± 12.0% vs 10.7 ± 9.4%; p < 0.001). The DC vaccinations were safe with liver function tests showing no difference before and after immunization. Unfortunately in the study, no immune responses were monitored; thus, it was not possible to correlate the antitumor effect to the vaccine-induced immune activation. In another Phase II clinical trial, lysates from the established allogenic HCC tumor cell line HepG2 were used to pulse autologous DCs [27] for use as cancer vaccines. A total of 35 patients with advanced HCC, who were not suitable for radical or locoregional therapies, received at least three DC vaccinations. The disease control rate (partial response and stable disease ≥3 months) determined by imaging was 28%. In 17 patients, the baseline serum α-fetoprotein (AFP) was ≥1000 ng/ml; in four of these patients, it fell to <30% of baseline following vaccination. In one patient, there was a radiological partial response that was associated with a reduction of serum AFP to <10% of baseline. In addition, the immune responses were assessed in this study by IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assay. In several cases, there was an induction of T-cell responses to the vaccine and/or AFP antigens following vaccinations. Thus, autologous DC vaccination in HCC patients can be considered safe and well tolerated, with evidence of antitumor efficacy and generation of antigen-specific immune responses in some cases. The advantage of using tumor lysate for immunization is the inclusion of a broad spectrum of antigens that should induce a wide range of immune responses against HCC. However, the immune responses activated by such vaccines are mostly weak, generating modest antitumor effect. In addition, the inclusion of all antigens in the hepatocytes has the risk of generating autoimmune hepatitis.
Antigen-defined HCC cancer vaccines
The most promising and exciting HCC immunotherapy approach involves targeting antigens that are specifically expressed or overexpressed in HCC tumor cells. Several HCC-specific tumor-associated antigens have been identified over the last few decades, including oncofetal proteins of AFP [11,12] and glypican (GPC)3 [28], cancer/testis antigens of MAGE-A [29] and NY-ESO [30], and the widely overexpressed antigens such as telomerase reverse transcriptase [31]. HCC cancer vaccines based on defined HCC-associated antigens have been tested in animal models and clinical trials [11,12,31,32] in the form of peptides, proteins or genes with and without DCs. In this review, we are focusing on AFP- and GPC3-based HCC vaccines owing to their high specificity to HCC.
AFP-based HCC vaccines
AFP is highly re-expressed in 70–80% of HCC [33], and has been used not only as a biomarker for diagnosis, but also as a target for T cell-mediated immunotherapy in animal studies [34,35] and clinical investigations [11,32,36].
Animal studies
In animal models, native mouse AFP plasmid DNA [37–39], DCs transduced with viral vectors [35,40] or pulsed with peptides [41] has been utilized to induce AFP-specific immune responses. However, only low levels of immune activation and antitumor effects have been observed. For example, using the ELISPOT assay, only 10 spots/1 × 106 splenocytes were detected after two intramuscular immunizations with mouse AFP plasmid DNA [37]. In another study, three immunizations with bone marrow-derived DCs expressing mouse AFP only protected one out of four mice from AFP-positive tumor challenge [35]. Mouse AFP DNA formulated with cationic polymers generated moderate T-cell responses, although a more pronounced antitumor overall effect was observed [38]. Xenogenic AFP peptides [41] or proteins [42] have also been used to increase AFP-specific immune responses. In addition, a combination of AFP with heat shock protein [43,44] or stimulatory cytokines [34,45] was employed to enhance the AFP-specific responses. However, such efforts only resulted in marginal improvement of immune activation. The prime-boost immunization such as DNA prime followed by vaccinia vector [46] or adenoviral vector [47] boost generated a higher immune response to 100–200 spots/1 × 106 splenocytes. However, the overall antitumor effect was modest and inconsistent. In general, the HCC vaccines based on wild-type AFP demonstrated promising yet insufficient immune activation and limited antitumor responses in animal models. This weak immune activation may also explain why no H-2b restricted AFP epitopes have been identified, which could hinder the progress of HCC vaccine development.
Human studies
Butterfield and her group pioneered the AFP-based human HCC vaccine studies [48]. They first identified four HLA-A2-restricted AFP epitopes in HLA-A2 transgenic mice and in cultured human CD8 T cells [49,50]. Butterfield et al. then conducted the first clinical trial in heavily pretreated stage IV patients with three biweekly vaccinations of four different AFP peptides emulsified in incomplete Freund’s adjuvant [36]. They found that all six patients generated T-cell responses to most or all of the four peptides as measured by ELISPOT and tetramer assays. Thus, the human T-cell repertoire is capable of recognizing AFP in the context of MHC class I even in an environment of high circulating serum levels of AFP. In a follow-up Phase I/II trial, DCs pulsed with the AFP peptides were further used [32]. Although AFP-specific responses were detected, no significant antitumor effect was observed. It appeared that the immune responses were not potent enough or irrelevant to generate an antitumor effect. To improve the antitumor effect, an additional clinical trial was initiated to include AFP DNA vaccines followed by an adenoviral vector boost (Clnicaltrials.gov identifier: NCT00669136). In addition, cytokine granulocyte–macrophage colony-stimulating factor was also incorporated into a second new clinical trial (Clinicaltrials.gov identifer: NCT00093548). The hope is that these added measures will generate stronger immune responses and improve antitumor effects.
GPC-based vaccines
In the late 1990s, another oncofetal protein, GPC3, was found to be highly associated with HCC [51]. In contrast to the secreted AFP, the C-terminus of GPC3 is attached to the cell membrane via a glycophos-phatidylinositol anchor. Thus, both the cellular and humoral responses can be generated against GPC3 + HCC tumor cells. Several anti-GPC3 antibodies for HCC immunotherapy are being developed and some have achieved clinical trial status. Readers are recommended to examine a recent review on this topic [15]. Regarding the cellular arm of GPC3-specific immune responses, both H-2b [52] and H-2d [53]-restricted CD8 epitopes have been identified in mice and have been employed to activate mouse GPC3-specific T-cell responses in order to generate an antitumor effect. Human HLA-A2 (GPC3144–152) and HLA-A24 (GPC3298–306) restricted human GPC3 epitopes have also been identified [54]. In a recent clinical trial [55], immunization with GPC3144–152 and GPC3298–306 peptides induced GPC3-specific cytotoxic T lymphocytes in 30 out of 33 advanced HCC patients; furthermore, 19 out of 33 patients had stabilized disease for longer than 2 months. Most importantly, the overall survival was found to be correlated with the frequency of cytotoxic T lymphocytes in the patients. Thus, GPC3-based HCC vaccines have shown great promise in treating human HCC.
Antigen engineering to create more effective HCC vaccines
AFP and GPC3 are self/tumor proteins being highly expressed during fetal development and gradually disappearing after birth. Thus, the high-affinity, self-reactive immune effector cells should be depleted in the thymus during development through the mechanism of central tolerance. Previous studies demonstrated that immunization with native self/tumor antigens is usually unable to activate the host T-cell repertoire [56–58]. Consistent with these reports, immunization with vaccinia vector-expressing, native, full-length mouse AFP was incapable of inducing AFP-specific CD8 responses in mice [59]. We reason that in order to effectively activate immune effector cells that recognize and kill tumor cells, engineering of the weakly immunogenic self/tumor proteins to create highly immunogenic antigens may be required.
Altered peptide ligands induce more robust immune responses
The ‘altered peptide ligand (APL) (or heteroclitic peptides)’ approach has been extensively studied [58, 60–62] as a means of activating self/tumor antigen reactive T cells. The principle of APL design is to increase the stability of the APL and MHC complexes [63], which can then optimally engage with TCR to activate cognate T cells. In the design of APLs, the changes in the peptides must be significant enough to activate the T-cell clones that cannot be activated by a native antigen, but at the same time, the change must be subtle enough to allow the activated T cells to cross-recognize the native self/tumor antigen. However, even with careful design, the T-cell repertoire activated by APLs may be skewed [64] and the quality of clonotypic T cells may be suboptimal [65] in generating an antitumor effect. Furthermore, some of the synthetic short peptide epitopes predicted by computer may not indeed be processed and presented by target tumor cells [50]. These drawbacks of APLs may explain why recent APL clinical trials failed to show significant anti-tumor effects [66]. To our best knowledge, there is only one report demonstrating that the APL technology was used to activate AFP-specific T cells [67]. Unfortunately, no antitumor effect was reported in the study.
Gene vaccine encoding epitope-optimized antigen (heteroclitic gene vaccine) activates potent CD8 responses to generate strong antitumor effect
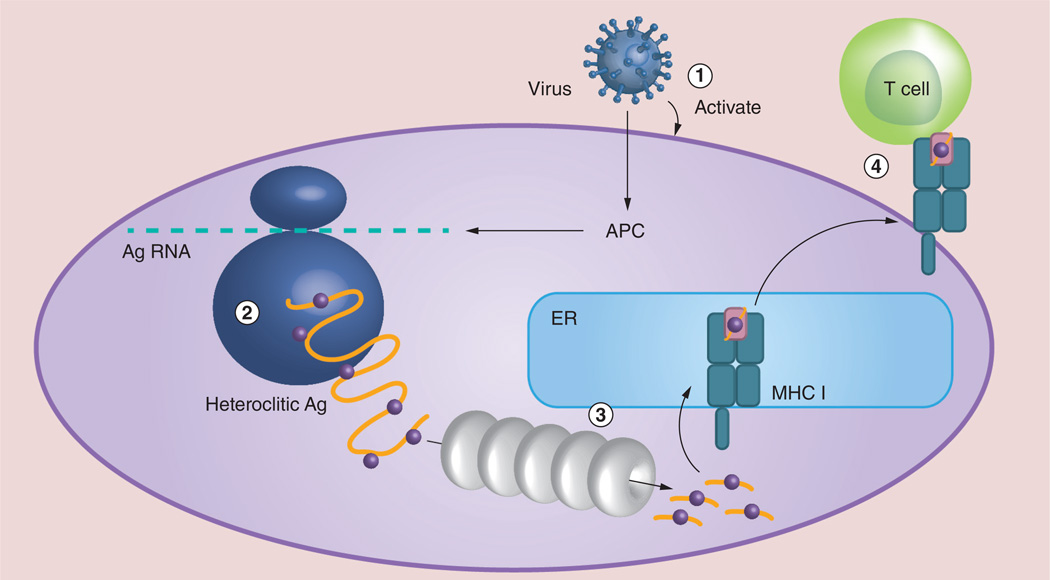
To enhance the tumor antigen-specific CD8 responses that are correlated to the antitumor effect, we reasoned that gene vaccines encoding epitope-optimized tumor antigens (heteroclitic gene vaccine) may produce a better chance of success than APLs (Figure 2). Unlike exogenously APL short peptides, gene vaccines in the format of recombinant virus may better recapitulate natural antigen processing and presentation. The tumor antigen expressed from gene vaccine and synthesized within antigen-presenting cells will probably traverse the same processing and presentation pathways as the native antigen expressed in target tumor cells. Thus, any immune cell activated by gene vaccine will probably recognize target tumor cells. In addition, the full-length antigen offers a much broader immune response than shorter APLs. It may have a better chance of generating an antitumor effect. Furthermore, one single immunization will allow us to examine immune responses against multiple epitopes, facilitating identification of antigen-specific immune responses. Consistent with this reasoning, it was found that plasmid DNA or recombinant lentivector encoding the full-length melanoma antigen of tyrosinase related protein 1 (TRP1), which contained multiple optimized CD8 epitopes, stimulated potent TRP1-specific CD8 responses [56, 57]. One single immunization with recombinant lentivector encoding the optimized TRP1 full-length antigen induced approximately 10% of circulating CD8 T cells to secrete IFN-γ in responses to native TRP1 antigen [57]. Thus, epitope-optimization of self/tumor antigen together with genetic immunization presents an effective approach to induce potent immune responses against self/tumor antigens.
Figure 2. Tumor antigen synthesis, processing and presentation by an antigen-presenting cell after viral transduction and T-cell activation.
Viral vector expressing offers several advantages in generating antitumor immunity: (1) the contact of virus optimally activates APCs; (2) multiple altered peptide ligands are integrated into the epitope-optimized heteroclitic full length Ag, activating a broader spectrum of immune responses and facilitating epitope identification; (3) Ag synthesized inside APCs may undergo the same Ag processing and presentation pathway as that in tumor cells; and (4) the activated T cells will have better chance of recognizing tumor cells. Ag: Antigen; APC: Antigen-presenting cell; ER: Endoplasmic reticulum.
Engineering AFP to create highly immunogenic HCC vaccines
Recently, using the strategy of epitope optimization and recombinant lentivector-mediated genetic immunization, we systematically studied whether HCC-associated self/tumor antigens of AFP can be engineered to become highly immunogenic so as to activate potent CD8 immune responses [68]. One major finding of our study is that the creation of epitope-optimized AFP is absolutely required to effectively activate AFP-specific CD8 T cells in mice. Once activated, however, these CD8 T cells induced by epitope-optimized AFP could efficiently cross-recognize native AFP epitopes on HCC tumor cells to generate a potent antitumor effect. In murine HCC models, including the carcinogen-induced autochthonous HCC model, we reported several important findings.
Epitope optimization creates highly immunogenic AFP that can effectively activate AFP-specific CD8 T cells, enabling one to identify three H-2b–restricted CD8 epitopes
Using an algorithmic program developed by Guevara-Patiño and colleagues [69], we identified ten putative H-2b (5 Db and 5 Kb)-restricted CD8 epitopes in the AFP protein, which were then optimized to increase their MHC I binding. Using a MHC stabilization assay, we found that the optimized epitopes of mouse AFP212opt and AFP499opt peptides significantly increased the affinity of binding to H-2b molecules in comparison to their native counterparts. To test if the computer- designed optimized AFP could induce more potent CD8 responses than native AFP, we made a lentivector to express the full-length AFP containing all ten optimized epitopes. We chose lentivector-mediated immunization because it has been proved to be one of the most effective approaches to activate CD8 and CD4 T cells [70–75]. We found that a significant proportion of CD8 T cells from the optimized AFP immunized mice were able to produce IFN-γ after stimulation with three native AFP peptides: AFP212, AFP419 and AFP499. As high as 1.66% of spleen CD8 T cells produced IFN-γ following AFP212 peptide stimulation. By contrast, there were no IFN-γ-producing CD8 cells detected in the mice immunized with recombinant lentivector-expressing native AFP. These data indicate that epitope optimization of AFP is required to effectively activate AFP-specific CD8 T cells, which are capable of cross-recognizing native AFP epitopes. We also noticed that the three native AFP epitopes identified in the study had an intermediate affinity for H-2b molecules; this agrees with the theory that T cells against the highest and lowest affinity antigens are deleted through positive and negative selection during thymus development [76].
CD8 T cells activated by optimized AFP recognize & effectively kill AFP+ tumor cells
One of the major drawbacks of APL technology is that CD8 T cells activated by APLs may not recognize antigen-expressing tumor cells because the native epitopes corresponding to the APL may not be naturally processed and presented by target tumor cells. This prompted us to study whether CD8 T cells activated by full-length optimized AFP expressed from gene vaccines could recognize native AFP epitopes on AFP-expressing tumor cells. Because the available B6 mouse HCC cell line, Hepa1–6, is MHC I-negative even after IFN-γ treatment, we utilized EL4-AFP tumor cells as surrogate target cells, similar to previously reported studies [35]. We found that the CD8 T cells activated by the optimized AFP gene vaccine could effectively recognize AFP+ EL4 tumor cells but not the parental EL4 tumor cells. Importantly, the CD8 T cells activated by the optimized AFP gene vaccine could effectively kill EL4-AFP target tumor cells. Even at the low effector/target ratio of 10:1, approximately 70% of EL4-AFP target cells were killed and eliminated within 6 h. These data strongly suggest that CD8 T cells activated by optimized AFP gene vaccine can recognize and kill tumor cells expressing native AFP. The data also support the hypothesis that the AFP CD8 epitopes identified in this study are presented by AFP+ tumor cells, making them recognizable by the activated CD8 T cells.
Immunization with optimized AFP but not native AFP gene vaccine completely protects mice from AFP+tumor cell challenge in a CD8-dependent manner
We then studied whether immunization with optimized AFP gene vaccine could prevent mice from the challenge of EL4-AFP tumor cells. Compared to naive control mice, native AFP immunization did not protect mice from tumor challenge, although tumor growth was slowed, a limited antitumor effect was found similar to a previous report [35]. By contrast, one single immunization of optimized AFP gene completely protected mice from a lethal dose of EL4-AFP tumor challenge. In addition, the antitumor effect of optimized AFP gene immunization was mediated by the activated CD8 T cells as CD8 depletion completely abolished the antitumor effect of optimized AFP gene vaccine, which is consistent with previous findings [35].
Immunization with optimized AFP gene vaccine significantly reduces carcinogen-induced autochthonous HCC & increases liver infiltration of functional AFP-specific CD8 T cells
We then studied the antitumor effect of optimized AFP gene vaccine in the clinically relevant carcinogen (diethylnitrosamine; DEN)-induced autochthonous HCC model in mice. Our data showed that immunization with optimized AFP but not native AFP gene vaccine significantly reduced the number of DEN-induced autochthonous HCC tumor nodules. Furthermore, immunological analysis demonstrated that the optimized AFP gene vaccine significantly increased the liver infiltration of functional AFP-specific CD8 T cells that produced high levels of IFN-γ in response to native AFP peptide stimulation.
Prime boost with optimized AFP gene vaccine increases the AFP-specific CD8 response & antitumor effect in the autochthonous HCC model, & the liver infiltration of AFP-specific CD8 T cells
Previously, we found that prime-boost immunization markedly increased the CD8 memory immune responses and better prevented mice from developing autochthonous melanoma [74]. In this study, we also utilized a prime-boost strategy to increase the magnitude of AFP-specific CD8 responses. We found that prime-boost with optimized AFP gene vaccine significantly enhanced the systemic AFP-specific CD8 effector responses. The mean fluorescent intensity of IFN-γ in the prime-boosted group was significantly higher compared with that of the prime-alone group, suggesting that the amount of IFN-γ produced is significantly greater. The AFP-specific CD8 responses returned to baseline 2 months after the boost. Importantly, the AFP-specific CD8 T-cell responses were markedly increased again at the age of 6 months, corresponding to the emerging DEN-induced HCC tumor and AFP expression. The spike of AFP-specific CD8 responses suggests that the AFP-specific memory CD8 T cells induced by vaccines may detect the emerging AFP antigen in the liver and may respond by expansion, which would then control autochthonous HCC growth. The mouse liver from the prime-boosted mice was infiltrated with a high percentage of AFP-specific CD8 T cells. Approximately 4.5% of the liver-infiltrated CD8 T cells were AFP212-specific CD8 T cells by AFP212-Db tetramer staining. In addition, compared with the spleen, the percentage of AFP212-specific CD8 T cells was significantly higher in the tumor, suggesting that either AFP-specific CD8 T cells are preferentially recruited into the liver tissue, or may simply reflect the fact that the AFP-specific CD8 memory T cells respond to the emerging HCC tumor cells in the liver by expansion; thus, they may be retained in liver tissue owing to the constant emerging AFP antigens. On the other hand, no AFP-specific CD8 T cells were found in the livers of native AFP gene vaccine prime-boosted mice. Thus, the creation of highly immunogenic AFP by epitope optimization is absolutely required to activate and increase liver infiltration of functional AFP-specific CD8 T cells to prevent autochthonous HCC in the DEN-treated mice.
Conclusion
In summary, three immunological approaches have been explored as adjuvant therapies to treat HCC following surgical or nonsurgical treatment in patients. These approaches include adoptive transfer of antigen-nonspecific CIK cells, antigen-undefined vaccines derived from HCC tumor cell lysate and antigen-defined cancer vaccines based on AFP and GPC3. The HCC cancer vaccines are based on native self-proteins associated with HCC (either as defined antigens of AFP and GPC3 or as undefined antigens of the total HCC tumor lysate). Owing to the self-nature of the HCC antigens, the immune activation and antitumor effects of these vaccines are weak. In order to improve the immune responses against HCC-associated antigens, we recently utilized the antigen engineering approach of epitope optimization to increase the immunogenicity of AFP and used the recombinant lentivector to deliver the engineered vaccines. We found that epitope-optimized AFP but not the native AFP gene vaccine potently activated AFP-specific CD8 T cells, which, critically, could cross-recognize native AFP peptides and kill AFP+ tumor cells; this generated a potent antitumor effect. More importantly, immunization with optimized AFP, but not native AFP, increased liver infiltration of AFP- specific CD8 T cells and prevented the carcinogen-induced autochthonous HCC. In addition, prime-boost immunization with epitope-optimized AFP but not native AFP gene vaccine generates long-lasting CD8 memory cells that could detect and respond to evolving HCC tumor cells, enhancing the prevention of autochthonous HCC. Our animal study provides a blueprint to create effective human HCC vaccines that could produce an improved success rate than the current HCC vaccines based on native tumor antigens; this could provide a means to prevent HCC relapse after liver resection and de novo tumor development in high-risk populations.
Future perspective
We predict that the following aspects may become the future frontlines of HCC immunotherapy, which could generate better antitumor effects.
Develop highly immunogenic human HCC vaccines
The novel findings of epitope optimization to create highly immunogenic HCC-associated AFP antigen made in our animal study provides strong evidence that it is possible to induce more potent immune responses against self/tumor antigen and generate much stronger antitumor effect. We expect that it will be translated into human studies to develop novel antigen-specific human HCC vaccines. Both AFP and GPC3 can be targeted to increase the chance of success. Targeting GPC3 may offer additional benefits because humoral immunity may also contribute to the antitumor effect.
Combination therapy with checkpoint blockade
In the last few years, checkpoint blockade of targeting cytotoxic T-lymphocyte antigen 4 and programmed death 1 generate impressing antitumor effects [77]. Our own previous study found that programmed death 1 blockade could increase efficacy of lentivector immunization and rescue the effector function of tumor-infiltrating T cells [78]. We envision that such a nonspecific approach will generate a synergistic effect when combined with antigen-specific novel HCC cancer vaccines, not only by increasing the magnitude of immune responses, but also by removing the immune blockade at the effector phase in the tumor lesions.
Immune prevention in high-risk population
Vaccines are most effective when they are used to prevent diseases. However, it has been a challenge to employ cancer vaccines to prevent cancer development owing to the difficulty of gauging the risk/benefit ratio of cancer vaccination. HCC may offer an excellent model for testing the idea of immune prevention by cancer vaccines. HBV and HCV patients have an approximately 146-fold higher chance of developing HCC [79]. Even worse, approximately 24% of male HBV patients infected by vertical transmission develop HCC by age 70 years [80]. These patients have a >10,000-times higher risk than HBV-negative people. There is no intervention to stop the progression from chronic HBV infection to HCC. These high-risk patients, living in the fear of HCC, will probably accept HCC vaccines and should benefit from them. In addition, the lack of treatment options and high recurrence rate of HCC after liver resection will expedite translation of research bench findings to become a form of adjuvant therapy to prevent HCC relapse.
Generation of reprogrammed autologous T cells that can specifically target tumor cells
Recent studies demonstrated that the delivery of antigen-specific TCR gene into autologous T cells to create TCR reprogrammed T cells that are specific for particular tumor antigen is a potent approach to achieve an antitumor effect [81]. In addition, chimeric antigen receptor that combines the specificity of an antibody and the potent tumor killing effect of T cells is used to engineer T cells to render novel antigen specificity and tumor-killing activity to autologous T cells for adoptive cell transfer [82,83]. Such approaches have been used successfully to treat blood cancer. However, to obtain a good high-affinity TCR or antibody genes for HCC tumor antigens of AFP and GPC3, appropriate potent vaccines may be required. Thus, our innovative vaccine design is critical not only for active immunotherapy of HCC, but also for obtaining high-affinity TCR and/or antibodies for the purpose of adoptive immunotherapy.
Executive summary.
Current immunotherapy approaches for hepatocellular carcinoma
Adoptive transfer of antigen target-undefined cytokine-induced killer (CIK) cells has been studied in clinical trials after liver resection of hepatocellular carcinoma (HCC) tumors and in combination with transcatheter arterial chemoembolization and radiofrequency ablation. Delay of HCC recurrence and diseases progression were observed in clinical trials.
- HCC vaccines: HCC cancer vaccines based on defined antigens such as α-fetoprotein (AFP) and glypican (GPC)-3 or undefined antigens of autologous or allogenic HCC tumor lysates have been used in clinical trials. However, enhancement of the immune activation is required to generate a significant antitumor effect.
- Antigen undefined: dendritic cells pulsed with either autologous or allogenic tumor lysate were used as vaccines.
- Antigen defined: AFP- and GPC3-based HCC vaccines have been employed.
Engineering self/tumor proteins to create highly effective HCC vaccines
Epitope optimization was reported as an effective approach to create highly immunogenic AFP antigens in mice.
Genetic immunization with recombinant lentivector expressing epitope-optimized mouse AFP effectively activated AFP-specific CD8 T cells that not only cross-recognize native AFP epitopes, but also recognize and kill AFP+ tumor cells.
The AFP CD8 epitopes identified in this study are naturally processed and presented by tumor cells, making them recognizable to CD8 T cells activated by optimized AFP.
The activated AFP-specific CD8 T cells play an important role in mediating antitumor effect of AFP vaccines.
Prime-boost immunization strategy induces long-lasting memory CD8 T cells that can detect and respond to evolving autochthonous HCC tumor induced by carcinogen and thus generate potent antitumor effects to prevent autochthonous HCC in mouse models.
Conclusion
Adoptive transfer of CIK immune cells activated in vitro delays HCC recurrence after surgery and enhances the outcome of conventional HCC treatments. Further understanding the mechanisms of how CIK cells work is required to improve its antitumor efficacy.
The efficacy of current HCC vaccines based on HCC tumor lysate and native AFP (or GPC3) protein needs improvement in order to generate meaningful antitumor effects.
Antigen engineering by epitope optimization and genetic immunization has great potential to create highly effective and antigen-defined HCC vaccines to potently activate immune cells to enhance antitumor effects.
Future perspective
Develop AFP- and GPC3-based human HCC vaccines by the antigen-engineering approach of epitope optimization and genetic immunization.
Identify high-affinity TCR genes and antibody genes that may be used to create reprogrammed T cells to treat HCC patients.
Explore combinatorial immunotherapy approach by incorporating the checkpoint blockade antibodies into HCC vaccination.
Conduct clinical trials to study the prevention of HCC in high-risk populations by means of administering HCC vaccines.
Acknowledgements
The authors wish to acknowledge the assistance of Grace Ho in the English editing of this manuscript.
Footnotes
Financial & competing interests disclosure
Research in Y He’s laboratory has been supported by NIH/NCI grants and Georgia Research Alliance. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.CDC. United States Cancer Statistics Public Information Data. http://wonder.cdc.gov/cancer.html.
- 3.Chang MH, You SL, Chen CJ, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J. Natl Cancer Inst. 2009;101(19):1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 4.Wright TL. Introduction to chronic hepatitis B infection. Am. J. Gastroenterol. 2006;101(Suppl. 1):S1–S6. doi: 10.1111/j.1572-0241.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 5.Sy T, Jamal MM. Epidemiology of hepatitis C virus (HCV) infection. Int. J. Med. Sci. 2006;3(2):41–46. doi: 10.7150/ijms.3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am. J. Gastroenterol. 2013;108(8):1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 8.Corey KE, Pratt DS. Current status of therapy for hepatocellular carcinoma. Ther. Adv. Gastroenterol. 2009;2(1):45–57. doi: 10.1177/1756283X08100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J. Surg. 2011;35(4):858–867. doi: 10.1007/s00268-010-0928-z. [DOI] [PubMed] [Google Scholar]
- 10.Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: unique challenges and clinical opportunities. Oncoimmunology. 2012;1(1):48–55. doi: 10.4161/onci.1.1.18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bei R, Mizejewski GJ. Alpha fetoprotein is more than a hepatocellular cancer biomarker: from spontaneous immune response in cancer patients to the development of an AFP-based cancer vaccine. Curr. Mol. Med. 2011;11(7):564–581. doi: 10.2174/156652411800615162. [DOI] [PubMed] [Google Scholar]
- 12.Buonaguro L, Petrizzo A, Tagliamonte M, Tornesello ML, Buonaguro FM. Challenges in cancer vaccine development for hepatocellular carcinoma. J. Hepatol. 2013;59(4):897–903. doi: 10.1016/j.jhep.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Zhu AX, Gold PJ, El-Khoueiry AB, et al. First-in-man Phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2013;19(4):920–928. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 14.Mizejewski GJ. Alpha-fetoprotein (AFP)-derived peptides as epitopes for hepatoma immunotherapy: a commentary. Cancer Immunol. Immunother. 2009;58(2):159–170. doi: 10.1007/s00262-008-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng M, Ho M. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett. 2013;588(2):377–382. doi: 10.1016/j.febslet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J. Transl. Med. 2013;11:83. doi: 10.1186/1479-5876-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesiano G, Todorovic M, Gammaitoni L, et al. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin. Biol. Ther. 2012;12(6):673–684. doi: 10.1517/14712598.2012.675323. [DOI] [PubMed] [Google Scholar]
- 18.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 19.Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig. Liver Dis. 2009;41(1):36–41. doi: 10.1016/j.dld.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Xie F, Zhang X, Li H, et al. Adoptive immunotherapy in postoperative hepatocellular carcinoma: a systemic review. PLoS ONE. 2012;7(8):e42879. doi: 10.1371/journal.pone.0042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong JH, Ma L, Wu LC, et al. Adoptive immunotherapy for postoperative hepatocellular carcinoma: a systematic review. Int. J. Clin. Pract. 2012;66(1):21–27. doi: 10.1111/j.1742-1241.2011.02814.x. [DOI] [PubMed] [Google Scholar]
- 22. Pan K, Li YQ, Wang W, et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann. Surg. Oncol. 2013;20(13):4305–4311. doi: 10.1245/s10434-013-3144-x. •• The latest data of a cytokine-induced killer cell clinical trial using a large population of patients, generating convincing data regarding to the efficacy of cytokine-induced killer cells.
- 23.Huang ZM, Li W, Li S, et al. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J. Immunother. 2013;36(5):287–293. doi: 10.1097/CJI.0b013e3182948452. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Carmona MA, Marten A, Hoffmann P, et al. Patient-derived dendritic cells transduced with an a-fetoprotein-encoding adenovirus and co-cultured with autologous cytokine-induced lymphocytes induce a specific and strong immune response against hepatocellular carcinoma cells. Liver Int. 2006;26(3):369–379. doi: 10.1111/j.1478-3231.2005.01235.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee WC, Wang HC, Jeng LB, et al. Effective treatment of small murine hepatocellular carcinoma by dendritic cells. Hepatology. 2001;34(5):896–905. doi: 10.1053/jhep.2001.29003. [DOI] [PubMed] [Google Scholar]
- 26.Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J. Immunother. 2005;28(5):496–504. doi: 10.1097/01.cji.0000171291.72039.e2. [DOI] [PubMed] [Google Scholar]
- 27. Palmer DH, Midgley RS, Mirza N, et al. A Phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49(1):124–132. doi: 10.1002/hep.22626. • Together with Lee et al. [25], these two reports are the representative antigen-undefined hepatocellular carcinoma (HCC) vaccines using autologous or allogenic tumor cell lysate.
- 28.Chen M, Li G, Yan J, et al. Reevaluation of glypican-3 as a serological marker for hepatocellular carcinoma. Clin. Chim. Acta. 2013;423:105–111. doi: 10.1016/j.cca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Tada F, Abe M, Hirooka M, et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012;41(5):1601–1609. doi: 10.3892/ijo.2012.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8 T-cell responses in hepatocellular carcinoma. Hepatology. 2013;59(4):1415–1426. doi: 10.1002/hep.26731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greten TF, Forner A, Korangy F, et al. A Phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. doi: 10.1186/1471-2407-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butterfield LH, Ribas A, Dissette VB, et al. A Phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin. Cancer Res. 2006;12(9):2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Xiao GQ, Yan LN, et al. Value of alpha-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J. Gastroenterol. 2013;19(11):1811–1819. doi: 10.3748/wjg.v19.i11.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm CF, Ortmann D, Mohr L, et al. Mouse alpha-fetoprotein-specific DNA-based immunotherapy of hepatocellular carcinoma leads to tumor regression in mice. Gastroenterology. 2000;119(4):1104–1112. doi: 10.1053/gast.2000.18157. [DOI] [PubMed] [Google Scholar]
- 35.Vollmer CM, Jr, Eilber FC, Butterfield LH, et al. Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res. 1999;59(13):3064–3067. [PubMed] [Google Scholar]
- 36. Butterfield LH, Ribas A, Meng WS, et al. T-cell responses to HLA-A•0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin. Cancer Res. 2003;9(16 Pt 1):5902–5908. • The first report of the α -fetoprotein-based HCC vaccines.
- 37.Tian G, Yi JL, Xiong P. Specific cellular immunity and antitumor responses in C57BL/6 mice induced by DNA vaccine encoding murine AFP. Hepatobiliary Pancreat. Dis. Int. 2004;3(3):440–443. [PubMed] [Google Scholar]
- 38.Cany J, Barteau B, Tran L, et al. AFP-specific immunotherapy impairs growth of autochthonous hepatocellular carcinoma in mice. J. Hepatol. 2011;54(1):115–121. doi: 10.1016/j.jhep.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Hanke P, Serwe M, Dombrowski F, Sauerbruch T, Caselmann WH. DNA vaccination with AFP-encoding plasmid DNA prevents growth of subcutaneous AFP-expressing tumors and does not interfere with liver regeneration in mice. Cancer Gene Ther. 2002;9(4):346–355. doi: 10.1038/sj.cgt.7700445. [DOI] [PubMed] [Google Scholar]
- 40.Tan XH, Zhu Q, Liu C, Liu XL, Shao XT, Wei B. Immunization with dendritic cells infected with human AFP adenovirus vector effectively elicits immunity against mouse hepatocellular carcinomas. Zhonghua Zhong Liu Za Zhi. 2006;28(1):13–16. [PubMed] [Google Scholar]
- 41.Pang XH, Chen MS, Jia WH, Zhou XX. Inhibitory effects of human AFP-derived peptide-pulsed dendritic cells on mouse hepatocellular carcinoma. Ai Zheng. 2008;27(12):1233–1238. [PubMed] [Google Scholar]
- 42.Zhang W, Liu J, Wu Y, et al. Immunotherapy of hepatocellular carcinoma with a vaccine based on xenogeneic homologous alpha fetoprotein in mice. Biochem. Biophys. Res. Commun. 2008;376(1):10–14. doi: 10.1016/j.bbrc.2008.08.061. [DOI] [PubMed] [Google Scholar]
- 43.Lan YH, Li YG, Liang ZW, et al. A DNA vaccine against chimeric AFP enhanced by HSP70 suppresses growth of hepatocellular carcinoma. Cancer Immunol. Immunother. 2007;56(7):1009–1016. doi: 10.1007/s00262-006-0254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Lin H, Wang Q. Specific genetic immunotherapy induced by recombinant vaccine alpha-fetoprotein-heat shock protein 70 complex. Phys. Proc. 2012;33:738–742. [Google Scholar]
- 45.Rodriguez MM, Ryu SM, Qian C, et al. Immunotherapy of murine hepatocellular carcinoma by alpha-fetoprotein DNA vaccination combined with adenovirus-mediated chemokine and cytokine expression. Hum. Gene Ther. 2008;19(7):753–759. doi: 10.1089/hum.2007.130. [DOI] [PubMed] [Google Scholar]
- 46.Meng WS, Butterfield LH, Ribas A, et al. Alpha-fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res. 2001;61(24):8782–8786. [PubMed] [Google Scholar]
- 47.Saeki A, Nakao K, Nagayama Y, et al. Diverse efficacy of vaccination therapy using the alpha-fetoprotein gene against mouse hepatocellular carcinoma. Int. J. Mol. Med. 2004;13(1):111–116. [PubMed] [Google Scholar]
- 48.Evdokimova VN, Butterfield LH. Alpha-fetoprotein and other tumour-associated antigens for immunotherapy of hepatocellular cancer. Expert Opin. Biol. Ther. 2008;8(3):325–336. doi: 10.1517/14712598.8.3.325. [DOI] [PubMed] [Google Scholar]
- 49.Butterfield LH, Koh A, Meng W, et al. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59(13):3134–3142. [PubMed] [Google Scholar]
- 50.Butterfield LH, Meng WS, Koh A, et al. T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein. J. Immunol. 2001;166(8):5300–5308. doi: 10.4049/jimmunol.166.8.5300. [DOI] [PubMed] [Google Scholar]
- 51.Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997;57(22):5179–5184. [PubMed] [Google Scholar]
- 52.Iwama T, Horie K, Yoshikawa T, et al. Identification of an H2-Kb or H2-Db restricted and glypican-3-derived cytotoxic T-lymphocyte epitope peptide. Int. J. Oncol. 2013;42(3):831–838. doi: 10.3892/ijo.2013.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakatsura T, Komori H, Kubo T, et al. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clin. Cancer Res. 2004;10(24):8630–8640. doi: 10.1158/1078-0432.CCR-04-1177. [DOI] [PubMed] [Google Scholar]
- 54.Komori H, Nakatsura T, Senju S, et al. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin. Cancer Res. 2006;12(9):2689–2697. doi: 10.1158/1078-0432.CCR-05-2267. [DOI] [PubMed] [Google Scholar]
- 55. Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin. Cancer Res. 2012;18(13):3686–3696. doi: 10.1158/1078-0432.CCR-11-3044. •• Promising clinical trial data showing that human glypican-3 peptide-based vaccines generate glypican-3-specific immune responses and clinical antitumor efficacy.
- 56.Guevara-Patino JA, Engelhorn ME, Turk MJ, et al. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. J. Clin. Invest. 2006;116(5):1382–1390. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Peng Y, Mi M, et al. Lentivector immunization stimulates potent CD8 T cell responses against melanoma self-antigen tyrosinase-related protein 1 and generates antitumor immunity in mice. J. Immunol. 2009;182(10):5960–5969. doi: 10.4049/jimmunol.0900008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Overwijk WW, Tsung A, Irvine KR, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of ‘self-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998;188(2):277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran L, Judor JP, Gauttier V, et al. The immunogenicity of the tumor-associated antigen alpha-fetoprotein is enhanced by a fusion with a transmembrane domain. J. Biomed. Biotechnol. 2012;878657(2012) doi: 10.1155/2012/878657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dyall R, Bowne WB, Weber LW, et al. Heteroclitic immunization induces tumor immunity. J. Exp. Med. 1998;188(9):1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slansky JE, Rattis FM, Boyd LF, et al. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13(4):529–538. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 62.Van Stipdonk MJ, Badia-Martinez D, Sluijter M, Offringa R, Van Hall T, Achour A. Design of agonistic altered peptides for the robust induction of CTL directed towards H-2Db in complex with the melanoma-associated epitope gp100. Cancer Res. 2009;69(19):7784–7792. doi: 10.1158/0008-5472.CAN-09-1724. [DOI] [PubMed] [Google Scholar]
- 63.Van Der Burg SH, Visseren MJ, Brandt RM, Kast WM, Melief CJ. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J. Immunol. 1996;156(9):3308–3314. [PubMed] [Google Scholar]
- 64.Ekeruche-Makinde J, Clement M, Cole DK, et al. T-cell receptor-optimized peptide skewing of the T-cell repertoire can enhance antigen targeting. J. Biol. Chem. 2012;287(44):37269–37281. doi: 10.1074/jbc.M112.386409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Speiser DE, Baumgaertner P, Voelter V, et al. Unmodified self antigen triggers human CD8 T cells with stronger tumor reactivity than altered antigen. Proc. Natl Acad. Sci. USA. 2008;105(10):3849–3854. doi: 10.1073/pnas.0800080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ilipazzi P, Pilla L, Mariani L, et al. Limited induction of tumor cross-reactive T cells without a measurable clinical benefit in early melanoma patients vaccinated with human leukocyte antigen class I-modified peptides. Clin. Cancer Res. 2012;18(23):6485–6496. doi: 10.1158/1078-0432.CCR-12-1516. [DOI] [PubMed] [Google Scholar]
- 67.Meng WS, Butterfield LH, Ribas A, et al. Fine specificity analysis of an HLA-A2.1-restricted immunodominant T cell epitope derived from human alpha-fetoprotein. Mol Immunol. 2000;37(16):943–950. doi: 10.1016/s0161-5890(01)00017-7. [DOI] [PubMed] [Google Scholar]
- 68. Hong Y, Peng Y, Guo ZS, et al. Epitope-optimized alpha-fetoprotein genetic vaccines prevent carcinogen-induced murine autochthonous hepatocellular carcinoma. Hepatology. 2014;59(4):1448–1458. doi: 10.1002/hep.26893. •• The most recent HCC vaccine study showing that epitope optimization and genetic immunization could generate much improved α-fetoprotein-specific CD8 responses, which is correlated to better antitumor effect in mice.
- 69.Houghton CS, Engelhorn ME, Liu C, et al. Immunological validation of the EpitOptimizer program for streamlined design of heteroclitic epitopes. Vaccine. 2007;25(29):5330–5342. doi: 10.1016/j.vaccine.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 70.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24(5):643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Y, Falo LD., Jr Lentivirus as a potent and mechanistically distinct vector for genetic immunization. Curr. Opin. Mol. Ther. 2007;9(5):439–446. [PMC free article] [PubMed] [Google Scholar]
- 72.Esslinger C, Chapatte L, Finke D, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J. Clin. Invest. 2003;111(11):1673–1681. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arce F, Breckpot K, Collins M, Escors D. Targeting lentiviral vectors for cancer immunotherapy. Curr. Cancer Ther. Rev. 2011;7(4):248–260. doi: 10.2174/157339411797642605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao H, Peng Y, Hong Y, et al. Lentivector prime and vaccinia virus vector boost generate high-quality CD8 memory T cells and prevent autochthonous mouse melanoma. J. Immunol. 2011;187(4):1788–1796. doi: 10.4049/jimmunol.1101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong Y, Peng Y, Mi M, et al. Lentivector expressing HBsAg and immunoglobulin Fc fusion antigen induces potent immune responses and results in seroconversion in HBsAg transgenic mice. Vaccine. 2011;29(22):3909–3916. doi: 10.1016/j.vaccine.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 77. Callahan MK, Wolchok JD. At the bedside: CTLA-4-and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013;94(1):41–53. doi: 10.1189/jlb.1212631. • A recent review on the topic of checkpoint blockade for tumor immunotherapy.
- 78.Zhou Q, Xiao H, Liu Y, et al. Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization. J. Immunol. 2010;185(9):5082–5092. doi: 10.4049/jimmunol.1001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ulcickas Yood M, Quesenberry CP, Jr, Guo D, et al. Incidence of hepatocellular carcinoma among individuals with hepatitis B virus infection identifed using an automated data algorithm. J. Viral. Hepat. 2008;15(1):28–36. doi: 10.1111/j.1365-2893.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 80.Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J. Natl Cancer Inst. 2000;92(14):1159–1164. doi: 10.1093/jnci/92.14.1159. [DOI] [PubMed] [Google Scholar]
- 81.Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011;29(11):550–557. doi: 10.1016/j.tibtech.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. •• Demonstrating the potent antitumor efficacy of chimeric antigen receptor-modified autologous T cells.