Abstract
IMPORTANCE
Although anti–vascular endothelial growth factor treatment of neovascular age-related macular degeneration (AMD) results in improved vision overall, loss of substantial vision can occur. Understanding the processes that lead to loss of vision may lead to preventive strategies.
OBJECTIVE
To determine the incidence, characteristics, causes, and baseline predictors of sustained visual acuity loss after 2 years of treatment with ranibizumab or bevacizumab for neovascular AMD.
DESIGN, SETTING, AND PARTICIPANTS
A cohort study within a randomized clinical trial of participants in the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT).
INTERVENTIONS
Participants were randomly assigned to treatment with ranibizumab or bevacizumab and to 2 years of monthly or as needed injections or monthly injections for 1 year and as needed injections the following year.
MAIN OUTCOMES AND MEASURES
Sustained visual acuity loss, defined as loss of 15 or more letters from baseline at weeks 88 and 104.
RESULTS
Among 1030 participants, 61 eyes (5.9%) developed sustained visual acuity loss in 2 years. Within this group, visual acuity decreased gradually over time, with a mean decrease of 2, 19, and 33 letters from baseline at 4 weeks, 1 year, and 2 years, respectively. At 2 years, eyes with sustained visual acuity loss had more scarring (60.0% vs 41.4%, P = .007), more geographic atrophy (GA) (31.6% vs 20.7%, P = .004), larger lesions (16 vs 8 mm2, P < .001), and higher proportions of intraretinal fluid (82.5% vs 51.0%, P < .001), subretinal hyperreflective material (84.5% vs 44.2%, P < .001), retinal thinning (43.3% vs 23.0%, P < .001), and thickening (20.0% vs 12.1%, P < .001). Likely causes of sustained visual acuity loss included foveal scarring (44.3%), pigmentary abnormalities (27.9%), and foveal GA (11.5%). Baseline factors independently associated with a higher incidence of sustained visual acuity loss were the presence of nonfoveal GA (odds ratio [OR], 2.86; 95% CI, 1.35–6.08; P = .006), larger area of choroidal neovascularization (OR for a >4-disc area vs ≤1-disc area, 3.91; 95% CI, 1.70–9.03; P = .007), and bevacizumab treatment (OR, 1.83; 95% CI, 1.07–3.14; P = .03).
CONCLUSIONS AND RELEVANCE
Sustained visual acuity loss was relatively rare in CATT. The development of foveal scar, pigmentary abnormalities, or GA contributed to most of the sustained visual acuity loss. Risk was 3% higher among eyes treated with bevacizumab. Treatment that targeted the prevention of scarring or GA may improve vision outcomes.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00593450
Anti–vascular endothelial growth factor (anti-VEGF) treatment is highly effective in preserving visual acuity in most eyes with neovascular age-related macular degeneration (AMD).1–7 However, despite therapy, some patients still lose a significant amount of vision. Two pivotal studies of ranibizumab, the Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD (MARINA) and Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR), reported a loss of 15 letters or more in 9% to 10% of eyes at 2 years.1,2 Furthermore, a comparison of eyes that lost 15 or more letters with those that gained 15 or more letters after monthly ranibizumab therapy suggested that increased areas of retinal pigment epithelium (RPE) abnormalities and increased total lesion areas at 2 years were associated with a worse visual acuity outcome.8 These data may not apply to current clinical practice because many ophthalmologists do not treat their patients monthly,9 and bevacizumab, rather than ranibizumab, is the most commonly used drug to treat neovascular AMD in the United States.10 Furthermore, there are insufficient data to evaluate reasons for sustained visual acuity loss and the association between vision loss and the specific anti-VEGF medication or treatment regimen.
The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) demonstrated that bevacizumab and ranibizumab are equally effective during a 2-year period to improve visual acuity of eyes with neovascular AMD.5,6 Although most participants in CATT (75.1%) had the same or improved visual acuity at 2 years relative to baseline acuity, 9.2% had lost 15 or more letters.6 Some of these visual acuity losses occurred early during follow-up and were sustained to the end of the study. Because eyes with sustained vision losses are unlikely to regain substantial amounts of vision, it is important to understand the incidence of this unfavorable outcome and the morphologic features and baseline predictors associated with sustained visual acuity loss. Using data from the CATT, we provide a comprehensive evaluation of morphologic features and likely causes of sustained visual acuity loss at 2 years and evaluate a full array of baseline candidate predictors of sustained visual acuity loss.
Methods
Details on the study design and methods for CATT have been reported previously5,6 and are available at clinicaltrials.gov (identifier NCT00593450).
Study Participants
Institutional review boards associated with each center approved the study protocol, and written informed consent was obtained from each participant. At enrollment, participants were randomized to 1 of the 4 treatment groups: (1) ranibizumab monthly, (2) bevacizumab monthly, (3) ranibizumab as needed, and (4) bevacizumab as needed. At 1 year, participants initially assigned to monthly treatment retained their drug assignment but were reassigned randomly to either monthly or as needed treatment. Participants initially assigned to as needed treatment retained both their drug and regimen for year 2.
Study enrollment criteria included age of 50 years or older, the presence in the study eye of previously untreated active choroidal neovascularization (CNV) due to AMD, absence of foveal center geographic atrophy (GA), and visual acuity of 20/25 to 20/320 on electronic visual acuity testing.11
Study Procedures
Certified photographers took color fundus photographs (CFPs) and fluorescein angiograms (FAs) at baseline, 1 year, and 2 years. Certified technicians performed optical coherence tomography (OCT) at baseline and follow-up visits every 4 weeks. Stratus time domain OCT systems (Carl Zeiss Meditec, Inc)were used at all year 1 visits and some year 2 visits, and spectral domain OCT systems were used at 2058 (22.6%) of the year 2 visits.
Two masked trained readers at the Fundus Photography Reading Center at the University of Pennsylvania independently evaluated CFPs and FAs for the lesion characteristics at baseline, 1 year, and 2 years, following the CATT grading protocol.12 Discrepancies between readers were adjudicated by the director of the reading center (J.E.G.). The area of the CNV and total area of the CNV lesion at baseline and the total area of the CNV lesion at 1 year and 2 years were measured with ImageJ (http://rsbweb.nih.gov/ij/).
Masked trained readers at the OCT Reading Center at Duke University evaluated the presence and location of fluid, the presence of subretinal hyperreflective material (SHRM), and RPE elevation, following a standard protocol.13 In addition, readers measured the thickness at the foveal center of the retina, subretinal fluid, and subretinal tissue complex (ie, the distance from the bound of the retina to the Bruch membrane that included pigment epithelial detachment, CNV, blood, and fibrosis). At all visits, masked certified visual acuity examiners measured visual acuity using the electronic visual acuity test with refraction only at weeks 4, 12, 24, 36, 52, 64, 76, 88, and 104.
Determination of Sustained Visual Acuity Loss and Its Likely Cause
To account for random visual acuity fluctuations over time or sporadic vision loss in eyes with AMD, sustained visual acuity loss was defined as the loss of 15 letters or more from baseline at weeks 88 and 104. Visual acuity at week 88 was not available (because of a missed visit) in 94 eyes (9.1%), and in these cases, the best-corrected visual acuity measured at week 76 was used to confirm a sustained decrease in vision. Fourteen eyes with measured visual acuity at week 104 but not at weeks 88 and 76 were defined as not having sustained visual acuity loss.
A retina specialist (B.J.K.) reviewed the CFPs and FAs to determine the most likely cause of sustained visual acuity loss. Priority was given to pathologic findings at the foveal center seen on images from the patient’s last visit. Gradings from the Fundus Photography Reading Center and OCT Reading Center were also taken into account. When CFPs and FAs were not sufficient to determine the cause of sustained visual acuity loss, the OCT retinal thickness was taken into account. This was the case when there were only RPE changes at the fovea because RPE changes can be associated with abnormally thin (ie, <120 μm) or thick (>212 μm) retinal tissue.14 The OCT retinal thickness was not labeled as the primary cause of vision loss if there was scarring, GA, RPE tear, hemorrhage, or active CNV at the foveal center. For 12 cases in which the cause of vision was still not clear or the recorded OCT retinal thickness seemed incongruous with the foveal pathologic findings, a multimodal imaging approach was taken and the OCT images were reviewed. Cases that did not have an obvious single cause for the vision loss were discussed among the clinical investigators (B.J.K. and J.E.G.) and the reading center director (E.D.). Some of these cases were classified as having no clear reason for visual acuity loss.
Statistical Analysis
The incidence of sustained visual acuity loss was calculated as the proportion of eyes with sustained visual acuity loss among patients who completed 2 years of follow-up. The visual acuity progression during 2 years was compared between eyes with and without sustained visual acuity loss. Time to the first development of a 3-line loss that resulted in sustained visual acuity loss was estimated using a Kaplan-Meier curve. Morphologic features determined by CFPs, FAs, and OCT images at baseline and at 2 years were compared between eyes with and without sustained visual acuity loss, using the Fisher exact test for comparison of proportions and the t test for comparison of means. Baseline predictors of sustained visual acuity loss were evaluated initially by univariate analysis followed by multivariate analysis using logistic regression models. The drug group, regimen group, and their interaction were tested in both univariate and multivariate analyses. Adjusted odds ratios (ORs) and associated 95% CIs were calculated from a multivariate logistic regression model. All data analyses were performed using SAS statistical software, version 9.2 (SAS Institute Inc), and a 2-sided P < .05 was considered statistically significant.
Results
Incidence of Sustained Visual Acuity Loss
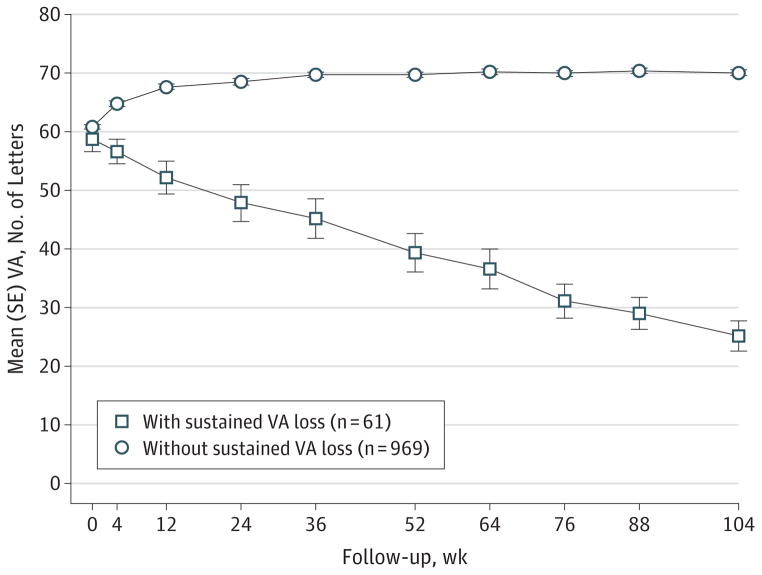
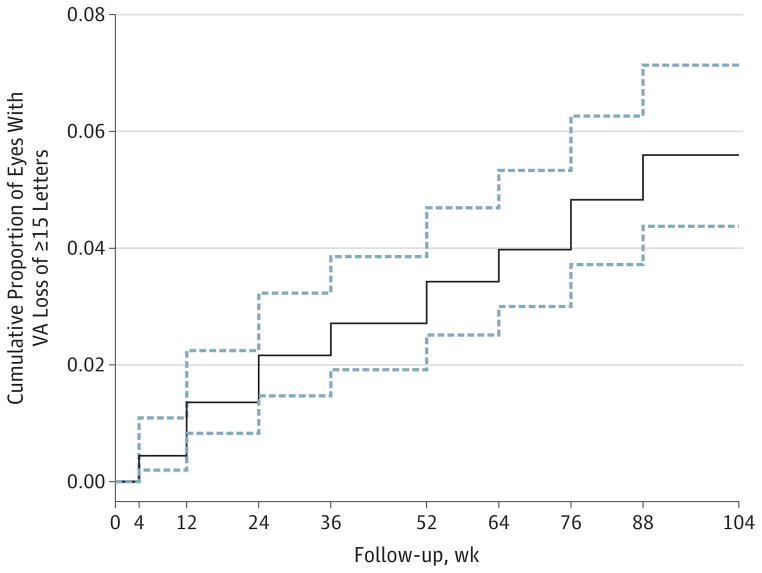
Among 1030 patients who completed 2 years of follow-up, 61 (5.9%; 95% CI, 4.6%–7.5%) developed sustained visual acuity loss of 15 letters or more, including 20 (1.9%; 95% CI, 1.3%–3.0%) with sustained visual acuity loss of 30 letters or more. Of the 61 eyes with sustained visual acuity loss, the mean visual acuity decreased gradually over time (Figure 1), with a mean decrease of 2 letters from baseline at 4 weeks, 19 letters at 1 year, and 33 letters at the end of 2 years compared with the mean gain of 4 letters from baseline at 4 weeks and 9 letters at both 1 year and 2 years among eyes without sustained visual acuity loss. Examination of visual acuity over time for each of the 61 eyes confirmed that visual acuity decreased gradually in 46 eyes (75.4%), and the remaining 15 eyes (24.6%) had an abrupt decrease (defined as a loss of ≥15 letters between 2 visits with visual acuity measurement after refraction) in visual acuity at some time in 2 years (eFigure 1 in the Supplement). At week 4, a total of 36 eyes (59.0%) with sustained visual acuity loss had no increase in visual acuity after one injection compared with 306 of the 969 eyes (31.6%) without sustained visual acuity loss (P < .001). Among the 61 eyes with sustained visual acuity loss, the mean visual acuity at year 2 was 25 letters (approximate Snellen equivalent of 20/320), with 38 eyes (62.3%) having lost 30 letters or more from baseline. Five eyes (8.2%) had visual acuity of 20/50 to 20/80, 14 (23.0%) had visual acuity of 20/100 to 20/160, 12 (19.7%) had visual acuity of 20/200 to 20/320, and 30 (49.2%) had visual acuity worse than 20/400. Kaplan-Meier curves for time to develop a loss of 15 or more letters in visual acuity that led to a sustained visual acuity loss (Figure 2) showed that the onset of visual acuity loss occurred throughout the 2-year follow-up period.
Figure 1. Visual Acuity (VA) Over Time.
Mean (SE) VA was plotted for patients with and without developing sustained VA loss.
Figure 2. Kaplan-Meier Curve for Time to First Loss of 3 Lines of Visual Acuity (VA).
The solid stepwise line is the point estimate of the proportion of eyes with VA loss of 15 letters or more. The dashed lines are the 95% CIs.
Features Associated With Sustained Visual Acuity Loss at 2 Years
Morphologic features in eyes with and without sustained visual acuity loss at 2 years are listed in Table 1. At 2 years, eyes with sustained visual acuity loss were more likely to have scarring (P = .007), GA (P = .004), and hemorrhage (P = .03). The total area of the CNV lesion increased more (P < .001) in eyes with sustained visual acuity loss and was approximately twice as large (P < .001) as eyes without sustained visual acuity loss. Eyes with sustained visual acuity loss were more likely to have intraretinal fluid (P < .001), SHRM (P < .001), retinal thinning or thickening (P < .001), thicker subretinal tissue complex (P = .02), and thinner subretinal fluid (P = .04), and were less likely to have subretinal fluid (P = .006). The eyes that developed sustained visual acuity loss were not significantly different from eyes without sustained visual acuity loss in the mean number of visits completed in 2 years (23.6 vs 24.1, P = .07) and mean number of treatments received (15.2 vs 16.8, P = .08).
Table 1.
Comparison of Features at 2 Years in Eyes With and Without Sustained Visual Acuity Loss
| Feature | Sustained Visual Acuity Lossa | P Value | |
|---|---|---|---|
| With (n = 61) | Without (n = 969) | ||
| Color fundus photographic or fluorescein angiogram features at 2 years | |||
| Scar | 36 (60.0) | 391 (41.4) | .007 |
| Atrophy | |||
| Geographic | 19 (31.6) | 197 (20.7) | .004 |
| Nongeographic | 22 (37.3) | 457 (49.0) | .08 |
| Hemorrhage contiguous with lesion | 5 (8.3) | 25 (2.6) | .03 |
| Total area of CNV lesion, mean (SE), mm2 | 15.9 (1.8) | 7.8 (0.2) | <.001 |
| Change in total area of CNV lesion from baseline, mean (SE), mm2 | 7.8 (1.9) | 1.6 (0.2) | <.001 |
| OCT image features at 2 years | |||
| Fluid | |||
| Intraretinal | 47 (82.5) | 479 (51.0) | <.001 |
| Subretinal | 10 (19.2) | 343 (36.8) | .006 |
| Sub-RPE | 16 (32.7) | 348 (37.9) | .13 |
| Presence of subretinal hyperreflective material | 49 (84.5) | 418 (44.2) | <.001 |
| Retinal thickness at foveal center, μm | |||
| <120 | 26 (43.3) | 220 (23.0) | <.001 |
| 120–212 | 22 (36.7) | 620 (64.9) | |
| >212 | 12 (20.0) | 116 (12.1) | |
| Mean (SE) | 187 (23.1) | 158 (2.1) | .004 |
| Subretinal fluid thickness at foveal center, mean (SE), μm | 0.4 (0.3) | 9.2 (1.1) | .04 |
| Subretinal tissue complex thickness at foveal center, mean (SE), μm | 157 (19.1) | 125 (3.3) | .02 |
| Follow-up and treatment in 2 years | |||
| No. of visits completed, mean (SE) | 23.6 (0.3) | 24.1 (0.1) | .07 |
| No. of anti-VEGF treatments, mean (SE) | 15.2 (0.7) | 16.8 (0.2) | .08 |
Abbreviations: CNV, choroidal neovascularization; OCT, optical coherence tomography; RPE, retinal pigment epithelium; VEGF, vascular endothelial growth factor.
Data are presented as number (percentage) of patients unless otherwise indicated.
Likely Causes of Sustained Visual Acuity Loss
The most likely causes of sustained visual acuity loss, as determined from review of CFPs, FAs, and OCT images, were foveal scarring in 27 eyes (44.3%), pigmentary abnormalities in 17 eyes (27.9%), foveal GA in 7 eyes (11.5%), RPE tear in 4 eyes (6.6%), active CNV in 3 eyes (4.9%), and hemorrhage in 1 eye (1.6%) (Table 2). One of the eyes with foveal scarring also had endophthalmitis at week 60. Among 17 eyes with pigmentary abnormalities, 9 eyes (52.9%) had retinal thinning (<120 μm), and 4 eyes (23.5%) had retinal thickening (>212 μm). Representative eyes with pigmentary abnormalities that had normal retinal thickness, retinal thinning, or retinal thickening are presented in eFigure 2 in the Supplement. When the most likely causes were compared between ranibizumab and bevacizumab, foveal scar tended to be the cause more often in the bevacizumab-treated group (51.4% vs 33.3%, P = .19), and foveal GA tended to be the cause more often in the ranibizumab-treated group (5.4% vs 20.8%, P = .10).
Table 2.
Frequency of Likely Causes of Sustained Visual Acuity Loss Overall and by Drug Group
| Likely Cause of Sustained Visual Acuity Loss | No. (%) of Eyes | ||
|---|---|---|---|
| Ranibizumab (n = 24) | Bevacizumab (n = 37) | All Combined (N = 61) | |
| Scar at foveal center | 8 (33.3) | 19 (51.4) | 27 (44.3) |
| Geographic atrophy at foveal center | 5 (20.8) | 2 (5.4) | 7 (11.5) |
| Pigmentary abnormalities | 7 (29.2) | 10 (27.0) | 17 (27.9) |
| Retinal thinning (<120 μm) | 3 (12.5) | 6 (16.2) | 9 (14.8) |
| Retinal thickening (>212 μm) | 2 (8.3) | 2 (5.4) | 4 (6.6) |
| Normal retinal thickness (120–212 μm) | 2 (8.3) | 2 (5.4) | 4 (6.6) |
| RPE tear | 2 (8.3) | 2 (5.4) | 4 (6.6) |
| Hemorrhage at foveal center | 0 | 1 (2.7) | 1 (1.6) |
| Active CNV | 2 (8.3) | 1 (2.7) | 3 (4.9) |
| No clear reason | 0 | 2 (5.4) | 2 (3.3) |
Abbreviations: CNV, choroidal neovascularization; RPE, retinal pigment epithelium.
Baseline Predictors for Sustained Visual Acuity Loss
In univariate analysis, baseline factors significantly associated with increased risk of sustained visual acuity loss were presence of nonfoveal GA, larger area of CNV, larger total area of CNV lesion, poor baseline vision (visual acuity of ≤20/200) in the study eye, baseline visual acuity of 20/50 or worse in the fellow eye, and presence of intraretinal fluid not at the foveal center (eTable in the Supplement). The incidence of sustained visual acuity loss did not differ among the 3 treatment regimen groups (P = .20), with 4.2% in eyes treated monthly for 2 years, 7.9% in eyes switched from monthly in year 1 to as needed in year 2, and 5.8% in eyes treated as needed for 2 years. The treatment drug was marginally associated with sustained visual acuity loss; bevacizumab-treated eyes had a higher incidence of sustained visual acuity loss than ranibizumab-treated eyes (7.4% vs 4.5%, P = .06). The dosing regimen and the interaction between drug and dosing regimen were not statistically significant (P = .71 and P = .18, respectively). In multivariate analysis (Table 3), the baseline predictors independently associated with increased risk of sustained visual acuity loss were nonfoveal GA (P = .006), larger CNV area (P = .007), and bevacizumab treatment (P = .03).
Table 3.
Multivariate Analysis of Baseline Factors Associated With Sustained Visual Acuity Loss
| Factor | Patients at Risk, No.a | Sustained Visual Acuity Loss, No. (%) | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Baseline nonfoveal geographic atrophy | ||||
| None or questionable | 959 | 51 (5.3) | 1 [Reference] | .006 |
| Present | 70 | 10 (14.3) | 2.86 (1.35–6.08) | |
| Baseline area of CNV (disc area) | ||||
| ≤1 | 417 | 14 (3.4) | 1 [Reference] | .007 |
| >1-≤2 | 203 | 10 (4.9) | 1.63 (0.70–3.76) | |
| >2-≤4 | 190 | 14 (7.4) | 2.44 (1.13–5.28) | |
| >4 | 100 | 11 (11.0) | 3.91 (1.70–9.03) | |
| Unknown | 119 | 12 (10.1) | 3.30 (1.47–7.44) | |
| Drug group | ||||
| Ranibizumab | 528 | 24 (4.6) | 1 [Reference] | .03 |
| Bevacizumab | 501 | 37 (7.4) | 1.83 (1.07–3.14) | |
| Regimen group | ||||
| Monthly always | 262 | 11 (4.2) | 1 [Reference] | .24 |
| Monthly in year 1, as needed in year 2 | 252 | 20 (7.9) | 1.92 (0.89–4.15) | |
| As needed always | 515 | 30 (5.8) | 1.37 (0.67–2.81) | |
Abbreviation: CNV, choroidal neovascularization.
One patient was excluded for an unknown status on baseline nonfoveal geographic atrophy.
Discussion
Our study evaluated the incidence, causes, and baseline predictors of sustained visual acuity loss among the 1030 participants in CATT treated for 2 years with either ranibizumab or bevacizumab. Sustained visual acuity loss of 15 letters or more occurred in 5.9% of participants at 2 years of treatment. Foveal scar, pigmentary abnormalities, and GA contributed to most cases (83.7%). In addition, presence of baseline GA, larger CNV baseline area, and bevacizumab treatment were independently associated with higher risk of sustained visual acuity loss.
The characteristics of eyes that lost vision when receiving monthly ranibizumab as part of the ANCHOR and MARINA trials were evaluated by Rosenfeld et al.8 Our study differs from that report in several ways. First, to minimize the effect of temporary or sporadic vision loss, our definition of sustained visual acuity loss required that the vision loss was present at both weeks 88 and 104 rather than week 104 only. Second, we compared morphologic features between eyes with sustained visual acuity loss to all other eyes without sustained visual acuity loss, whereas Rosenfeld et al used eyes that gained 15 letters or more as a comparison group. We chose to include all eyes in our analyses to increase generalizability. Third, we evaluated the OCT features of the eyes of CATT participants, whereas OCT images were generally not available for the ANCHOR and MARINA trials. Despite these differences, both studies found that increased total area of CNV lesion and RPE abnormalities are associated with worse vision outcome at 2 years.
Our study found that eyes with sustained visual acuity loss were more likely to have intraretinal fluid, SHRM, abnormal retinal thickness, and thicker subretinal tissue complex, and less likely to have subretinal fluid. In most eyes, SHRM, which comprises CNV (blood and fibrosis, when present), was seen at baseline and disappeared in some eyes after anti-VEGF treatment. However, at 2 years, a higher percentage of eyes with sustained visual acuity loss had SHRM (80.3% vs 43.1%, P < .001) or developed new SHRM (14.8% vs 4.2%, P < .001) compared with eyes without sustained visual acuity loss. The finding that intraretinal fluid, a thick subretinal lesion, and an abnormally thin or thick retina were each associated with sustained visual acuity loss is consistent with our recent observations that these factors are associated with worse visual acuity, irrespective of whether the visual acuity decline is sustained.14 These findings highlight the usefulness of OCT to detect morphologic AMD features associated with worse vision outcomes.
In addition, eyes that developed sustained visual acuity loss had poor final visual acuity outcomes. Among the 61 eyes with sustained visual acuity loss, most (68.9%) had visual acuity of 20/200 or worse, and 20% of eyes had visual acuity worse than 20/800 at 2 years. Our determination of the likely causes of sustained visual acuity loss, based on CFPs, FAs, and OCT images, were foveal scar (44.3%), pigmentary abnormalities (27.9%), and foveal GA (11.5%). Some less common ocular events, such as endophthalmitis, retinal hemorrhage, and RPE tear, can cause severe visual acuity loss (but not necessarily sustained visual acuity loss). Only 1 of 11 eyes with endophthalmitis developed sustained visual acuity loss, and the likely cause of visual acuity loss was foveal fibrotic scar. An RPE tear occurred in 23 study eyes, and only 4 of these eyes developed sustained visual acuity loss.
The percentage that developed sustained visual acuity loss was higher in eyes treated with bevacizumab than those treated with ranibizumab (4.6% vs 7.4%, P = .03 in multivariate analysis); the regimen and interaction between the drug and treatment regimen were not statistically significant (P = .71 and P = .18, respectively). With only 61 eyes with sustained visual acuity loss, we were not able to detect any difference between bevacizumab and ranibizumab in the causes of vision loss. We note that foveal scarring was more frequent among those treated with bevacizumab and foveal GA was more common in those treated with ranibizumab, but not to a statistically significant degree. Replication in other studies and longer follow-up of eyes of CATT participants would be required to determine whether the path to sustained visual acuity loss differs between the 2 drugs.
The presence of nonfoveal GA, larger CNV area at baseline, and bevacizumab treatment were independently associated with a higher risk of sustained visual acuity loss. However, other risk factors, such as age, baseline visual acuity, RPE elevation, lesion type, retinal angiomatous proliferation lesion, and retinal thickness on OCT image, that were associated with visual acuity outcomes (eg, final visual acuity, change in visual acuity, 3-line improvement in visual acuity) at 1 year15 were not significant predictors of sustained visual acuity loss. This difference may be due to the limited statistical power from the small number of cases with sustained visual acuity loss.
Conclusions
A total of 5.9% of eyes of CATT participants developed sustained visual acuity loss of 15 letters or more in 2 years of treatment with either ranibizumab or bevacizumab, and their visual acuity decreased gradually over time. Most sustained visual acuity loss was due to foveal scar, pigmentary abnormalities, or GA. The presence of baseline nonfoveal GA, larger lesion size, and bevacizumab treatment are predictive of sustained visual acuity loss. Visual acuity deteriorated gradually during 2 years in most eyes that developed sustained visual acuity loss, most likely due to progressive scarring or RPE changes, some of which developed over time into GA. New treatments that target the prevention of scar or GA formation may improve the visual acuity outcomes of anti-VEGF treatment.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by cooperative agreements U10 EY017823, U10 EY017825, U10 EY017826, and U10 EY017828 from the National Eye Institute, National Institutes of Health, US Department of Health and Human Services.
Footnotes
Supplemental content at jamaophthalmology.com
Group Information: Members of the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group are listed in the eAppendix in the Supplement.
Conflict of Interest Disclosures: Dr Kim was an advisory board participant in 2012 for Allergan and Eyetech. Dr Regillo has served as a consultant to, received grants from, and served on the speaker’s bureaus for Genentech and Regeneron. Dr Blinder has served as a consultant to Ocusoft and served on the speaker’s bureaus for Bausch & Lomb and Genentech. No other disclosures were reported.
Previous Presentation: This study was presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology; May 7, 2013; Seattle, Washington.
Author Contributions: Dr Ying had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ying, Kim, Maguire, Flaxel, Martin.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Ying, Grunwald, Flaxel, Regillo.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Ying, Maguire, Huang, Rahhal. Obtained funding: Maguire, Jaffe, Grunwald, Martin.
Administrative, technical, or material support: Daniel, Flaxel, Regillo.
Study supervision: Ying, Maguire, Daniel, Jaffe, Grunwald, Blinder, Regillo.
Role of the Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 4.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363–372. e5. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin DF, Maguire MG, Fine SL, et al. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravarthy U, Harding SP, Rogers CA, et al. IVAN Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B MARINA and ANCHOR Study Groups. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118 (3):523–530. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Curtis LH, Hammill BG, Qualls LG, et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 Medicare beneficiaries. Am J Ophthalmol. 2012;153(6):1116–1124. e1. doi: 10.1016/j.ajo.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Brechner RJ, Rosenfeld PJ, Babish JD, Caplan S. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 Medicare fee-for-service part B claims file. Am J Ophthalmol. 2011;151(5):887–895. e1. doi: 10.1016/j.ajo.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 12.Grunwald JE, Daniel E, Ying GS, et al. CATT Research Group. Photographic assessment of baseline fundus morphologic features in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119(8):1634–1641. doi: 10.1016/j.ophtha.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeCroos FC, Toth CA, Stinnett SS, Heydary CS, Burns R, Jaffe GJ CATT Research Group. Optical coherence tomography grading reproducibility during the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2012;119(12):2549–2557. doi: 10.1016/j.ophtha.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe GJ, Martin DF, Toth CA, et al. Association of macular morphology with visual acuity in the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Ophthalmology. 2013;120 (9):1860–1870. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying GS, Huang J, Maguire MG, et al. Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122–129. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.