Abstract
Background
Neutrophil expression of the Fcγ receptor I (CD64) is upregulated in adult patients with clinically active inflammatory bowel disease (IBD). We tested the relationship of CD64 with mucosal inflammation and clinical relapse in pediatric Crohn's disease (CD).
Methods
In a cohort of 208 newly diagnosed CD and 43 non-IBD controls, ileal expression of FcγRI/S100A9 was determined by RNA sequencing from biopsies obtained at ileocolonoscopy. In a second cohort, we tested for the peripheral blood polymorphonuclear neutrophil (PMN) CD64 index from 26 newly diagnosed CD, 30 non-IBD controls and 83 children with established CD.
Results
Ileal FcγRIA mRNA expression was significantly elevated in CD at diagnosis compared with non-IBD controls (p<0.001), and correlated with ileal S100A9 (calprotectin) expression (r=0.83, p<0.001). The median(range) PMN CD64 index for newly diagnosed CD was 2.3(0.74-9.3) compared with 0.76(0.39-1.2) for non-IBD controls (p<0.001) with 96% sensitivity and 90% specificity at the cut point of 1.0. The PMN CD64 index significantly correlated with mucosal injury as measured by the Simple Endoscopic Score-CD (SES-CD, r=0.62, p<0.001). CD patients in clinical remission receiving maintenance therapy with a PMN CD64 index <1.0 had a sustained remission rate of 95% over the following 12 months compared with 56% in those with a PMN CD64 index >1.0 (p<0.01).
Conclusions
An elevated PMN CD64 index is associated with both mucosal inflammation and an increased risk for clinical relapse in pediatric CD. The PMN CD64 index is a reliable marker for sustained remission in CD patients receiving maintenance therapy.
Keywords: innate immune system in IBD, Fc gamma receptor, CD64 index, pediatric Crohn's disease, biomarker
Introduction
Ongoing mucosal injury in Crohn's disease (CD) is currently assessed indirectly with subjective disease specific clinical activity indices, by relatively insensitive blood biomarkers or via gastrointestinal-specific fecal markers. Although fecal calprotectin and lactoferrin have been shown to correlate with intestinal inflammation,1,2 C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) remain the most commonly utilized markers to detect inflammation in CD. Despite continued advances, limitations of the current CD biomarkers (blood or fecal) cause clinicians to rely on costly tests including magnetic resonance enterography (MRE) or invasive endoscopy as both tools provide supportive evidence of intestinal inflammation during periods of overt clinical relapses. However, persistent intestinal inflammation can also be seen during periods of clinical remission.3,4 Treatment decisions that rely on clinical symptoms alone without objective, less invasive markers of persistent intestinal inflammation may increase the risk of CD progression and disease related complications.5
As a chronic inflammatory disorder with periods of clinical remission and relapses, the goal of CD therapy is to improve the patient's quality of life and prevent persistent inflammation which can lead to CD complications including strictures, fistulae and abscesses. There is growing evidence that achievement of intestinal healing alters the natural course of CD. Mucosal healing (MH) has been associated with reduced rates of hospitalization, lower relapse rates and decreased need for surgery.6-8 Although a single established definition for this important clinical endpoint has not been formed, MH is usually defined as complete resolution of intestinal ulcerations during repeat endoscopy.9,10 Equipped with a cost effective, sensitive and rapid biomarker to detect MH, clinicians could potentially improve and eventually optimize CD therapeutic regimens and reduce the need for MRE and endoscopy to detect clinically silent but ongoing intestinal inflammation. In addition, with a reliable surrogate marker, clinicians could also more accurately differentiate a clinical relapse from irritable bowel syndrome (IBS) or the presence of other comorbid functional gastrointestinal disorders (FGID) in quiescent CD which remains a challenge with the current subjective disease activity indices, especially in pediatric CD.
The Fcγ receptors are expressed by innate immune cells including neutrophils and monocytes and serve to unite antibody specificity and effector cell functions. Quiescent polymorphonuclear neutrophils (PMNs) constitutively express the Fcγ receptor II (CD32) and Fcγ receptor III (CD16) with minimal levels of Fcγ receptor I (CD64) expressed on neutrophils under basal conditions.11 With active inflammation, neutrophil CD64 is upregulated early in the innate immune response by interferon (IFN)-γ and granulocyte-colony stimulating factor (G-CSF).12,13 The central role of CD64, as a high affinity receptor for immunoglobulin (Ig)G1 and IgG3, is recognition and subsequent clearance of immune complexes by phagocytosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and the respiratory burst.11 Upregulation of neutrophil CD64 has been shown to differentiate symptomatically active from quiescent inflammatory bowel disease (IBD) and relapsing IBD from infectious enterocolitis in adults.14 However, whether up-regulation of either tissue or circulating neutrophil CD64 correlated with endoscopic evidence of ongoing mucosal inflammation or risk of imminent relapse has not been examined.
The primary goal of our investigation was to determine the utility of measuring neutrophil CD64 expression as a surrogate marker for ongoing intestinal inflammation and whether the presence of a normal PMN CD64 index during a clinical remission in those receiving CD maintenance therapy would be associated with sustained remission. We first set out to explore the relative expression of the Fcγ receptor I (FcγRI) from inflamed CD mucosa (ileum and rectal). This was followed up by hypothesizing that the PMN CD64 index (a whole blood assay) could be utilized as a biomarker to test for mucosal inflammation by correlating the PMN CD64 index with the ileal mRNA expression of FcγRIA and an endoscopic score of intestinal injury. Finally, we investigated the utility of the PMN CD64 index as a screening biomarker to delineate new diagnosis pediatric CD from non-IBD related conditions such as IBS.
Materials and Methods
Patient populations
We studied three groups of patients from two distinct cohorts. Group 1 consisted of children and adolescents enrolled in the Pediatric Resource Organization for Kids with Inflammatory Intestinal Diseases (PRO-KIIDS) network RISK Stratification inception cohort study. The RISK Stratification consortium study is a multicenter cohort of 1794 subjects with newly diagnosed IBD (of which 1112 patients were diagnosed with CD) and non-IBD controls (n=373) that were enrolled from 2008-2012 at 28 sites in North America. All RISK subjects have been well characterized with endoscopic, histologic and disease activity scores and provided blood, stool and intestinal biopsy specimens at time of diagnosis. For our study, we have included the 251 and 137 patients for which the respective ileal and rectal biopsy specimens were obtained during the diagnostic ileocolonoscopy and RNA sequencing (RNAseq) was performed.
Groups 2 and 3 were enrolled at Cincinnati Children's Hospital Medical Center (CCHMC) from September 2011 to January 2014 and are referred to collectively as the CCHMC cohort. Group 2 consisted of patients referred for colonoscopy for the suspicion of IBD. The patients meeting clinical and histologic criteria for CD15 were entered as newly diagnosed CD. Patients with chronic gastrointestinal symptoms who were referred for colonoscopy for the suspicion of IBD and were found to have normal ileocolonoscopic findings by endoscopy and did not have any chronic evidence of intestinal inflammation on histological exam were included as the non-IBD controls. Group 3 consisted of CD patients receiving maintenance therapy that were enrolled either during a routine clinic visit or prior to a follow up colonoscopy. Blood samples were obtained from each participant. Clinical tests, including stool samples to determine fecal calprotectin levels or presence of intestinal pathogens were collected at the discretion of the primary gastroenterologist and were included in our analysis. The diagnosis of CD and determination of disease phenotype (Paris classification16) was made by standard clinical, radiological, histological and endoscopic criteria.15 Disease severity was evaluated by the short pediatric CD activity index (short PCDAI) with inactive disease defined as a score of less than 15, mild activity defined by 15-30 and scores greater than 30 as moderate to severe.17
Ileal and Rectal Fcγ Receptor I and S100A9 mRNA Expression by RNAseq
Ileal and rectal biopsy specimens were obtained during the diagnostic ileocolonoscopy from newly diagnosed CD patients and non-IBD controls from the RISK cohort (Group 1). The biopsy specimens were preserved in RNAlater® (Life Technologies) and then processed for mRNA utilizing a Qiagen kit (Qiagen, Hilden, Germany). The RNA were then poly(A) selected and subjected to single-end RNAseq by the Illumina HiSeq 2000 in a CCHMC NIH supported Digestive Health Center core lab. Reads were aligned using TopHat.18 The aligned reads were quantified by Avadis® NGS software, (Version 1.3.0, Strand Scientific Intelligence Inc., San Francisco, CA), using Hg19 as the reference genome and Reads Per Kilobase per Million mapped reads (RPKM) as an output. The DESeq algorithm was used for RPKM normalization within Avadis® NGS software and the normalized counts were log2-transformed and base-lined to the median expression of control samples. Our study was specifically interested in the expression of FcγRIA (CD64) and S100A9 (calprotectin) in CD patients.
Ileal FcγRIA and S100A9 mRNA expression by RT-PCR
Formalin fixed, paraffin embedded (FFPE) ileal biopsy specimens that were previously obtained during the diagnostic ileocolonoscopy from newly diagnosed CD and non-IBD controls (Cohort 2) were processed for RNA expression by reverse transcriptase-polymerase chain reaction (RT-PCR).19 Total RNA was extracted from these clinically archived FFPE samples by Qiagen miRNeasy FFPE RNA mini kit. With RNA purity verified (~1.9-2.0, 260/280), 500ng of RNA was reverse transcribed into cDNA. Quantitative PCR amplification was performed with ABI 7900HT amplification system (ABI/Life Technologies, New York). A set of pre-designed taqman individual expression assays were used following manufacturers suggested procedures, namely (ABI/Life Technologies FcγRIA Hs 00417598 and S100A9 Hs 00610058). The expression levels were quantified after normalizing to the housekeeping gene GAPDH.
PMN CD64 index
Each blood sample from Groups 2 and 3 was processed within 24 hours from collection for the measurement of the CD64 expression on granulocytes performed by quantitative flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA) using the Leuko64™ assay kit (Trillium Diagnostics, Brewer, ME20). The kit includes fluorescent beads and antibodies to CD64 and CD163. The lymphocyte, monocyte and granulocyte populations are defined by their forward and side scatter characteristics along with surface CD163 staining to further define the monocyte population. The CD64 index is calculated by the ratio of the mean fluorescent intensity (MFI) of the granulocytes to that of the calibration beads. The granulocyte specific index is referred as the PMN CD64 index.
Endoscopic Evaluation
Mucosal inflammation was evaluated by the Simple Endoscopic Score-CD21 (SES-CD) for CD patients who were referred for colonoscopy and had complete endoscopic imaging data available (Groups 2 & 3). The SES-CD evaluates 5 segmental areas of the ileum and colon on 4 parameters (ulcer size, percent of ulcerated and affected surfaces, and stenosis) with each parameter scored from 0-3. The total score from each area is tabulated with inactive defined as a score of 0-3, mild 4-10, moderate 11-19 and a score of >20 defined as severe inflammation as has been suggested in a previous investigation.4 The SESCD was tabulated by the same pediatric gastroenterologist from a streaming video monitor in a retrospective manner blinded to the results of the PMN CD64 index and disease activity index. For further analysis of endoscopic scoring assessments and RNA expression, CD patients who had rectal biopsies obtained for RNAseq analysis were divided into (1, normal) if there were no histologic and endoscopic abnormality noted, (2, Micro) if the endoscopic exam was normal but the histology was consistent with CD and (3, Macro) if they had both histologic and endoscopic findings of CD.
Statistical Analyses
All statistical analyses were performed using GraphPad PRISM© Version 5. Mann-Whitney U test was used for comparisons between two groups while the Kruskal Wallis analysis of variance with Dunn's test for multiple comparisons was used for assessments between more than two groups. The association between the PMN CD64 index and the short PCDAI, laboratory assessments, and endoscopic scores were evaluated by the Spearman's rank correlation coefficient (r) for nonparametric correlations. Receiver operator characteristic (ROC) curves were performed by plotting sensitivity vs. 1-specificity. Kaplan-Meier survival curve analysis was performed to test for differences in the rate of clinical relapse in CD patients over the subsequent 12 months and was stratified by the baseline PMN CD64 index. Data are expressed as median (minimum to maximum range). A p value <0.05 was considered to be statistically significant.
Ethical Considerations
All subjects and/or their parents/guardians provided informed consent, with subjects over the age of 11 providing assent prior to collection of study data. The study was approved by the Institutional Review Board at CCHMC. For the RISK subjects, the Institutional Review Board at each participating center approved the protocol.
Results
Study Population
Group 1: RISK Cohort
RNAseq analysis was performed from ileal biopsy specimens from 208 Caucasian patients with newly diagnosed CD and 43 non-IBD controls as well as from rectal biopsy specimens obtained from 109 newly diagnosed CD and 28 non-IBD controls. The clinical and demographic characteristics are in Table 1.
Table 1.
RISK cohort clinical and demographic characteristics.
| Group 1 | ||||
|---|---|---|---|---|
| Ileal mRNA | Rectal mRNA | |||
| Non-IBD Controls (n=43) | Crohn's Disease (n=208) | Non IBD Controls (n=28) | Crohn's Disease (n=109) | |
| Gender M/F | 28/15 | 128/80 | 17/11 | 73/36 |
| Median Age (range) | 11 (2-17) | 12 (2-17) | 11 (2-17) | 12 (2-17) |
| Age at diagnosis | ||||
| 0-<10 years | 58 | 26 | ||
| 10-<17 years | 150 | 83 | ||
| Location | ||||
| Distal 1/3 ileum | 46 | 23 | ||
| Colonic | 37 | 19 | ||
| Ileocolonic | 125 | 67 | ||
| Behavior | ||||
| Inflammatory (Nonstricturing & Nonpenetrating) | 208 | 109 | ||
Groups 2 & 3: CCHMC Cohort
Group 2 consisted of 56 patients referred for colonoscopy for the clinical and/or laboratory suspicion of IBD. 26 patients met criteria for CD and 30 patients were included as non-IBD controls as they had no histological findings of IBD and had a normal ileocolonoscopy. Patients with mild non-specific acute gastric or esophageal histologic inflammation were not excluded. Small bowel imaging was performed in 3/30 non-IBD controls. The final diagnosis for the non-IBD controls included irritable bowel syndrome (IBS)-constipation (9/30), IBS-diarrhea (3/30), chronic abdominal pain (9/30), gastroesophageal reflux disease (7/30) and post-infectious (2/30). Group 3 consisted of 83 patients receiving maintenance therapy for CD. The clinical and demographic characteristics of the 139 CCHMC patients are in Table 2.
Table 2.
CCHMC cohort clinical and demographic characteristics.
| Group 2 | Group 3 | ||
|---|---|---|---|
| Non-IBD Controls (n=30) | New Diagnosis Crohn's (n=26) | Crohn's Disease (n=83) | |
| Gender M/F | 14/16 | 19/7 | 51/32 |
| Median Age (range) | 14 (6-18) | 13 (6-18) | 15 (1-24) |
| Active/inactive | 26/0 | 43/40 | |
| Crohn's Disease | |||
| Age at diagnosis | |||
| 0-<10 years | 7 | 28 | |
| 10-<17 years | 17 | 52 | |
| 17-40 years | 2 | 3 | |
| Location | |||
| Distal 1/3 ileum | 5 | 11 | |
| Colonic | 7 | 22 | |
| Ileocolonic | 14 | 50 | |
| Behavior | |||
| Inflammatory (Nonstricturing & Nonpenetrating) | 23 | 53 | |
| Stricturing | 0 | 10 | |
| Penetrating | 3 | 8 | |
| Both penetrating/Stricturing | 0 | 12 | |
| Current Therapy | |||
| Corticosteroids | 24 | ||
| IM (6-MP, MTX) | 49 | ||
| Anti-TNFα | 39 | ||
| Anti-TNFα + IM | 17 | ||
| 5-ASA | 38 | ||
Disease activity was defined using the short pediatric Crohn's disease activity index. Crohn's disease age at diagnosis, disease location and behavior were defined using the Paris classification. IM, immunomodulator; 6-MP, 6-mercaptopurine; MTX, methotrexate; TNF, tumor necrosis factor; 5-ASA, 5-aminosalicylic acid
Fcγ Receptor I is Upregulated in Ileal and Rectal Tissue as well as Circulating Neutrophils in Newly Diagnosed Crohn's Disease
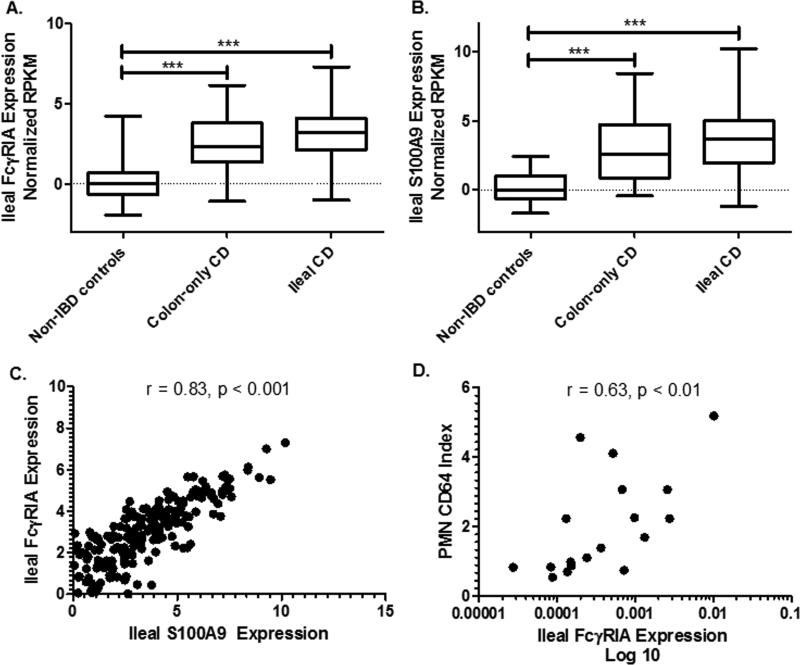
We first asked whether patients undergoing diagnostic ileocolonoscopy for suspected IBD and found to have CD would up-regulate FcγRIA in intestinal tissues and CD64 in circulating neutrophils compared to non-IBD controls. Ileal biopsy specimens obtained from Group 1 colon-only (Paris classification L2) CD patients and ileal CD (Paris classification L1/L3) showed a significant difference in the median normalized RPKM FcγRIA mRNA expression compared to the non-IBD control patients (p<0.001, Figure 1a). We found there wasn’t a significant difference in ileal FcγRIA expression between L2 CD and L1/L3 CD. Further, we found a similar relationship among the ileal expression of S100A9 (calprotectin) among these groups (Figure 1b). We further investigated the relationship between ileal expression of FcγRIA and S100A9 and found a significant correlation (Spearman r = 0.83, p<0.001, Figure 1c). To determine if ileal tissue expression levels of FcγRIA correlated with CD64 expression on the surface of circulating PMN's in the blood, we performed RTPCR from RNA obtained from FFPE on 14 newly diagnosed CD patients and 6 non-IBD controls. We found a significant Spearman correlation between the PMN CD64 index from circulating neutrophils and tissue CD64 expression (r = 0.63, p<0.01, Figure 1d).
Figure 1. Ileal Fcγ Receptor I expression in newly diagnosed Crohn's disease.
mRNA expression level of FcγRIA and S100A9 were determined by RNA sequencing of ileal biopsy specimens obtained from newly diagnosed colon-only CD (n=37), ileal/ileocolonic CD (n=171) and non-IBD controls (n=43). A) Ileal FcγRIA expression from CD patients was compared to non-IBD controls while B) S100A9 expression was also evaluated in the same groups. C) The association between ileal FcγRIA and S100A9 expression in newly diagnosed CD was tested. D) Ileal expression of FcγRIA was determined by RT-PCR and obtained from biopsy specimens on newly diagnosed CD and non-IBD controls. The FcγRIA expression was then compared to the surface expression of CD64 on circulating neutrophils. RNA sequencing values are normalized log2 transformed. Data are shown as the median and interquartile range. Differences between the groups were tested by Kruskal Wallis with Dunn's test for multiple comparisons. Spearman rank (r) coefficient performed for correlations. ***p<0.001.
Rectal FcγRIA mRNA expression was also determined from 109 newly diagnosed CD and compared to 28 non-IBD controls. The findings were similar to the ileal expression data. We found the expression of FcγRIA from CD with either endoscopic or histologic proctitis was significantly elevated compared to non-IBD controls (p<0.001, Figure 2a). We did not find a significant difference in the median normalized RPKM between the CD patients with a histologic and endoscopic normal rectum (n=34) and the non-IBD controls. Further, we found a significant difference between CD with normal rectum and CD with microscopic proctitis (determined by the histological evaluation, n=20, p<0.05). The S100A9 mRNA expression displayed a similar pattern to FcγRIA with the exception that there was no difference in S100A9 expression between CD with microscopic proctitis and CD with a non-inflamed rectum (Figure 2b). We also found a significant correlation of rectal FcγRIA and S100A9 among the CD patients (Spearman r = 0.73, p<0.001, Figure 2c).
Figure 2. Rectal Fcγ Receptor I expression in newly diagnosed Crohn's disease.
mRNA expression level of FcγRIA and S100A9 were determined by RNA sequencing of rectal biopsy specimens obtained from newly diagnosed CD and non-IBD controls. CD normal rectum (n=34), CD microscopic rectal inflammation (n=20), and CD macroscopic rectal inflammation (n=55). A) Rectal FcγRIA expression from CD patients was compared to non-IBD controls while B) S100A9 expression was also evaluated in the same groups. C) The association between rectal FcγRIA and S100A9 expression in newly diagnosed CD was tested. RNAseq values are normalized log2 transformed. Data are shown as the median and interquartile range. Differences between groups were tested by Kruskal Wallis with Dunn's test for multiple comparisons. Spearman rank (r) coefficient performed for correlations. Normal, no endoscopic or histological features of Crohn's, Micro, endoscopic normal with histologic features of Crohn's. Macro, both endoscopic and histologic features of Crohn's. *<0.05, **<0.01, ***<0.001, ns, non-significant.
We then asked whether CD64 would be up-regulated on the surface of circulating neutrophils from peripheral blood in patients having ileocolonoscopy for suspected IBD (Group 2). In this group, 26 patients were diagnosed with CD with a median PMN CD64 index of 2.3 (range 0.74-9.3) and a median PMN CD64 index of 0.76 (range 0.39-1.2) in the 30 non-IBD controls (p<0.001, Figure 3a). In the same cohort, 32/56 patients referred for endoscopy had fecal calprotectin collected. The median(range) fecal calprotectin for 14 non-IBD controls was 147 mcg/gm (16-670) compared to 1318 mcg/gm (226-2500) for new diagnosis CD (p<0.001). To evaluate both biomarkers in this cohort, we performed receiver operator characteristic (ROC) curve analysis. At a cut-off of 1.0, the PMN CD64 index was 96% sensitive and 90% specific in differentiating new diagnosis IBD from non-IBD with a positive predictive value (PPV) of 89%, a 96% negative predictive value (NPV) and an area under the curve (AUC) of 0.98 (95% confidence internal, CI of 0.94-1.02, Figure 3b). By comparison, we performed ROC curve analysis on the 32/56 (57%) patients in Group 2 who had a fecal calprotectin collected prior to endoscopy. Prior analysis of fecal calprotectin has suggested that a cut off of >50 mcg/gm should be used to distinguish IBD from IBS with >250 mcg/gm utilized to determine the presence of large ulcers in CD.1 In our cohort, at a cut off of 50 mcg/gm, fecal calprotectin was 100% sensitive, 29% specific with a PPV of 64% and a NPV of 100% (AUC 0.96, 95% CI 0.91-1.02) for new diagnosis CD. At a cut off of 250 mcg/gm, it was 94% sensitive, 64% specific with a PPV of 77% and a NPV of 90%. In order to compare the test characteristics of both biomarkers in the same patients, we performed ROC curve analysis for the PMN CD64 index on the 32/56 patients who also had a fecal calprotectin collected. We found the PMN CD64 index to be 94% sensitive, 93% specific with a PPV of 94% and a NPV of 93% at the cut off of 1.0.
Figure 3. Peripheral blood neutrophil CD64 expression for newly diagnosed Crohn's disease.
Circulating neutrophil expression of CD64 was determined by flow cytometry and is reported as the PMN CD64 index. A).The PMN CD64 index was measured from 30 non-IBD controls and 26 newly diagnosed CD patients. B) Receiver operator characteristic analysis was performed for the PMN CD64 index to determine the cut point for this marker relative to the diagnosis of CD by ileocolonoscopy. At a CD64 index of 1.0, the PMN CD64 index was 96% sensitive and 90% specific. Data shown as the median and interquartile range. Differences between the groups were tested by Mann Whitney U. AUC, area under the curve. CI, confidence interval. ***p<0.001.
The PMN CD64 Index Correlates with Mucosal Inflammation
We next assessed peripheral blood neutrophil CD64 expression as a marker of endoscopic evidence of mucosal inflammation in newly diagnosed and CD patients receiving maintenance therapy. 36 CD patients (20 patients in Group 2 and 16 patients in Group 3) had an ileocolonoscopy performed with video recording to determine the SES-CD. The PMN CD64 index correlated with the SES-CD (Spearman r = 0.62, p<0.001, Figure 4a). In addition, there was a significant difference in the median PMN CD64 index and the degree of endoscopic severity as defined by the SES-CD (Kruskal-Wallis p<0.01, Figure 4b). Next, we evaluated the correlation of the SES-CD and fecal calprotectin collected in this group (16/36 CD patients) and found a significant Spearman correlation (r = 0.65, p<0.01). Lastly, the SES-CD correlated with the short PCDAI (Spearman r = 0.45, p<0.01) and the ESR (Spearman r = 0.59, p<0.05, n=16). However, CRP did not correlate with the SES-CD (Spearman r = 0.34, p = not significant).
Figure 4. Relationship of the PMN CD64 index and mucosal inflammation in Crohn's disease.
Circulating neutrophil expression of CD64 was determined by flow cytometry and is reported as the PMN CD64 index while endoscopic disease severity was determined by the Simple Endoscopic Score-Crohn's disease (SES-CD). A) The association between the PMN CD64 index and the SES-CD (n=36). B) The PMN CD64 index stratified by the intensity of intestinal inflammation as determined by the SES-CD. Spearman rank (r) coefficient was performed for correlation. Differences between groups were tested by Kruskal Wallis analysis of variance with Dunn's test for multiple comparisons and shown as median and interquartile range. *p<0.05***p<0.001
The PMN CD64 Index is Elevated in Clinically Active CD Compared to Quiescent CD
To evaluate the utility of the PMN CD64 index for disease activity (which was determined by the short PCDAI) we enrolled 83 CD patients receiving maintenance therapy (Group 3). The median(range) PMN CD64 index for the 43 active CD was 1.6 (0.77-8.2) which was significantly elevated compared with the 40 quiescent CD (median 0.97, range 0.63-2, p<0.001, Figure 5a). Interestingly, when we compared quiescent CD to the non-IBD controls (median 0.76, range 0.39-1.2), the index also differed significantly between the two groups (p<0.001).
Figure 5. The PMN CD64 index and clinical disease activity.
The PMN CD64 index was determined by flow cytometry. Disease quiescence for CD was defined by a short PCDAI <15. A) PMN CD64 index for quiescent and active CD is shown. B) Correlation between the PMN CD64 index and the short PCDAI. Data shown as the median and interquartile range. Statistical significance was determined by Mann Whitney U. Spearman rank coefficient (r) performed for correlation. ***p<0.001.
Next we determined whether the degree of elevation in the PMN CD64 index would be associated with the clinical severity of IBD flares as assessed by the short PCDAI. Comparing the neutrophil CD64 expression across disease severity in CD, we found that the median PMN CD64 index for mild activity (short PCDAI 15-30) was 1.2 (0.77-3.9) and for moderate-severe disease activity (short PCDAI >30) the median was 2.2 (0.85-8.2, p<0.05). With further analysis, the PMN CD64 index was shown to significantly correlate with the short PCDAI (Spearman r = 0.56, p<0.001, Figure 5b).
We also determined whether the PMN CD64 index from Groups 2 & 3 would be associated with other laboratory markers of inflammation. The PMN CD64 index directly correlated with CRP (Spearman r = 0.76, p<0.001) and ESR levels (Spearman r = 0.66, p <0.001 Figure 6a, 6b). The CD64 index modestly correlated with the absolute neutrophil count (ANC, Spearman r = 0.32, p<0.01). Given the correlation between ileal and rectal expression of FcγRIA and S100A9, we examined the relationship of the PMN CD64 index with fecal calprotectin in the CCHMC cohort (Groups 2 & 3) and also detected a significant correlation (Spearman r = 0.54, p<0.001, Figure 6c) in 53 patients that had both biomarkers measured. We found that the median PMN CD64 index did not differ by CD location (ileal, colonic or ileocolonic).
Figure 6. CD64 index and inflammatory Blood and Fecal Biomarkers utilized in Crohn's.
PMN CD64 index was determined by flow cytometry. The inflammatory markers ESR, CRP and fecal calprotectin were measured in the CCHMC clinical laboratory. Analyses were performed from Cohorts 2 and 3 to compare the PMN CD64 index with A) CRP, B) ESR and C) fecal calprotectin. D) Receiver operator characteristic analysis for the PMN CD64 index for clinically active CD as determined by short PCDAI. At a CD64 index of 1.0 the test is 85% sensitive and 56% specific (p<0.001) for active CD. Spearman rank (r) coefficient performed for correlations. AUC, area under the curve; CI, confidence interval.
We also performed ROC analysis to determine the utility of the PMN CD64 index to accurately identify clinically active disease as determined by the short PCDAI and found at a cut point of 1.0, the sensitivity was 85% with a specificity of 55%, PPV of 74% and a NPV of 71%. (AUC 0.83, 95% CI 0.75-0.91, p<0.001 Figure 6d). Likewise using the short PCDAI to define active inflammation, a fecal calprotectin of >250 mcg/gm was 86% sensitive and 50% specific with a PPV of 89% and a NPV of 43% (AUC 0.85, 95% CI 0.7-1.0).
PMN CD64 Index and Clinical Activity Index Predict Likelihood of Sustained Remission
Given the correlation between the PMN CD64 index and endoscopic severity in CD, we determined whether normalization of the index would be associated with sustained remission. In Group 3, 40 CD patients had clinically inactive disease (defined by a short PCDAI <15) at time of the CD64 index measurement. Of those 40 patients, we had complete follow up data for the following 12 months on 37 patients. We found that CD patients receiving maintenance therapy in clinical remission who had a PMN CD64 index > 1.0 experienced a relapse rate of 44% during one year follow-up, compared with a relapse rate of 5% in those with a PMN CD64 index < 1.0 (p<0.01, Figure 7).
Figure 7. The PMN CD64 index and sustained clinical remission.

The PMN CD64 index was determined by flow cytometry in 37 CD patients receiving maintenance therapy and in clinical remission (short PCDAI < 15). Rates of clinical relapse were determined during one year follow-up. Kaplan-Meier survival curve analysis was performed and is shown for CD patients stratified by a CD64 index of 1.0.
Discussion
Activated neutrophils play a crucial role in host defense via recognition, phagocytosis, and elimination of invading pathogens. The neutrophil phagocytic responses are initiated by specific receptors that recognize intestinal microbes such as the toll like receptors (TLR) and C-type lectins or by Igopsonized bacteria through the Fcγ receptors.22 Likewise, CD is characterized by a mixed inflammatory cell infiltrate including activated neutrophils that as a result of a dysregulated response to the local gut microbiota produce proinflammatory cytokines, chemokines, T cell activation and reactive oxygen species that contribute to mucosal injury.23-25 Our investigation demonstrated that active CD was significantly associated with an upregulation of CD64 on circulating neutrophils and an increased expression of FcγRIA from ileal and rectal biopsy specimens.
Prior investigations have shown that quantitative analysis of CD64 can be used to distinguish chronic inflammatory conditions from healthy controls.14,26-28 Tillinger et al. not only evaluated CD64 as a marker to differentiate acute, intestinal infection from clinically active IBD, but also showed that CD64 discriminated clinically active disease from quiescent IBD in adult patients.14 Although they did show evidence of CD64 positive neutrophils infiltrating the lamina propria of the colon in active CD by immunohistochemistry,14 our investigation further demonstrates that the mRNA expression of FcγRIA from ileal and rectal tissue is also significantly elevated above controls. More recently, Wojtal et al. showed that non-response to anti-TNFα therapy in IBD patients was associated with an elevation of CD64 mRNA expression from post-treatment colonic biopsy specimens.29 Whether mucosal expression of FcγRIA can provide prognostication for anti-TNFα response will need to be further investigated. Although limited to 20 patients, our correlation between the PMN CD64 index and ileal expression of FcγRIA suggests that the PMN CD64 index can be utilized as a marker of mucosal treatment response.
Fecal calprotectin was developed as a non-invasive surrogate biomarker of intestinal inflammation as it correlated with the fecal excretion of indium-111-labeled granulocytes30,31 and has been validated as a surrogate stool marker for endoscopic lesions in IBD.1 However, fecal calprotectin has also been associated with elevations with non-steroidal anti-inflammatory drug (NSAID) use, celiac disease, allergic colitis and colonic polyps.32,33 Similarly, the PMN CD64 index has been utilized in other disciplines, most notably in the early recognition of neonatal sepsis34 and other bacterial infections in children.35 When we compared these two biomarkers in their ability to identify new diagnosis CD, the PMN CD64 demonstrated a superior specificity when a fecal calprotectin cut off of 50 mcg/gm was utilized (90% specificity for PMN CD64 index compared to 29% specificity for fecal calprotectin). While a fecal calprotectin level of >50 mcg/gm has been suggested as the cut point to distinguish IBD from non-IBD, we found that only 4/14 non-IBD controls had a fecal calprotectin <50 mcg/gm. We found 9/14 non-IBD controls had a fecal calprotectin >100 mcg/gm (median 299, range 105-670) and 5/14 >250 mcg/gm. The final diagnosis for those 9 patients with a fecal calprotectin >100 mcg/gm included GERD (5/9), IBS-constipation (2/9) and chronic abdominal pain (2/9). Evaluation of the small bowel was at the discretion of the treating clinician and was not completed in any of the 9 subjects with an elevated fecal calprotectin. For cohort 2, the mean time of fecal calprotectin collection prior to endoscopy was 18 days (standard deviation 15).
With the increased use of anti-TNFα therapy, clinical trials have shown that MH is associated with improved outcomes and proposed by some investigators that MH should be the primary endpoint for all IBD therapeutics.36,37 Taken one step further in CD, deep remission (defined as a CD activity index less [CDAI] than 150 and complete mucosal healing as defined by endoscopy) has been shown in adults with CD to be related with improved long term disease specific quality of life, fewer flares and hospitalizations, greater work productivity and less activity impairment compared to MH alone.38 With these goals, several biomarkers and testing modalities (disease index, and imaging,) have been proposed to less invasively evaluate for MH. Fecal calprotectin levels have proven to be superior to either CRP or the subjective clinical indices in identifying endoscopic remission.39 In our study, the PMN CD64 index correlated well with the degree of macroscopic injury in CD (r = 0.62) and is similar to the published data with fecal calprotectin and the SES-CD(r = 0.75).4 With a closer review of our cohort with endoscopic scoring, we found the largest discrepancies between SES-CD and the PMN CD64 index was seen in those CD patients with significant small bowel disease who had a significantly elevated PMN CD64 index but a relatively low SES-CD score. In fact, in our final analysis, we excluded 2 newly diagnosed CD patients who both had a calculated SES-CD score of only 2, which by the Schoepfer et al40 scoring system would have been classified as inactive. Upon further imaging of the small bowel, both of these treatment naïve CD patients were found to have extensive small bowel inflammation as well as a markedly elevated PMN CD64 index and fecal calprotectin. This inconsistency illustrates the limitation of the SES-CD and other endoscopic scores with their failure to account for extensive small bowel inflammation in CD. There has been great interest in developing a CD intestinal score which will take into account both ileocolonoscopic findings as well as small bowel imaging techniques41
Studies have found that 57-59% of CD patients have concurrent IBS.42,43 Moreover, with the similarities of gastrointestinal symptoms shared in active CD and IBS, determining disease activity in a patient with a comorbidity of CD and IBS poses a significant diagnostic dilemma. An objective biomarker such as the PMN CD64 index provides a potentially improved opportunity to determine MH or clinically silent CD with ongoing intestinal inflammation without relying on endoscopy and disease specific activity indices. Our study was not designed to differentiate active CD without IBS from quiescent CD with IBS, however, the significant correlation seen between the PMN CD64 index and both the SES-CD and ileal expression of FcγRIA suggests additional utility of the CD64 index as a screen for active intestinal inflammation in CD.
Our investigation has demonstrated that the PMN CD64 index determined by whole blood analysis can provide useful data to the clinician treating pediatric CD. Larger, prospective studies will be needed to determine if improved outcomes are seen by defining deep remission in pediatric CD as a short PCDAI <15 and a PMN CD64 index <1.0. We found that those CD meeting these parameters sustained a 95% remission rate over the following year. We were not able to perform a similar analysis for fecal calprotectin in our cohort given the small number of patients with the biomarker collected, but studies have shown that a fecal calprotectin >400 mcg/gm in those with clinically quiescent IBD is associated with increased risk of disease relapse in the following year.44,45
The major strength of our study is the large sample size of patients who had RNAseq performed from the ileum and rectum. Given the heterogeneity of collecting ileal biopsy across this large multicenter inception cohort, we could not confirm if ileal biopsies sent for RNAseq analysis included Peyer's patches. However, given the large numbers of non-IBD controls and the similarities between the rectal and ileal RNA expression for both genes in CD, this limitation may not be contributory in the final interpretation of the ileal RNAseq data. The relative small sample size (n=36) and the retrospective viewing of the ileocolonoscopy to determine the SES-CD by a single endoscopist as well as the inability of the SES-CD to reflect the total inflammatory burden will require larger, prospective validation prior to widespread use of the PMN CD64 index as surrogate marker of MH. Although the PMN CD64 index assay is a commercially available assay and can be processed in less than one hour, it requires the use of a flow cytometer which may not be available at every laboratory. The patient charge at our institution to perform the PMN CD64 index in our CLIA approved clinical laboratory is $159.00 with a <24 hour turnaround time compared to an institution charge to the patient of $477.00 for fecal calprotectin with results arriving <5 days. Our institution does not currently offer point-of-care fecal calprotectin testing, however, this technology is available with much faster results and good correlation to the standard ELISA assays.46
Although CD64 has been investigated in adult IBD, this is the first study to investigate CD64 up-regulation in pediatric CD by ileal and rectal RNAseq as well as to correlate the PMN CD64 index on circulating neutrophils and the degree of endoscopic evidence of mucosal inflammation. The significant correlation between the PMN CD64 index and the SES-CD, ileal CD64 expression and sustained remission offers an exciting opportunity to monitor the PMN CD64 index as the standardized marker of treatment response and deep remission in CD.
Acknowledgments
Authors would like to thank Ramona Bezold and Kathleen Lake for their dedication for patient recruitment, Megan Hale for her work on the RNA extraction as well as the CCFA-Risk study publication committee for a thorough review of this manuscript. We would also like to thank the following RISK study investigators and institutions: Scott B. Snapper, MD, Boston Children's Hospital, Boston, MA; Richard Kellermayer, MD, Baylor, Houston, TX; Michael D. Kappelman, MD, University of North Carolina at Chapel Hill, Chapel Hill, NC; Anthony Otley, MD, IWK Health Centre, Halifax, Nova Scotia, Canada; Marian D. Pfefferkorn, MD, Riley Children's Hospital, Indianapolis, IN; Stanley A. Cohen, MD, Children's Center for Digestive Health Care, LLC, Atlanta, GA; Stephen L. Guthery, MD, University of Utah and Primary Children's Medical Center, Salt Lake City, UT; Neal S. LeLeiko, MD, PhD, Hasbro Children's Hospital, Providence, RI; Maria Oliva-Hemker, MD, Johns Hopkins Medical Center, Baltimore, MD; David J. Keljo, MD, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA; Dedrick E. Moulton, MD, Vanderbilt Children's Hospital, Nashville, TN; Barbara S. Kirschner, MD, University of Chicago, Chicago, IL; Ashish S. Patel, MD, Texas Children's Hospital, Dallas, TX; David A. Ziring, MD, Children's Hospital of Los Angeles, Los Angeles, CA.
Grant Support: This work was supported by NIH training grant T32 DK007727 (PM), NIH R01 DK078683 (LAD), the Integrative Morphology and Flow Cytometry cores of the National Institutes of Health (NIH)-supported Cincinnati Children's Hospital Research Foundation Digestive Health Center (1P30DK078392-01), and the Crohn's & Colitis Foundation of America sponsored RISK study through PRO-KIIDS.
Financial disclosures: The authors have no financial arrangement(s) with a company whose product figures prominently in the submitted manuscript or with a company making a competing product.
References
- 1.D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012 Dec;18(12):2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 2.Abraham BP, Kane S. Fecal markers: calprotectin and lactoferrin. Gastroenterology clinics of North America. 2012 Jun;41(2):483–495. doi: 10.1016/j.gtc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflammatory bowel diseases. 2012 Sep;18(9):1634–1640. doi: 10.1002/ibd.21925. [DOI] [PubMed] [Google Scholar]
- 4.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. The American journal of gastroenterology. 2010 Jan;105(1):162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 5.Panaccione R, Colombel JF, Louis E, Peyrin-Biroulet L, Sandborn WJ. Evolving definitions of remission in Crohn's disease. Inflammatory bowel diseases. 2013 Jul;19(8):1645–1653. doi: 10.1097/MIB.0b013e318283a4b3. [DOI] [PubMed] [Google Scholar]
- 6.Peyrin-Biroulet L, Ferrante M, Magro F, et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. Journal of Crohn's & colitis. 2011 Oct;5(5):477–483. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010 Feb;138(2):463–468. doi: 10.1053/j.gastro.2009.09.056. quiz e410-461. [DOI] [PubMed] [Google Scholar]
- 8.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012 Nov;61(11):1619–1635. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 9.D'Haens G, Van Deventer S, Van Hogezand R, et al. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999 May;116(5):1029–1034. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 10.De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn's disease: a systematic review. Inflammatory bowel diseases. 2013 Feb;19(2):429–444. doi: 10.1002/ibd.22977. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmeyer F, Witte K, Schmidt RE. The high-affinity Fc gamma RI on PMN: regulation of expression and signal transduction. Immunology. 1997 Dec;92(4):544–552. doi: 10.1046/j.1365-2567.1997.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckle AM, Hogg N. The effect of IFN-gamma and colony-stimulating factors on the expression of neutrophil cell membrane receptors. J Immunol. 1989 Oct 1;143(7):2295–2301. [PubMed] [Google Scholar]
- 13.Kerst JM, de Haas M, van der Schoot CE, et al. Recombinant granulocyte colony-stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells. Blood. 1993 Dec 1;82(11):3265–3272. [PubMed] [Google Scholar]
- 14.Tillinger W, Jilch R, Jilma B, et al. Expression of the High-Affinity IgG Receptor FcRI (CD64) in Patients With Inflammatory Bowel Disease: A New Biomarker for Gastroenterologic Diagnostics. American Journal of Gastroenterology. 2009 Jan;104(1):102–109. doi: 10.1038/ajg.2008.6. [DOI] [PubMed] [Google Scholar]
- 15.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007 Nov;133(5):1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflammatory bowel diseases. 2011 Jun;17(6):1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 17.Kappelman MD, Crandall WV, Colletti RB, et al. Short pediatric Crohn's disease activity index for quality improvement and observational research. Inflammatory bowel diseases. 2011 Jan;17(1):112–117. doi: 10.1002/ibd.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trapnell C, Salzberg SL. How to map billions of short reads onto genomes. Nature biotechnology. 2009 May;27(5):455–457. doi: 10.1038/nbt0509-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen T, Stucke EM, Grotjan TM, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology. 2013 Dec;145(6):1289–1299. doi: 10.1053/j.gastro.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. http://www.trilliumdx.com/24/Leuko64.
- 21.Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004 Oct;60(4):505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 22.Ravetch JV, Bolland S. IgG Fc receptors. Annual review of immunology. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 23.Yamada T, Grisham MB. Role of neutrophil-derived oxidants in the pathogenesis of intestinal inflammation. Klinische Wochenschrift. 1991 Dec 15;69(21-23):988–994. doi: 10.1007/BF01645144. [DOI] [PubMed] [Google Scholar]
- 24.Abraham C, Cho JH. Inflammatory bowel disease. The New England journal of medicine. 2009 Nov 19;361(21):2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. The Journal of clinical investigation. 2007 Mar;117(3):514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ureten K, Ertenli I, Ozturk MA, et al. Neutrophil CD64 expression in Behcet's disease. The Journal of rheumatology. 2005 May;32(5):849–852. [PubMed] [Google Scholar]
- 27.Hussein OA, El-Toukhy MA, El-Rahman HS. Neutrophil CD64 expression in inflammatory autoimmune diseases: its value in distinguishing infection from disease flare. Immunological investigations. 2010;39(7):699–712. doi: 10.3109/08820139.2010.491520. [DOI] [PubMed] [Google Scholar]
- 28.Reinisch W, Gasche C, Lichtenberger C, et al. Altered Expression of Fc-Gamma-Receptors on Circulating Polymorphonuclear Leukocytes in Active Crohns-Disease. Gastroenterology. 1995 Apr;108(4):A901–A901. [Google Scholar]
- 29.Wojtal KA, Rogler G, Scharl M, et al. Fc Gamma Receptor CD64 Modulates the Inhibitory Activity of Infliximab. PLoS One. 2012;7(8):e43361. doi: 10.1371/journal.pone.0043361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roseth AG, Fagerhol MK, Aadland E, Schjonsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scandinavian journal of gastroenterology. 1992 Sep;27(9):793–798. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 31.Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scandinavian journal of gastroenterology. 1999 Jan;34(1):50–54. doi: 10.1080/00365529950172835. [DOI] [PubMed] [Google Scholar]
- 32.Tibble JA, Sigthorsson G, Foster R, et al. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut. 1999 Sep;45(3):362–366. doi: 10.1136/gut.45.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kristinsson J, Roseth A, Fagerhol MK, et al. Fecal calprotectin concentration in patients with colorectal carcinoma. Diseases of the colon and rectum. 1998 Mar;41(3):316–321. doi: 10.1007/BF02237485. [DOI] [PubMed] [Google Scholar]
- 34.Streimish I, Bizzarro M, Northrup V, et al. Neutrophil CD64 as a diagnostic marker in neonatal sepsis. The Pediatric infectious disease journal. 2012 Jul;31(7):777–781. doi: 10.1097/INF.0b013e318256fb07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudensky B, Sirota G, Erlichman M, Yinnon AM, Schlesinger Y. Neutrophil CD64 expression as a diagnostic marker of bacterial infection in febrile children presenting to a hospital emergency department. Pediatric emergency care. 2008 Nov;24(11):745–748. doi: 10.1097/PEC.0b013e31818c2679. [DOI] [PubMed] [Google Scholar]
- 36.Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointestinal endoscopy. 2006 Mar;63(3):433–442. doi: 10.1016/j.gie.2005.08.011. quiz 464. [DOI] [PubMed] [Google Scholar]
- 37.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. The New England journal of medicine. 2005 Dec 8;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 38.Sandborn W, Colombel JF, Lomax K, et al. Achievement of early deep remission is associated with lower rates of weekly dosing for adalimumab treated patients with Crohn's disease; data from EXTEND. Gut. 2011;60:A136–A137. [Google Scholar]
- 39.af Bjorkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Farkkila M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn's disease. Scandinavian journal of gastroenterology. 2012 May;47(5):528–537. doi: 10.3109/00365521.2012.660542. [DOI] [PubMed] [Google Scholar]
- 40.Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflammatory bowel diseases. 2008 Jan;14(1):32–39. doi: 10.1002/ibd.20275. [DOI] [PubMed] [Google Scholar]
- 41.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflammatory bowel diseases. 2011 Jun;17(6):1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simren M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Bjornsson ES. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. The American journal of gastroenterology. 2002 Feb;97(2):389–396. doi: 10.1111/j.1572-0241.2002.05475.x. [DOI] [PubMed] [Google Scholar]
- 43.Keohane J, O'Mahony C, O'Mahony L, O'Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? The American journal of gastroenterology. 2010 Aug;105(8):1788, 1789–1794. doi: 10.1038/ajg.2010.156. quiz 1795. [DOI] [PubMed] [Google Scholar]
- 44.Samson CM, Morgan P, Williams E, et al. Improved outcomes with quality improvement interventions in pediatric inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2012 Dec;55(6):679–688. doi: 10.1097/MPG.0b013e318262de16. [DOI] [PubMed] [Google Scholar]
- 45.Walkiewicz D, Werlin SL, Fish D, Scanlon M, Hanaway P, Kugathasan S. Fecal calprotectin is useful in predicting disease relapse in pediatric inflammatory bowel disease. Inflammatory bowel diseases. 2008 May;14(5):669–673. doi: 10.1002/ibd.20376. [DOI] [PubMed] [Google Scholar]
- 46.Sydora MJ, Sydora BC, Fedorak RN. Validation of a point-of-care desk top device to quantitate fecal calprotectin and distinguish inflammatory bowel disease from irritable bowel syndrome. Journal of Crohn's & colitis. 2012 Mar;6(2):207–214. doi: 10.1016/j.crohns.2011.08.008. [DOI] [PubMed] [Google Scholar]