Abstract
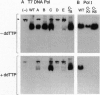
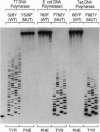
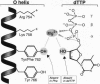
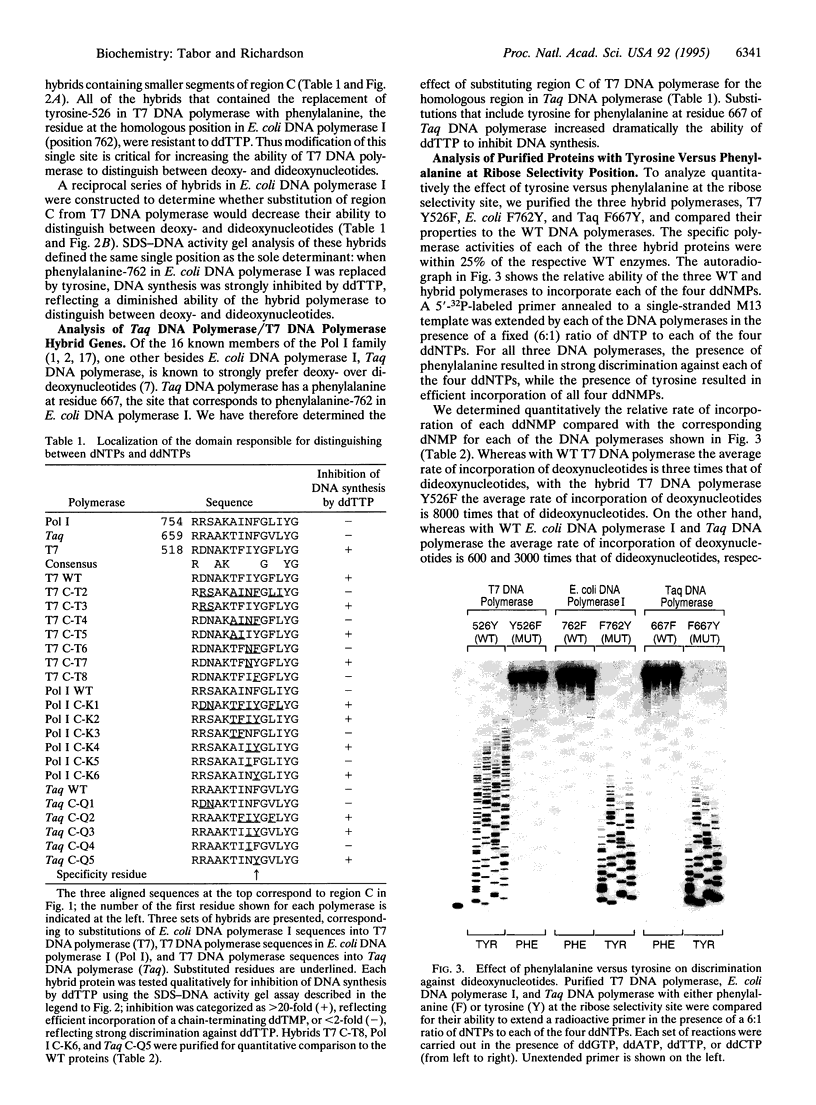
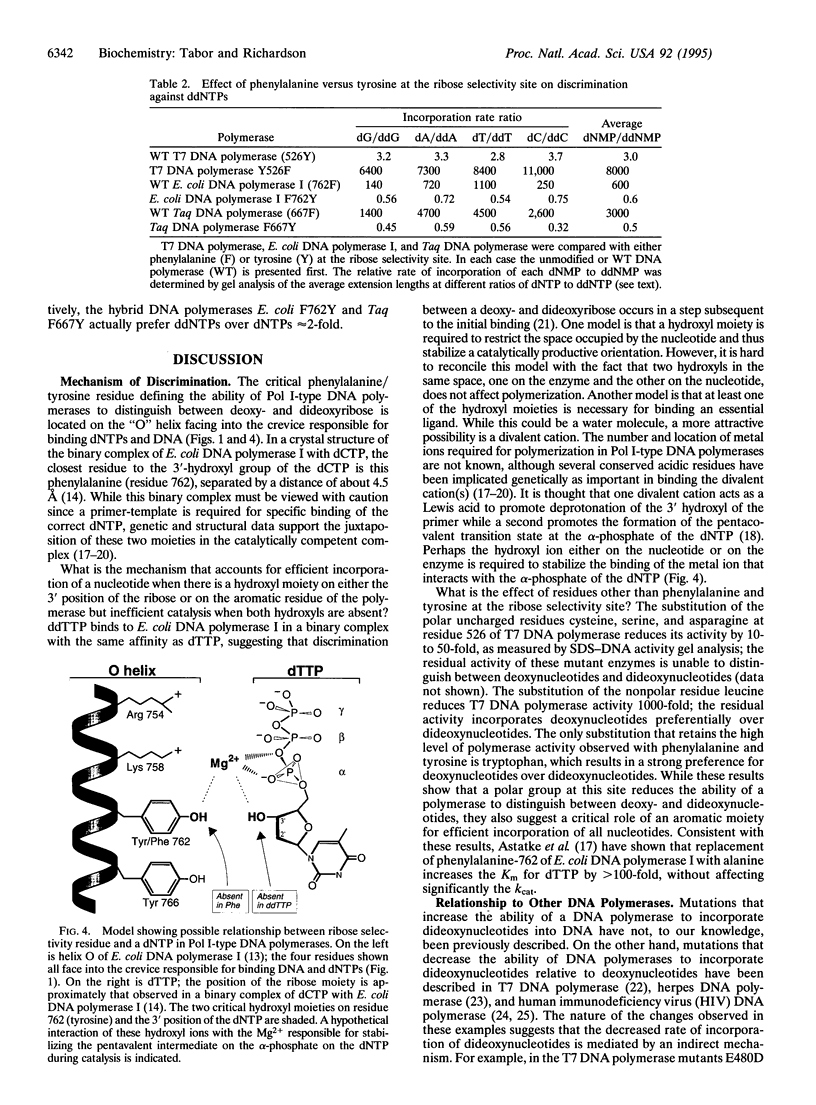
Bacteriophage T7 DNA polymerase efficiently incorporates a chain-terminating dideoxynucleotide into DNA, in contrast to the DNA polymerases from Escherichia coli and Thermus aquaticus. The molecular basis for this difference has been determined by constructing active site hybrids of these polymerases. A single hydroxyl group on the polypeptide chain is critical for selectivity. Replacing tyrosine-526 of T7 DNA polymerase with phenylalanine increases discrimination against the four dideoxynucleotides by > 2000-fold, while replacing the phenylalanine at the homologous position in E. coli DNA polymerase I (position 762) or T. aquaticus DNA polymerase (position 667) with tyrosine decreases discrimination against the four dideoxynucleotides 250- to 8000-fold. These mutations allow the engineering of new DNA polymerases with enhanced properties for use in DNA sequence analysis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astatke M., Grindley N. D., Joyce C. M. Deoxynucleoside triphosphate and pyrophosphate binding sites in the catalytically competent ternary complex for the polymerase reaction catalyzed by DNA polymerase I (Klenow fragment). J Biol Chem. 1995 Jan 27;270(4):1945–1954. doi: 10.1074/jbc.270.4.1945. [DOI] [PubMed] [Google Scholar]
- Atkinson M. R., Deutscher M. P., Kornberg A., Russell A. F., Moffatt J. G. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2',3'-dideoxyribonucleotide. Biochemistry. 1969 Dec;8(12):4897–4904. doi: 10.1021/bi00840a037. [DOI] [PubMed] [Google Scholar]
- Beese L. S., Derbyshire V., Steitz T. A. Structure of DNA polymerase I Klenow fragment bound to duplex DNA. Science. 1993 Apr 16;260(5106):352–355. doi: 10.1126/science.8469987. [DOI] [PubMed] [Google Scholar]
- Beese L. S., Friedman J. M., Steitz T. A. Crystal structures of the Klenow fragment of DNA polymerase I complexed with deoxynucleoside triphosphate and pyrophosphate. Biochemistry. 1993 Dec 28;32(51):14095–14101. doi: 10.1021/bi00214a004. [DOI] [PubMed] [Google Scholar]
- Boyer P. L., Tantillo C., Jacobo-Molina A., Nanni R. G., Ding J., Arnold E., Hughes S. H. Sensitivity of wild-type human immunodeficiency virus type 1 reverse transcriptase to dideoxynucleotides depends on template length; the sensitivity of drug-resistant mutants does not. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4882–4886. doi: 10.1073/pnas.91.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite D. K., Ito J. Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res. 1993 Feb 25;21(4):787–802. doi: 10.1093/nar/21.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Cheng Y. C. The role of cytoplasmic deoxycytidine kinase in the mitochondrial effects of the anti-human immunodeficiency virus compound, 2',3'-dideoxycytidine. J Biol Chem. 1992 Feb 15;267(5):2856–2859. [PubMed] [Google Scholar]
- Delarue M., Poch O., Tordo N., Moras D., Argos P. An attempt to unify the structure of polymerases. Protein Eng. 1990 May;3(6):461–467. doi: 10.1093/protein/3.6.461. [DOI] [PubMed] [Google Scholar]
- Derse D., Bastow K. F., Cheng Y. Characterization of the DNA polymerases induced by a group of herpes simplex virus type I variants selected for growth in the presence of phosphonoformic acid. J Biol Chem. 1982 Sep 10;257(17):10251–10260. [PubMed] [Google Scholar]
- Donlin M. J., Johnson K. A. Mutants affecting nucleotide recognition by T7 DNA polymerase. Biochemistry. 1994 Dec 13;33(49):14908–14917. doi: 10.1021/bi00253a030. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce C. M., Steitz T. A. Function and structure relationships in DNA polymerases. Annu Rev Biochem. 1994;63:777–822. doi: 10.1146/annurev.bi.63.070194.004021. [DOI] [PubMed] [Google Scholar]
- Karawya E., Swack J. A., Wilson S. H. Improved conditions for activity gel analysis of DNA polymerase catalytic polypeptides. Anal Biochem. 1983 Dec;135(2):318–325. doi: 10.1016/0003-2697(83)90689-9. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Structural and functional properties of calf thymus DNA polymerase delta. Prog Nucleic Acid Res Mol Biol. 1981;26:83–96. doi: 10.1016/s0079-6603(08)60396-7. [DOI] [PubMed] [Google Scholar]
- Ollis D. L., Brick P., Hamlin R., Xuong N. G., Steitz T. A. Structure of large fragment of Escherichia coli DNA polymerase I complexed with dTMP. 1985 Feb 28-Mar 6Nature. 313(6005):762–766. doi: 10.1038/313762a0. [DOI] [PubMed] [Google Scholar]
- Ollis D. L., Kline C., Steitz T. A. Domain of E. coli DNA polymerase I showing sequence homology to T7 DNA polymerase. 1985 Feb 28-Mar 6Nature. 313(6005):818–819. doi: 10.1038/313818a0. [DOI] [PubMed] [Google Scholar]
- Polesky A. H., Dahlberg M. E., Benkovic S. J., Grindley N. D., Joyce C. M. Side chains involved in catalysis of the polymerase reaction of DNA polymerase I from Escherichia coli. J Biol Chem. 1992 Apr 25;267(12):8417–8428. [PubMed] [Google Scholar]
- Polesky A. H., Steitz T. A., Grindley N. D., Joyce C. M. Identification of residues critical for the polymerase activity of the Klenow fragment of DNA polymerase I from Escherichia coli. J Biol Chem. 1990 Aug 25;265(24):14579–14591. [PubMed] [Google Scholar]
- Prasad V. R., Lowy I., de los Santos T., Chiang L., Goff S. P. Isolation and characterization of a dideoxyguanosine triphosphate-resistant mutant of human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11363–11367. doi: 10.1073/pnas.88.24.11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober J. M., Trainor G. L., Dam R. J., Hobbs F. W., Robertson C. W., Zagursky R. J., Cocuzza A. J., Jensen M. A., Baumeister K. A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science. 1987 Oct 16;238(4825):336–341. doi: 10.1126/science.2443975. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos A., Hübscher U. Recovery of functional proteins in sodium dodecyl sulfate gels. Methods Enzymol. 1983;91:263–277. doi: 10.1016/s0076-6879(83)91024-8. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Effect of pyrophosphorolysis and metal ions. J Biol Chem. 1990 May 15;265(14):8322–8328. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Effect of manganese ions on the incorporation of dideoxynucleotides by bacteriophage T7 DNA polymerase and Escherichia coli DNA polymerase I. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4076–4080. doi: 10.1073/pnas.86.11.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Selective inactivation of the exonuclease activity of bacteriophage T7 DNA polymerase by in vitro mutagenesis. J Biol Chem. 1989 Apr 15;264(11):6447–6458. [PubMed] [Google Scholar]