Abstract
Background
Wheezing illnesses cause major morbidity in infants and are frequent precursors to asthma.
Objective
To examine environmental factors associated with recurrent wheezing in inner-city environments.
Methods
The Urban Environment and Childhood Asthma (URECA) study examined a birth cohort at high risk for asthma (n=560) in Baltimore, Boston, New York, and St. Louis. Environmental assessments included allergen exposure, and in a nested case-control study of 104 children, the bacterial content of house dust collected in the first year of life. Associations were determined among environmental factors, aeroallergen sensitization, and recurrent wheezing at age three.
Results
Cumulative allergen exposure over the first three years was associated with allergic sensitization, and sensitization at age three was related to recurrent wheeze. In contrast, first year exposure to cockroach, mouse and cat allergens was negatively associated with recurrent wheeze (OR 0.60, 0.65, and 0.75, p≤0.01). Differences in house-dust bacterial content in the first year, especially reduced exposure to specific Firmicutes and Bacteriodetes, was associated with atopy and atopic wheeze. Exposure to high levels of both allergens and this subset of bacteria in the first year of life was most common among children without atopy or wheeze.
Conclusions
In inner-city environments, children with the highest exposure to specific allergens and bacteria during their first year were least likely to develop recurrent wheeze and allergic sensitization. These findings suggest that concomitant exposure to high levels of certain allergens and bacteria in early life may be beneficial, and suggest new preventive strategies for wheezing and allergic diseases.
Keywords: Asthma, atopy, allergen exposure, microbial exposure, inner city
INTRODUCTION
Wheezing illnesses affect 35–50% of children by the age of 3 years1, 2 and are a leading cause for outpatient visits and hospitalizations.3, 4 Wheezing in non-atopic children is often transient, but recurrent wheezing in children who develop early allergic sensitization or other signs of atopy during the preschool years is a risk factor for asthma.5 As prevalence and severity of asthma is high in inner cities in the USA, it is especially important to identify risk factors that contribute to the development of allergic sensitization and wheezing in this environment.
The indoor environment of poor urban neighborhoods can include adverse conditions that promote allergic sensitization and recurrent wheezing.6 Examples include stress, lack of biodiversity, and exposure to indoor pollutants and perennial allergens such as cockroach and mouse.7–10 Conversely, farm-related microbial exposures in early life have been linked to protection against allergic diseases.11, 12 Whether relationships exist between microbial exposure and allergic disease outcomes in urban settings is unknown.
To examine the relationship between these conditions and development of allergic sensitization and recurrent wheezing, we studied children enrolled in an ongoing birth cohort study (Urban Environment and Childhood Asthma, “URECA”). Per study design, the entire cohort was evaluated at age 3 to test the hypothesis that high levels of exposure to sensitizing allergens, especially those associated with cockroach and mouse, is associated with the development of allergic sensitization and recurrent wheezing. In addition, a nested case control study was designed to determine whether early life exposure to certain microbes in house dust obtained from inner city homes is associated with development of allergic sensitization and wheezing. The results of these two studies are presented in this report.
METHODS
Study design
URECA is a longitudinal birth cohort study in four urban areas: Baltimore, Boston, New York City and St. Louis.13 Selection criteria included residence in an area with >20% residents below the poverty level, mother or father with allergic rhinitis, eczema, and/or asthma and birth at ≥ 34 weeks gestation. Maternal questionnaires were administered prenatally and participant questionnaires were administered every three months thereafter. Clinic visits occurred at 12, 24, 33, and 36 months, and homes were visited annually beginning at age 3 months for an environmental survey and house dust collection. Between February 2005 and March 2007, 1850 families were screened, 889 met eligibility criteria, and 560 were enrolled. Informed consent was obtained from the parent or legal guardian of the infant.
Study assessments
Allergen specific IgE (ImmunoCAP, Phadia, Uppsala Sweden) was measured annually for milk, egg, peanut, and German cockroach. At 2 and 3 years of age specific IgE for dust mites, dog, cat, mouse, and Alternaria was also measured. Prick skin testing was performed at age 33 months for 14 common indoor and outdoor allergens.13
Household dust samples from the living room (chair or sofa and floor) and child’s bedroom (mattress and floor) were collected as described in the Online Repository, and assayed for allergenic proteins including Bla g 1 (cockroach), Can f 1 (dog), Fel d 1 (cat), Der f 1 and Der p 1 (house dust mites), and Mus m 1 (mouse) by ELISA (Indoor Biotechnologies, Charlottesville, VA). A subsample (N=104) of living room dust specimens collected at 3 months of age underwent culture-independent microbiome profiling using a 16S rRNA-based phylogenetic microarray (G3 PhyloChip, Second Genome, San Bruno, CA; see Online Repository for details), to generate a high-resolution profile of both dominant and rare microbiota members in each sample for comparative and correlative analyses. An approximately equal number of dust samples were randomly selected from each of four categories defined by clinical outcomes at age 3 years: 1) recurrent wheeze and aeroallergen sensitivity, 2) recurrent wheeze alone, 3) aeroallergen sensitivity alone, and 4) neither outcome (Table E1, Online Repository). This sub-study population did not differ from the remainder of the cohort with respect to demographic characteristics or environmental exposures in the first year (Table E2).
Definitions
Aeroallergen sensitization was defined by a wheal ≥ 3mm larger than the saline control on prick skin testing or specific IgE ≥ 0.35 kU/L. Recurrent wheeze was defined as parental report of at least two wheezing episodes, with at least one episode occurring in the third year. Eczema was defined as a score ≥ 1.0 on the Eczema Area and Severity Index (EASI)14 at age 3 years. Children at higher risk for developing asthma were identified using the modified asthma predictive index (mAPI).15
Statistical analysis
Demographic comparisons between recurrent wheezers and non-wheezers were tested using Wilcoxon tests for continuous data and chi-squared tests for binary data. Univariate and multivariate analyses to determine association of exposures to sensitivity and recurrent wheeze were performed using logistic regression. Based on this and previous analyses,16 multivariate models were adjusted for race/ethnicity (strongly correlated with site), gender, mean perceived stress of the mother in the year after birth,17 and number of smokers in the home.
The three allergen exposures showing a strong inverse relationship to recurrent wheeze (cockroach, mouse, and cat; see below) were combined into a single allergen exposure index based on tertiles of exposure to individual allergens (see Online Repository). In addition, a dichotomous variable was created for exposure to each allergen (cockroach, mouse, and cat) to indicate if the levels were above standard cutoffs (Bla g 1, 2 U/g; Mus m 1, 0.5 µg/g; Fel d 1, 2 µg/g).18
Methods used to filter and analyze microbiome data are described in the Online Repository.
RESULTS
Factors related to recurrent wheeze in urban children
Of the 560 children in the URECA cohort, 478 (86%) remained in the study at age 3 years; 467 (83%) had sufficient data to assess recurrent wheeze and 383 (68%) had serum IgE data available at age 3. Children included in the primary analysis (with complete follow-up data on wheeze, sensitization, and home allergen exposure data) differed from those not included in terms of study site and race/ethnicity, but not allergen exposure (Table E3). Of these, 44% were sensitized to at least one aeroallergen, 36% had recurrent wheeze, and 9% had eczema (Figure E1, Online Repository). Furthermore, 12% of the cohort met the criteria for mAPI, indicating a high risk for subsequent asthma. Factors related to recurrent wheeze were annual family income <$15,000, lower birth weight and gestational age, the number of smokers in the household, and maternal prenatal depression (Table I). Children with recurrent wheeze had a median of 6 wheezing episodes (range 2–26) by age 3 years, and 77% had been prescribed albuterol, 27% an inhaled corticosteroid, and 33% at least one course of oral corticosteroid for wheezing.
Table I.
Characteristics of study participants.
| Overall (n=467) |
Recurrent Wheeze 36% (n=166) |
No Recurrent Wheeze 64% (n=301) |
|
|---|---|---|---|
| Mother completed high school | 58% (273) | 55% (92) | 61% (181) |
| Mother married | 14% (64) | 13% (21) | 14% (43) |
| Mother has ever had asthma | 50% (234) | 55% (91) | 48% (143) |
| Household income < $15,000* | 69% (321) | 75% (124) | 65% (197) |
| Number of smokers in home* | 0.93 ± 1.0 | 1.1 ± 1.2 | 0.84 ± 0.91 |
| Child’s race/ethnicity | |||
| Black | 71% (333) | 72% (120) | 71% (213) |
| Hispanic | 20% (92) | 16% (27) | 21% (65) |
| Other | 9% (42) | 12% (19) | 8% (23) |
| Child’s gender: Male | 51% (240) | 56% (93) | 49% (147) |
| C-section delivery | 33% (154) | 36% (60) | 31% (94) |
| Child’s birth weight (g)* | 3258.0 ± 504.4 | 3203.8 ± 567.0 | 3287.9 ± 464.6 |
| Gestational age* | 38.8 ± 1.5 | 38.4 ± 1.6 | 39.0 ± 1.4 |
| Breastfed child at 3 months | 24% (100) | 20% (29) | 26% (71) |
| BMI at age 3 | 16.7 ± 2.4 | 16.9 ± 2.9 | 16.7 ± 2.1 |
Significant difference (p<0.05) between recurrent wheeze group and children without recurrent wheeze.
Allergen levels from the first year dust samples varied by site (Figure E2) and there was also moderate within-individual variation over the three years (intraclass correlation 0.32–0.56). Cumulative exposure (summed over the three years) to cockroach, mouse and dust mite (D. farinae) was positively associated with sensitization to those allergens at age 3 (OR 1.27–1.68, Table E4), and, as expected, allergic sensitization was positively associated with recurrent wheeze (Table II). In contrast, exposure to allergens in the first year had little or no association with sensitization at year 3 (Table E4).
Table II.
Association between allergic sensitization in year 3 with recurrent wheeze in year 3.
| Sensitized | Unadjusted | Adjusted (+ race, gender) |
Adjusted (+ race, gender, PSS, ETS) |
|||||
|---|---|---|---|---|---|---|---|---|
| Sensitizations | n | % (n) | Odds ratio (95% CI) |
P | Odds ratio (95% CI) |
p | Odds ratio (95% CI) |
P |
| Any food | 383 | 40% (155) | 1.74 (1.14 – 2.67) | 0.01 | 1.81 (1.18 – 2.79) | 0.007 | 1.94 (1.25 – 3.03) | 0.003 |
| Any aeroallergen | 356 | 46% (163) | 1.58 (1.02 – 2.46) | 0.04 | 1.53 (0.98 – 2.39) | 0.06 | 1.63 (1.03 – 2.56) | 0.04 |
| Cat | 362 | 17% (61) | 1.78 (1.02 – 3.12) | 0.04 | 1.80 (1.03 – 3.17) | 0.04 | 1.93 (1.09 – 3.44) | 0.03 |
| Dog | 359 | 12% (44) | 1.91 (1.01 – 3.60) | 0.05 | 1.86 (0.97 – 3.55) | 0.06 | 2.22 (1.14 – 4.34) | 0.02 |
| Cockroach | 359 | 14% (52) | 1.62 (0.89 – 2.94) | 0.11 | 1.58 (0.87 – 2.89) | 0.14 | 1.74 (0.94 – 3.21) | 0.08 |
| Mouse | 366 | 20% (74) | 1.58 (0.94 – 2.66) | 0.09 | 1.60 (0.95 – 2.72) | 0.08 | 1.74 (1.01 – 2.97) | 0.04 |
| Dust mite (Der f) | 364 | 11% (41) | 2.18 (1.13 – 4.20) | 0.02 | 2.08 (1.07 – 4.03) | 0.03 | 2.40 (1.22 – 4.72) | 0.01 |
| Dust Mite (Der p) | 360 | 13% (45) | 1.48 (0.79 – 2.80) | 0.23 | 1.44 (0.76 – 2.73) | 0.27 | 1.57 (0.82 – 3.03) | 0.17 |
PSS=Perceived Stress Scale, ETS=Environmental tobacco smoke.
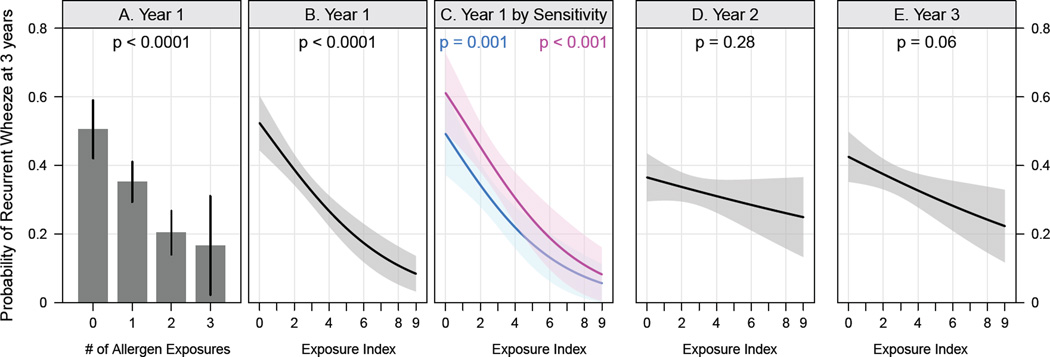
However, in contrast to our expectations, significant inverse relationships were found between first year exposure to cockroach, mouse, and cat – but not house dust mite or dog – allergens, and recurrent wheeze at age 3 (OR 0.60, 0.65, 0.75 and 0.97 and 1.01, respectively, Table III). An additive reduction in wheeze was observed with exposure to more than one of these three allergens (Figure 1). When categorized as exposed or not exposed using standard cut-offs, recurrent wheeze fell from 51% in those with no exposures (n=96) to 17% in those exposed to all three allergens (n=18) (Figure 1A). Using an index that reflects exposure to all three of these allergens in the first year of life, the prevalence of recurrent wheeze was inversely related to the index over the entire range of exposures (Figure 1B). The negative relationship between first year exposures and wheeze persisted when stratified by aeroallergen sensitization, and when adjusted for covariates (Figure 1C, Table III). Our data revealed that the timing of allergen exposure was important; the inverse relationship with recurrent wheeze at age 3 years was significant only for exposures in the first year of life, and not for those encountered in the second or third year (Figures 1D and 1E).
Table III.
Association between bedroom dust allergen exposure in the first year of life with recurrent wheeze in year 3.
| Unadjusted | Adjusted (+ sensitization*) | Adjusted (+ race, gender) | Adjusted (+ race, gender, PSS, ETS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Odds ratio (95% CI) |
P | Odds ratio (95% CI) |
p | Odds ratio (95% CI) |
p | Odds ratio (95% CI) |
P | |
| Exposures (year 1)† | |||||||||
| Cat | 362 | 0.75 (0.61 – 0.92) | 0.005 | 0.74 (0.61 – 0.91) | 0.004 | 0.73 (0.60 – 0.90) | 0.003 | 0.71 (0.58 – 0.88) | 0.001 |
| Dog | 359 | 1.01 (0.80 – 1.27) | 0.97 | 1.01 (0.80 – 1.27) | 0.97 | 1.04 (0.82 – 1.32) | 0.76 | 1.00 (0.79 – 1.28) | 0.98 |
| Cockroach | 359 | 0.60 (0.45 – 0.80) | <0.001 | 0.59 (0.44 – 0.79) | <0.001 | 0.61 (0.45 – 0.81) | <0.001 | 0.59 (0.44 – 0.80) | <0.001 |
| Mouse | 366 | 0.65 (0.52 – 0.82) | <0.001 | 0.64 (0.50 – 0.81) | <0.001 | 0.64 (0.51 – 0.81) | <0.001 | 0.65 (0.51 – 0.82) | <0.001 |
| Dust mite (Der f) | 364 | 0.97 (0.78 – 1.20) | 0.78 | 0.96 (0.77 – 1.19) | 0.69 | 0.95 (0.76 – 1.18) | 0.63 | 0.92 (0.73 – 1.15) | 0.45 |
| Model using allergen exposure index‡ | |||||||||
| Exposure Index (0–9) | 356 | 0.73 (0.64 – 0.83) | <0.001 | 0.73 (0.64 – 0.83) | <0.001 | 0.73 (0.64 – 0.84) | <0.001 | 0.73 (0.64 – 0.83) | <0.001 |
| Allergen exposure index stratified by aeroallergen sensitivity‡ | |||||||||
| Not sensitive | 234 | 0.73 (0.61 – 0.88) | 0.95∫ | n/a | n/a | 0.73 (0.60 – 0.88) | 0.97∫ | 0.72 (0.60 – 0.88) | 0.91∫ |
| Sensitive | 122 | 0.73 (0.61 – 0.88) | n/a | n/a | 0.74 (0.61 – 0.89) | 0.73 (0.61 – 0.89) | |||
Individual exposure models are adjusted for the respective sensitization. The Exposure Index is adjusted for overall aeroallergen sensitization.
Odds of recurrent wheeze for each 1-log increase in allergen level.
Odds of recurrent wheeze for a 1-unit increase in the allergen exposure index.
Interaction p-value
PSS=Perceived Stress Scale, ETS=Environmental tobacco smoke.
Figure 1.
Relationships between specific allergen exposures and recurrent wheezing. (A) Probability of recurrent wheeze (95% confidence intervals [CI]) according to the number of allergen exposures (cockroach, mouse and/or cat). (B–E) Probability of recurrent wheeze (95% CI) determined by logistic regression; (C) is stratified by aeroallergen sensitivity (pink=sensitive, blue=not sensitive), (D) and (E) show the probability of recurrent wheeze for allergen exposures during years 2 and 3.
Relationship of microbial exposure to atopy and to recurrent wheeze
To test the hypothesis that early life exposure to certain microbes in house dust is associated with protection against allergic sensitization and wheezing, we conducted a nested case-control study of the microbes present in house dust of 104 URECA households. The number of samples were relatively evenly distributed across four distinct groups: children with recurrent wheeze alone (“Wheeze”, n=26), atopy alone (“Atopy”, n=25), both recurrent wheeze and atopy (“Both”, n=24), or neither recurrent wheeze nor atopy (“Neither”, n=29). The inverse association between first-year allergen exposure and recurrent wheeze at age 3 years in the nested case-control study population was similar to that observed in the whole population (Table E5).
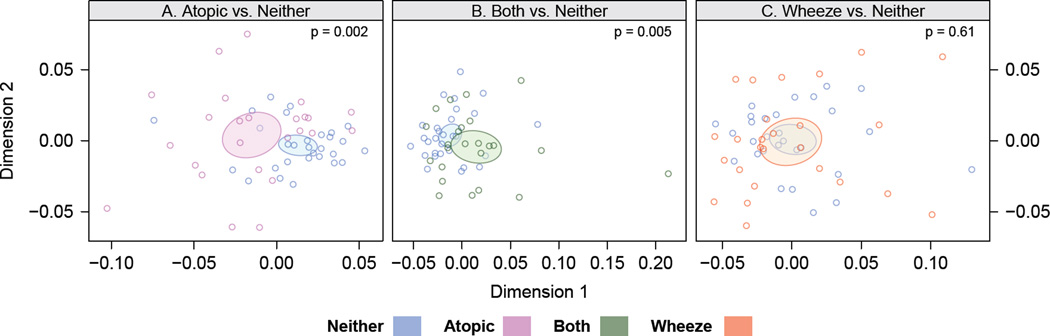
Relative bacterial richness, a measure of the number of bacterial taxa detected in each sample, was significantly different among the four groups (p < 0.02) and was considerably lower in first-year dust samples from the Atopy or Both groups relative to the Neither group (Figure E3), indicating reduced bacterial exposure in these environments. Multivariate analysis of house dust bacterial community composition further demonstrated that the Neither group house dust microbiome was compositionally distinct from that of the Atopy (p=0.002, Figure 2A) and Both groups (p=0.005, & Figure 2B), but not from the Wheeze group (Figure 2C).
Figure 2.
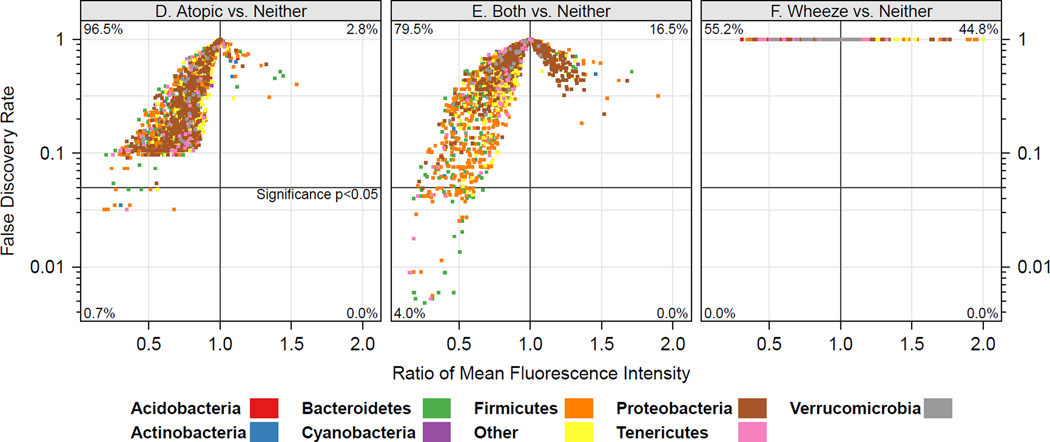
House dust microbiome composition is associated with clinical outcomes. (A–C) Similarity or dissimilarity of house dust microbiota composition is indicated by the distance between samples (colored dots); samples plotted close together are compositionally similar, greater inter-sample distance indicates compositionally distinct bacterial communities. Ellipses represent 95% CIs for each group. (D–F) Mean taxon relative abundance across house dust samples from the Neither group were compared to those of the Atopy (D), Both (E) and Wheeze (F) groups to identify specific bacterial taxon associated with clinical outcomes. Taxa in the left lower quadrant are underrepresented in the Atopy (D) and Both (E) groups compared to the Neither group. Colors indicate phylum-level classification of individual taxa, and the horizontal lines indicate p<0.05 after correction for false discovery rates.
To identify the specific taxa within the house dust microbiome that discriminated among these groups, we next compared taxon relative abundance between the Neither group and each of the other groups. The greatest number of taxa exhibiting significant difference in relative abundance was identified in the Both vs. Neither comparison (82 taxa, Figure 2E). All of these “taxa of interest” were significantly increased in the first year dust samples of the Neither group, raising the possibility that exposure to certain bacteria in house dust during the first year of life may play a role in protection against atopic wheeze. These putatively protective bacteria belonged to several phyla, but were primarily members of the Bacteroidetes and Firmicutes, in particular the Prevotellaceae, Lachnospiraceae and Ruminococcaceae families (Table E6). Fewer taxa showed significant differences in relative abundance in the Neither vs Atopy comparison (Figure 2D), but again, of those that did, all were relatively more abundant in the Neither group and were thus associated with a reduction in the risk of atopy. These taxa largely belonged to the same families as those identified from the Neither versus Both comparison (Table E6). No taxa were found to differ significantly in relative abundance between the Neither and the Wheeze groups (Figure 2F).
Relationships between allergen and microbial exposures
We next asked whether bacteria that were inversely related to atopy and atopy with wheeze were also related to allergen exposure. Of the 82 taxa of interest significantly enriched in the Neither group, 14 and 10 were also significantly correlated with the levels of Mus m 1 and Bla g 1 allergens respectively, and these taxa were primarily members of the Bacteroidetes (e.g. Prevotellaceae and Rikenellaceae; Table E7). Blattabacteria, known endosymbionts of cockroaches,19 were highly positively correlated with Bla g 1 levels, indicating that some of these bacterial constituents of house dust may originate from the enteric contents of cockroaches. None of the 82 taxa of interest were associated with Fel d 1 allergen levels.
Relationship of combined effects of allergens and bacteria to study outcomes
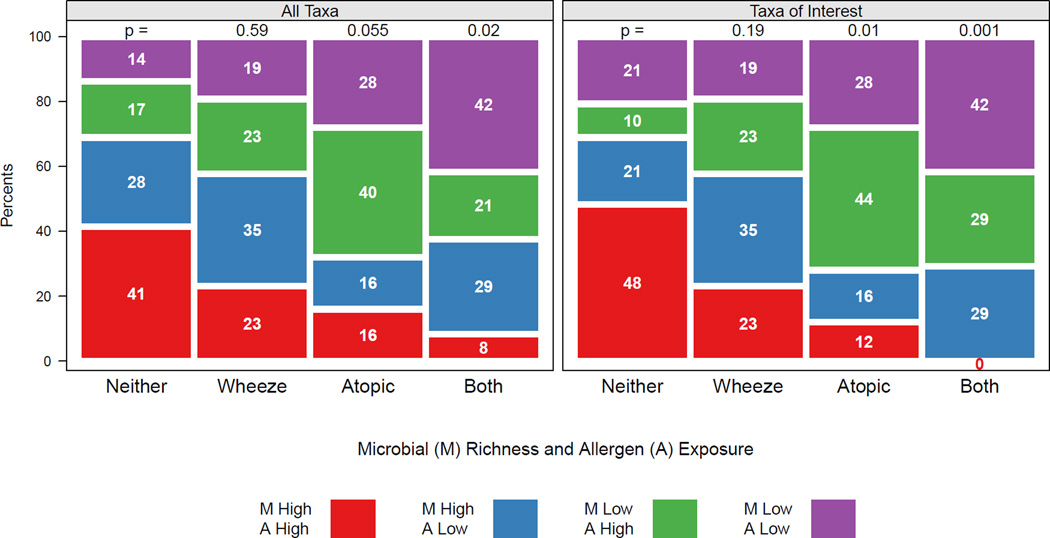
Given the above associations, we thought it important to examine how combined early life exposures to the selected allergens (cockroach, mouse, and cat) and to bacteria (richness of either the total community or the 82 taxa of interest) impacted clinical outcomes at 3 years. Our analysis of the nested case control study population showed that the combined exposure patterns differed significantly across the four outcome groups, and this was true whether the richness of the total bacterial community (Figure 3A) or of the 82 taxa of interest (Figure 3B) were considered in the analysis. Of the children in the Both group, the largest proportion (42%) were exposed to low levels of allergens and low bacterial community richness, while the smallest proportion (8%) of this group had been exposed to high levels of selected allergens and bacterial richness. Conversely, the children in the Neither group were more likely to have been exposed to high levels of allergens and high bacterial richness (41%), and were least likely (14%) to have had low level allergen and bacterial exposure (Figure 3). These comparisons reached higher levels of significance when only the 82 taxa of interest were used in the analysis (Figure 3B). For example, the largest proportion (42%) of children in the Both group had low level exposure to both the specified allergens and the 82 bacteria of interest, while none of the children in this group were exposed to high levels of allergen and of these specific bacteria.
Figure 3.
Distribution of allergen and bacterial exposure among children with atopy, recurrent wheeze, atopy with wheeze and neither outcome. Exposure to allergen is classified as high or low with respect to the median of the allergen exposure index, and richness of exposure to all microbes (A) or taxa of interest (B). The distribution of exposure in each outcome group is compared to the distribution in the "Neither" group.
DISCUSSION
Using a birth cohort and a nested case control study, we assessed the relationships between exposure to allergens and bacteria over the first three years of life and the development of recurrent wheeze and atopy. As hypothesized, cumulative allergen exposure over the first 3 years of life was associated with allergic sensitization, and allergic sensitization was associated with recurrent wheeze. However, the major finding from the birth cohort study was that high levels of cockroach, mouse and, to a lesser extent, cat allergen in the first-year inner city house dust samples had a strong inverse relationship with recurrent wheeze at age 3 years. This association was strongest for allergen levels in the first year dust samples, suggesting that the first few months of life is a critical time period in childhood allergic disease development. In addition, changes in the bacterial house dust environment, characterized by reduced exposure to bacterial richness as well as specific bacteria, were significantly associated with the development of atopy with wheezing or atopy alone. Some of the bacterial taxa related to favorable clinical outcomes were positively associated with mouse and cockroach allergen levels, raising the possibility that household pests may be the source of some of the beneficial bacteria in the inner city environment. Finally, combined analysis of exposure to both allergens and bacteria revealed that the group of children with neither wheeze nor atopy had the highest first year exposure to allergens and the bacterial species identified in this study as potentially protective against atopy and recurrent wheeze. This is, to our knowledge, the first scientific report of exposure to high levels of allergens combined with an environment rich in specific bacterial families as having a protective effect against atopy and atopy with wheezing in early childhood.
A prevalent conceptual model is that exposure to perennial indoor allergens contributes to the development of allergic sensitization with subsequent development of wheezing.20–22 Earlier cross-sectional studies of inner city populations supported this concept by showing a dose-response relationship between Bla g 1 levels in house dust and allergic sensitization to cockroach.23 Our findings are in some ways consistent with this model in showing that cumulative exposure to cockroach and mouse allergens in the dust of inner city houses over the first three years of life is associated with allergic sensitization, and that allergic sensitization is associated with recurrent wheeze.
Where our findings importantly differ from this conceptual model is in showing an inverse relationship between the levels of cockroach, mouse, and cat allergen in dust samples collected in the first months of life and recurrent wheeze at age 3. The temporal nature of the relationship is similar to that previously observed between early-life exposure to pets and reduced rates of allergic sensitization and asthma,24–26 and supports a mounting body of evidence that exposures in the first few months of life are important in shaping allergic and respiratory outcomes. This counter-intuitive effect of early life exposure to certain allergens may help explain the failure of several previous studies to find straightforward relationships between allergen exposure and the development of atopy and asthma27–30 or to demonstrate that allergen avoidance protects against development of these conditions.31–34 Notably, in contrast to other reports,25, 35 dog exposure was not protective for atopy or wheeze in this study. We speculate that the reason for the lack of protection in urban homes may be that dogs may be kept for security and thus interact less with young children, and may also be less likely to bring soil into the home from outdoor sources, which are sparse in urban neighborhoods.
Our findings point to particular families of bacteria as being protective against the development of atopy and atopic wheeze. These findings are in line with experimental data in animals and observational studies in Western Europe showing that microbial exposures in early life are critical contributors to respiratory health.11, 19 Certainly, the URECA findings have a very different context; overall rates of wheezing and allergic sensitization in low income, urban US populations are high, likely influenced by adverse factors related to the urban environment (e.g. indoor and outdoor pollutants) and increased rates of low birth weights, prematurity, household tobacco smoking and maternal stress and depression. Among families exposed to these adverse conditions, it is remarkable that the general tenets of the “Hygiene Hypothesis” are applicable, in that some of the residences in this environment contain bacteria to which exposure reduces the risk of developing allergic sensitization with or without recurrent wheezing. Whether these bacteria are similar in phylogeny, quantity, or function to those found in farmhouses remains to be determined.
The URECA study is the first to consider exposures to both allergens and bacteria in the same population. The effects of high specific allergen and specific bacterial exposure appear to be at least partially independent. High cockroach, mouse and cat allergen exposure was associated with reduction in recurrent wheezing regardless of atopy, whereas higher exposure to specific bacterial families in house dust appeared to target atopy. Interpretation of these effects is complicated by the fact that some of the “protective” bacteria were positively related to allergen levels. The combined analysis revealed potential synergism between high allergen and bacterial exposures: the children with neither atopy nor wheezing at age 3 had high early-life exposure to both allergens and the bacterial families of interest. In contrast, children with atopy alone were most often exposed to high levels of allergens and low levels of the putatively protective bacterial families. These clinical findings are consistent with experimental models in which induction of tolerance is achieved by administration of allergen together with a tolerizing signal provided by specific microbes.36–38 Our observations raise the possibility that the optimal conditions to promote tolerance may be early-life antigenic exposure to allergen in conjunction with exposure to particular bacteria such as those identified in this study.
There are several possible explanations for the associations between early microbial and allergen exposures and the three-year outcomes. The infant microbiome exhibits a temporal program of community assembly over the first year of life, and patterns of early-life gastrointestinal colonization have previously been linked to immune development,39 allergic disease,40, 41 and the response to viral respiratory infection.42, 43 Most of the “protective” taxa identified in our study belong to bacterial families (e.g. Prevotellaceae, Lachnospiraceaea and Ruminococcaceae,) which are human colonizers and important producers of immunomodulatory metabolites such as short chain fatty acids.44 This suggests that early-life exposure to house dust containing these bacteria may inoculate the developing gastrointestinal microbiome with species that produce metabolites that protect against development of atopy and atopic wheeze. It is also plausible that these microbial exposures could exert a similar effect at the airway mucosal surfaces. Accordingly, the respiratory microbiome is altered in asthma,45, 46 and experiments in mice demonstrate that environmental microbes in early life can modify lung mucosal immunity to reduce inflammatory responses to allergens.47 How allergen exposure modifies the risk of recurrent wheeze, which frequently is caused by viral respiratory infections, is unknown, but the mechanisms could be related to direct biological effects of allergens (e.g. activation of innate immune receptors) or allergen-associated biologics (e.g. proteases, chitin, DNA).48–50 Whether exposure to these immunologically active substances in early life affects the development of mucosal immune and antiviral responses remains to be determined.
Strengths of our investigation include its examination of an inner-city population at high risk for respiratory morbidity, high rate of study participant retention, and prospective assessments of environmental exposures. Previous clinical studies of the microbiome have used lower resolution culture- or molecular-based approaches to distinguish a limited selection of microbes. The URECA study used a phylogenetic microarray to profile thousands of organisms in parallel, and provided data at the phylum, family and species level. This is a multicenter study with site differences in exposure, race/ethnicity, and in the prevalence of recurrent wheeze, but the main study findings were unaffected by adjustment for these factors. One limitation of the study is that the microbial analysis was performed in a subset of homes, and relationships between specific bacterial taxa and outcomes, though FDR corrected, await confirmation in other populations. Furthermore, the array-based method in this study neither provides information about absolute quantity of bacteria, nor functional analysis of metabolic activity that could mediate effects on immune development. Finally, it is important to consider that effects of environmental exposures could change with age, and also in relationship to age-dependent phenotypes (e.g. recurrent wheezing vs. persistent asthma).
In summary, the inner city environment includes adverse exposures that promote allergic disease and wheezing. Even so, our results demonstrate that within this environment, early life exposures to certain allergens and bacteria are associated with significant reductions in recurrent wheeze and atopy. While allergen avoidance can benefit children with established allergic asthma, our findings imply that different strategies will be needed to prevent atopy and wheeze in preschool children. Conceptually, in environments characterized by high allergen exposure, our results raise the possibility that enhancing microbial exposures could be more effective than allergen abatement. Moving forward, the challenge will be to define whether specific allergen- and bacteria-associated mechanisms account for these effects to enable formulation of evidence-based interventions.
Supplementary Material
CLINICAL IMPLICATIONS.
Concomitant exposure to high levels of certain allergens and bacteria in early life may be beneficial, which suggests new preventive strategies for wheezing and allergic diseases.
Acknowledgements
The Urban Environment and Childhood Asthma Study is a collaboration of the following institutions and investigators (principal investigators are indicated by an asterisk; protocol chair is indicated by double asterisks):
Johns Hopkins University, Baltimore, MD – R Wood*, E Matsui, H Lederman, F Witter, J Logan, S Leimenstoll, D Scott, L Daniels, L Miles, D Sellers, A Swift; Boston University School of Medicine, Boston, MA – G O’Connor*, W Cruikshank, M Sandel, A Lee-Parritz, C Stamoulos, E Gjerasi, P. Price-Johnson, B Caldwell, M Tuzova; Harvard Medical School, Boston, MA – D Gold, R Wright; Columbia University, New York, NY – M Kattan*, C Lamm, N Whitney, P Yaniv, C Sanabia, A Valones; Mount Sinai School of Medicine, New York, NY – H Sampson, M Mishoe; Washington University School of Medicine, St Louis, MO – G Bloomberg*, L Bacharier, Y Sadovsky, E Tesson, C Koerkenmeier, R Sharp, K Ray, J Durrange, I Bauer; Statistical and Clinical Coordinating Center - Rho, Inc, Chapel Hill, NC – H Mitchell*, P Zook, C Visness, M Walter, R Bailey, S. Hicks, W Taylor, R Budrevich; Scientific Coordination and Administrative Center – University of Wisconsin, Madison, WI – W Busse*, J Gern**, P Heinritz, C Sorkness, WM Lee, K Grindle, A Dresen, T Pappas; National Institute of Allergy and Infectious Diseases, Bethesda, MD – P Gergen, A Togias, E Smartt, K Thompson.
Funding:
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contract numbers NO1-AI-25496, NO1-AI-25482, HHSN272200900052C, and HHSN272201000052I. Additional support was provided by the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, and 5UL1RR024992-02.
ABBREVIATIONS
- EASI
Eczema Area and Severity Index
- FDR
False Discovery Rate
- mAPI
Modified Asthma Predictive Index
- URECA
Urban Environment and Childhood Asthma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lau S, Illi S, Sommerfeld C, Niggemann B, Volkel K, Madloch C, et al. Transient early wheeze is not associated with impaired lung function in 7-yr-old children. Eur Respir J. 2003;21:834–41. doi: 10.1183/09031936.03.00037203. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 3.Carroll KN, Gebretsadik T, Griffin MR, Wu P, Dupont WD, Mitchel EF, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122:58–64. doi: 10.1542/peds.2007-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelletier AJ, Mansbach JM, Camargo CA., Jr Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics. 2006;118:2418–2423. doi: 10.1542/peds.2006-1193. [DOI] [PubMed] [Google Scholar]
- 5.Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 7.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 8.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Exposure to cockroach allergen in the home is associated with incident doctor-diagnosed asthma and recurrent wheezing. J Allergy Clin Immunol. 2001;107:41–47. doi: 10.1067/mai.2001.111143. [DOI] [PubMed] [Google Scholar]
- 9.Phipatanakul W, Celedon JC, Hoffman EB, Abdulkerim H, Ryan LM, Gold DR. Mouse allergen exposure, wheeze and atopy in the first seven years of life. Allergy. 2008;63:1512–1518. doi: 10.1111/j.1398-9995.2008.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phipatanakul W, Celedon JC, Sredl DL, Weiss ST, Gold DR. Mouse exposure and wheeze in the first year of life. Ann Allergy Asthma Immunol. 2005;94:593–599. doi: 10.1016/S1081-1206(10)61139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 12.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 13.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O'Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 15.Castro-Rodriguez JA. The Asthma Predictive Index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol. 2010;126:212–216. doi: 10.1016/j.jaci.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 18.Matsui EC, Hansel NN, McCormack MC, Rusher R, Breysse PN, Diette GB. Asthma in the inner city and the indoor environment. Immunol Allergy Clin North Am. 2008;28:665–686. x. doi: 10.1016/j.iac.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Sanchez MJ, Neef A, Patino-Navarrete R, Navarro L, Jimenez R, Latorre A, et al. Blattabacteria, the endosymbionts of cockroaches, have small genome sizes and high genome copy numbers. Environ Microbiol. 2008;10:3417–3422. doi: 10.1111/j.1462-2920.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 20.Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr Opin Allergy Clin Immunol. 2009;9:128–135. doi: 10.1097/aci.0b013e32832678b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuehr J, Frischer T, Meinert R, Barth R, Forster J, Schraub S, et al. Mite allergen exposure is a risk for the incidence of specific sensitization. J Allergy Clin Immunol. 1994;94:44–52. doi: 10.1016/0091-6749(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 22.Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, et al. House dust mite allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996;153:141–146. doi: 10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- 23.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 24.Bufford JD, Reardon CL, Li Z, Roberg KA, DaSilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38:1635–1643. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 25.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 26.Remes ST, Castro-Rodriguez JA, Holberg CJ, Martinez FD, Wright AL. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol. 2001;108:509–515. doi: 10.1067/mai.2001.117797. [DOI] [PubMed] [Google Scholar]
- 27.Chen CM, Rzehak P, Zutavern A, Fahlbusch B, Bischof W, Herbarth O, et al. Longitudinal study on cat allergen exposure and the development of allergy in young children. J Allergy Clin Immunol. 2007;119:1148–1155. doi: 10.1016/j.jaci.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000;356:1392–1397. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 29.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 30.Tovey ER, Almqvist C, Li Q, Crisafulli D, Marks GB. Nonlinear relationship of mite allergen exposure to mite sensitization and asthma in a birth cohort. J Allergy Clin Immunol. 2008;122:114–118. doi: 10.1016/j.jaci.2008.05.010. 8 e1-5. [DOI] [PubMed] [Google Scholar]
- 31.Corver K, Kerkhof M, Brussee JE, Brunekreef B, van Strien RT, Vos AP, et al. House dust mite allergen reduction and allergy at 4 yr: follow up of the PIAMA-study. Pediatr Allergy Immunol. 2006;17:329–336. doi: 10.1111/j.1399-3038.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 32.Horak F, Jr, Matthews S, Ihorst G, Arshad SH, Frischer T, Kuehr J, et al. Effect of mite-impermeable mattress encasings and an educational package on the development of allergies in a multinational randomized, controlled birth-cohort study -- 24 months results of the Study of Prevention of Allergy in Children in Europe. Clin Exp Allergy. 2004;34:1220–1225. doi: 10.1111/j.1365-2222.2004.02024.x. [DOI] [PubMed] [Google Scholar]
- 33.Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol. 2006;118:53–61. doi: 10.1016/j.jaci.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, et al. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med. 2004;170:433–439. doi: 10.1164/rccm.200401-083OC. [DOI] [PubMed] [Google Scholar]
- 35.Gern JE, Reardon CL, Hoffjan S, Nicolae D, Li Z, Roberg KA, et al. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol. 2004;113:307–314. doi: 10.1016/j.jaci.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Haapakoski R, Karisola P, Fyhrquist N, Savinko T, Lehtimaki S, Wolff H, et al. Toll-like receptor activation during cutaneous allergen sensitization blocks development of asthma through IFN-gamma-dependent mechanisms. J Invest Dermatol. 2013;133:964–972. doi: 10.1038/jid.2012.356. [DOI] [PubMed] [Google Scholar]
- 37.Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, et al. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A. 2013;110:3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penders J, Stobberingh EE, Thijs C, Adams H, Vink C, van Ree R, et al. Molecular fingerprinting of the intestinal microbiota of infants in whom atopic eczema was or was not developing. Clin Exp Allergy. 2006;36:1602–1608. doi: 10.1111/j.1365-2222.2006.02599.x. [DOI] [PubMed] [Google Scholar]
- 41.van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128:948–955. e1–e3. doi: 10.1016/j.jaci.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasui H, Kiyoshima J, Hori T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin Diagn Lab Immunol. 2004;11:675–679. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 45.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2010;127:372–81. e1–e3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ries M, Schuster P, Thomann S, Donhauser N, Vollmer J, Schmidt B. Identification of novel oligonucleotides from mitochondrial DNA that spontaneously induce plasmacytoid dendritic cell activation. J Leukoc Biol. 2013;94:123–135. doi: 10.1189/jlb.0612278. [DOI] [PubMed] [Google Scholar]
- 50.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.