Abstract
We performed a retrospective cohort analysis of 701 (533 White and 144 Black) patients with DLBCL treated at two referral centers in southern United States between 1981-2010. Median age of diagnosis for Blacks was 50 years vs. 57 years for Whites (p<0.001). A greater percentage of Blacks presented with elevated lactate dehydrogenase levels, B-symptoms, and performance status≥2. More Whites (8%) than Blacks (3%) had positive family history of lymphoma (p=0.048). There were no racial differences in the use of R-CHOP (52% Black vs. 47% White, p=0.73). While black race predicted worse survival among patients treated with CHOP (Hazard ratio [HR] 1.8, p<0.001), treatment with R-CHOP was associated with improved survival irrespective of race (HR 0.61, p=0.01). Future studies should examine biological differences that may underlie the observed racial differences in presentation and outcome.
Keywords: Diffuse large B-cell lymphoma, Race, Disparities, Outcomes, CHOP regimen, R-CHOP regimen
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common pathological subtype of non-Hodgkin lymphoma (NHL) in the Western world, comprising approximately one-third of all adult lymphomas.[1,2] The incidence of NHL and DLBCL increased 3-4% per year from the 1970s until the mid 1990s. This dramatic rise in incident cases of DLBCL is comparable only to the rise in skin cancers and occurred in both genders, across racial categories, and across all age groups except the very young.[3] Although the median age at diagnosis for DLBCL is in the sixth decade,[4] it can present over a broad age range and is heterogeneous in histologic appearance, immunophenotype, response to treatment, and clinical outcome. While DLBCL is associated with a median survival of less than 1 year in untreated patients,[5] DLBCL is potentially curable with conventional anthracycline-based chemotherapy. Until 2002, the combination chemotherapy regimen of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) remained the standard therapy for DLBCL following its development in the 1970s.[5,6] A series of randomized clinical trials demonstrated that adding the anti-CD20 antibody, rituximab (R), to CHOP chemotherapy significantly improved overall survival (OS), [7-9] leading R-CHOP to become the standard therapy for DLBCL.[3]
While it has been well established that there are significant racial differences in NHL[10,11] and DLBCL incidence[1,11] few studies have investigated the relationships between race, the patterns of DLBCL presentation, treatment selection, and clinical outcomes with modern therapies.[1,12-14] Data from 38,522 incident cases of DLBCL diagnosed from 1992 to 2007 encoded in Surveillance, Epidemiology and End Results (SEER) registries across the United States demonstrated that the incidence rates for DLBCL were significantly lower for black patients than white patients.[1] This study also indicated that there are racial differences in DLBCL patterns of presentation and outcomes. However, all prior registry-based studies have been critically limited by lack of uniform pathology review,[15] disagreement between computer-converted codes for DLBCL that have changed over time,[16] and incomplete or missing data on stage, race, important clinical and laboratory prognostic factor such as lactate dehydrogenase (LDH), treatment, treatment response, and follow-up.[1,12-14] Moreover, prior institutional studies and even some population based studies have had insufficient numbers of black patients to perform comparisons across racial groups.
To overcome these limitations, we conducted a retrospective cohort study of consecutive patients with a confirmed diagnosis of DLBCL receiving care at two major academic medical centers in the southern United States. We constructed a large, comprehensive dataset of black and white patients with complete ascertainment of demographic, socioeconomic, disease, and treatment information in order to assess the impact of race and socioeconomic factors on disease presentation, treatment selection, and disease outcomes.
Methods
Study Population
After approval by the Emory University and University of Alabama at Birmingham (UAB) Institutional Review Boards, we utilized published methods[17,18] to identify from pathology and medical records, patients who were diagnosed with DLBCL as defined by the World Health Organization classification of Tumours of Haematopoietic and Lymphoid Tissues.[19] Patients with DLBCL who received care within the Emory University healthcare system (1981-2010) and the UAB healthcare system (1985-2010) were included in the study. Cases were included if there was a diagnosis of DLBCL confirmed by record review, available information on date of diagnosis, date of last contact or date of death, known race, known stage, known performance status (PS), and known first course of treatment. Pathology slides were not re-reviewed for this study. Figure 1 depicts the selection of DLBCL cases included in the analyses.
Figure 1. Selection of study cohort.
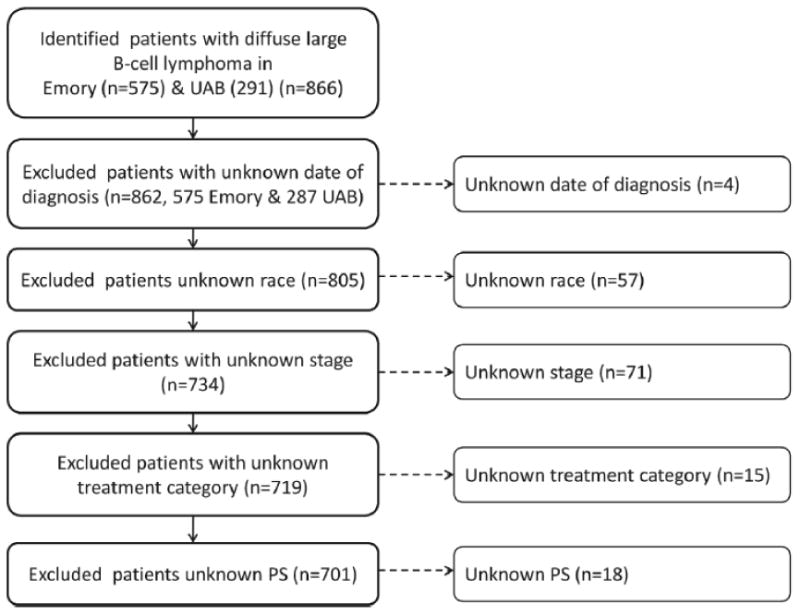
This figure provides an overview of the study cohort with reasons for inclusion/exclusion through the selection process.
Data Collection
Trained abstractors (PS, KB, UB, TDH, RG) collected clinical data from the time of diagnosis, during treatment, and at follow-up. Baseline demographic data collected at diagnosis were age, gender, race, marital status, employment status, and health insurance coverage. For these analyses, race was categorized as white, black, other, or unknown based on medical records and self-reported information. Health insurance coverage was categorized as Medicaid, Medicare +/- Medicaid, Private +/-Medicare/Medicaid, uninsured, or unknown. Employment status was used as a measure of socioeconomic status. Clinical data included in the analyses were family history of lymphoma, Eastern Cooperative Oncology Group (ECOG) PS score, serum LDH level, number of extranodal sites involved, Ann Arbor stage of disease, International Prognostic Index (IPI) risk category,[20] presence of B-symptoms, and first course of therapy.
Date of last contact and date of death due to any cause were collected. The date of death was verified using the social security death index. The primary outcome was OS defined as the time from date of diagnosis to the date of death and censored for living patients at the last date of contact. To evaluate the impact of changes in standard therapy for DLBCL on survival, we divided patients into treatment eras by year of diagnosis: 1981-1991 to reflect the early CHOP era,[6,21] 1992-1997 to reflect the years when CHOP was considered standard therapy,[5,22] 1998-2002 as the early years of R-CHOP use following the approval of rituximab for follicular NHL[23-25] and 2003-2010 to reflect the years when R-CHOP was widely utilized based on results of randomized control trials.[7-9]
Statistical Analysis
The primary aims of this work were to examine black/white differences in the age of onset of DLBCL, poor risk features contained within the IPI, and survival by treatment type. We hypothesized that black patients presented at a younger age, were more likely to have poor risk features, and would have poorer survival even when the same treatment was given. All other comparisons between black and white patients were part of exploratory analyses. Differences in baseline characteristics at diagnosis between racial groups were analyzed using two-sided t-tests and chi-square tests. Univariate and multivariable logistic regression models were used to identify predictors of receiving CHOP vs. R-CHOP as first course of treatment. Univariate and multivariable Cox proportional hazards models were used to determine predictors for survival. OS curves were constructed using the Kaplan-Meier method and were compared by a two-sided log-rank test. A level of significance (α) of 0.05 was defined as statistically significant. All statistics were computed using SAS software, version 9.0 (SAS Institute Inc. Copyright © 2002), and STATA 9.2 (StataCorp LP. Copyright © 1985-2006).
Results
Patient Population
We identified 866 consecutive patients (575 Emory and 291 UAB) with a confirmed diagnosis of DLBCL. Patients with missing data on date of diagnosis (4), race (57), stage (71), first course of treatment (15), or ECOG PS (18) were excluded, yielding a cohort of 701 cases (Figure 1). Cases with known race were grouped into white (533), black (144), and patients of other race (24). Due to small numbers of patients in other racial groups, the primary analyses focus on the differences between white and black patients.
Baseline Characteristics
Table I compares the baseline characteristics at diagnosis by race. Black patients were diagnosed at a significantly younger age compared to white patients (median age 50 vs. 57 years, p<0.001). Males comprised 58% of white and 48% of black patients (p=0.04). A greater proportion of black patients presented with an abnormal LDH level as compared to white patients (51% vs. 36%, p=0.03), and 42% of black compared to 31% of white patients presented with B-symptoms (p=0.02). Black patients were more likely to present with ECOG-PS ≥2 as compared to white patients (26% vs. 16%, p=0.01). There were no statistically significant differences in stage, extranodal site involvement, and IPI risk category at presentation. However, when IPI was age-adjusted (aaIPI), a greater proportion of black patients had high risk aaIPI as compared to white patients (aaIPI score 2 27% vs. 17%, aaIPI score 3 17% vs. 4%, p=0.03). The high risk scores for Black patients was especially true for the ≤60 years age group (aaIPI score 3 21% vs. 8%, p=0.02). More than twice as many white patients (8%) as black patients (3%) had a positive family history of lymphoma (p=0.048).
Table I. Racial differences in the presentation of DLBCL.
| Characteristic | Black | White | Other | p-value* comparing B vs. W |
|---|---|---|---|---|
| Total Number of Patients (%) | 144 (21) | 533 (76) | 24 (3) | |
| Age, years | ||||
| Mean | 50 | 57 | 46 | <0.001 |
| IQR | 37-61 | 45-69 | 33-62 | |
| ≤60 | 106 (74) | 305 (57) | 17 (71) | <0.001 |
| >60 | 38 (26) | 228 (43) | 7 (29) | |
| Male gender | 69 (48) | 307 (58) | 18 (75) | 0.038 |
| Family History of Lymphoma | ||||
| Yes | 4 (3) | 41 (8) | 0 (0) | 0.048 |
| No | 119 (83) | 442 (83) | 19 (79) | |
| Unknown | 21 (14) | 50 (9) | 5 (21) | |
| ECOG performance status | ||||
| 0-1 | 106 (74) | 446 (84) | 21 (88) | 0.006 |
| ≥2 | 38 (26) | 87 (16) | 3 (12) | |
| Disease stage | ||||
| I/II | 55 (38) | 231 (43) | 9 (37) | 0.267 |
| III/IV | 89 (62) | 302 (57) | 15 (63) | |
| LDH level | ||||
| Normal | 34 (23) | 151 (28) | 9 (38) | 0.026 |
| >ULN | 73 (51) | 193 (36) | 8 (33) | |
| Unknown | 37 (26) | 189 (36) | 7 (29) | |
| B-symptoms | ||||
| Absent | 71 (49) | 302 (57) | 14 (58) | 0.023 |
| Present | 61 (42) | 165 (31) | 8 (33) | |
| Unknown | 12 (8) | 66 (12) | 2 (8) | |
| No. of extranodal sites | ||||
| 0-1 | 94 (65) | 374 (70) | 16 (67) | 0.218 |
| ≥2 | 34 (24) | 92 (17) | 4 (17) | |
| Unknown | 16 (11) | 67 (13) | 4 (17) | |
| International Prognostic Index | ||||
| 0-1 | 45 (31) | 170 (32) | 7 (29) | 0.549 |
| 2 | 23 (16) | 78 (15) | 3 (13) | |
| 3 | 19 (13) | 65 (12) | 1 (4) | |
| 4-5 | 20 (14) | 48 (9) | 2 (8) | |
| Unknown | 37 (26) | 172 (32) | 11 (46) | |
| Insurance Type | ||||
| Medicaid | 26 (18) | 29 (5) | 2 (8) | <0.001 |
| Medicare +/- Medicaid | 20 (14) | 62 (12) | 3 (13) | |
| Private +/- M/M | 82 (57) | 424 (79) | 17 (71) | |
| Uninsured | 10 (7) | 10 (2) | 0 (0) | |
| Unknown | 6 (4) | 8 (2) | 2 (8) | |
| Employed | ||||
| Yes | 51 (35) | 205 (38) | 14 (58) | 0.684 |
| No | 36 (25) | 131 (25) | 5 (21) | |
| Unknown | 57 (40) | 197 (37) | 5 (21) | |
| First line of treatment | ||||
| R-CHOP | 75 (52) | 253 (47) | 14 (58) | 0.728 |
| CHOP | 45 (31) | 190 (36) | 6 (25) | |
| R+/- Other | 11 (8) | 37 (7) | 1 (4) | |
| Other | 13 (9) | 53 (10) | 3 (13) |
Variables unknown were excluded while calculating p-value
Abbreviations: B, Black; W, White; IQR, Interquartile Range; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; ULN, upper limit of normal; M/M, Medicare/Medicaid; R-CHOP, rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
An equal proportion of black and white patients declared their employment location at the time of presentation (35% vs. 38%, p=0.68). The majority of patients studied were insured, but fewer black patients were insured as compared to white patients (89% vs. 96%, p<0.001). The type of health insurance coverage also varied by racial groups with a greater portion of white patients having private insurance with/without supplemental Medicare/Medicaid as compared to black patients (79% vs. 57%; p<0.001). Greater numbers of black patients had Medicaid (18% vs. 5%) and Medicare (14% vs. 12%) as compared to white patients.
First Course of Treatment
First line regimens for patients in the study included CHOP (241), R-CHOP (342), and other regimens (118). As expected, the use of R-CHOP significantly increased over time after the approval of rituximab; patients diagnosed during 1998-2002 and 2003-2010 were more likely to receive R-CHOP (odds ratio [OR] 24.7 and 174.7 respectively, p<0.001) when compared to patients treated in the initial era from 1992-1997. Black and white patients showed similar trends in the rise of R-CHOP treatment (Figure 2). Patients presenting with ECOG PS ≥2 were less likely to receive R-CHOP (OR 0.59, p=0.009; Table II). Surprisingly, patients in this cohort who had no healthcare insurance coverage were more likely to receive R-CHOP compared to patients with private insurance (OR 10.8, p=0.002). Multivariable logistic regression models confirmed these findings.
Figure 2. Trends in use of CHOP and R-CHOP as first line of treatment of DLBCL.
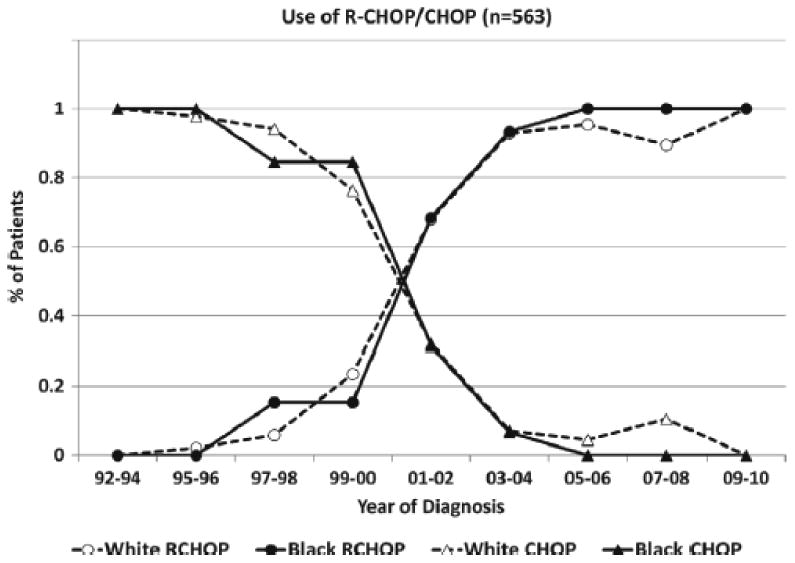
Plots the percentage of white and black patients with DLBCL that were treated with CHOP and R-CHOP across the years.
Table II. Logistic regression models of predictors of R-CHOP as first-line of treatment in DLBCL patients.
| Univariate model | Multiple variable model | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Factors | Odds Ratio | 95% CI | Odds Ratio | 95% CI | ||
| Age, years | ||||||
| ≤60 | Reference | |||||
| >60 | 0.93 | 0.68 | 1.26 | 0.81 | 0.53 | 1.24 |
| Gender | ||||||
| Female | Reference | |||||
| Male | 0.97 | 0.72 | 1.31 | 0.84 | 0.56 | 1.26 |
| Race | ||||||
| White | Reference | |||||
| Black | 1.20 | 0.83 | 1.74 | 0.91 | 0.55 | 1.52 |
| Other | 1.55 | 0.68 | 3.55 | 1.28 | 0.42 | 3.91 |
| ECOG performance status | ||||||
| 0-1 | Reference | |||||
| ≥2 | 0.59* | 0.40 | 0.88 | 0.55* | 0.33 | 0.93 |
| LDH level | ||||||
| Normal | Reference | |||||
| >ULN | 0.72 | 0.50 | 1.05 | 0.80 | 0.48 | 1.34 |
| Disease stage | ||||||
| I/II | Reference | |||||
| III/IV | 0.87 | 0.64 | 1.17 | 0.84 | 0.53 | 1.33 |
| No. of extranodal sites | ||||||
| 0-1 | Reference | |||||
| ≥2 | 0.75 | 0.51 | 1.12 | 0.90 | 0.52 | 1.57 |
| B-symptoms | ||||||
| Absent | Reference | |||||
| Present | 0.73 | 0.53 | 1.01 | 1.02 | 0.66 | 1.58 |
| Insurance Type | ||||||
| Private +/- M/M | Reference | |||||
| Medicaid | 1.08 | 0.62 | 1.86 | 1.23 | 0.58 | 2.63 |
| Medicare +/- Medicaid | 1.48 | 0.93 | 2.35 | 1.30 | 0.70 | 2.39 |
| Uninsured | 10.78* | 2.48 | 46.92 | 10.11* | 1.52 | 67.31 |
| Employed | ||||||
| No | Reference | |||||
| Yes | 0.67* | 0.45 | 0.99 | 0.83 | 0.50 | 1.40 |
| Diagnosis Era | ||||||
| 1992-1997 | Reference | |||||
| 1998-2002 | 24.67* | 7.62 | 79.82 | 25.50* | 7.78 | 83.57 |
| 2003-2010 | 174.67* | 53.82 | 566.90 | 153.23* | 46.12 | 509.06 |
Indicates statistical significance
Abbreviations: CI, Confidence Interval; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; ULN, upper limit of normal; M/M, Medicare/Medicaid.
Survival
With a median follow-up of 35 months (range 7 days – 240 months), univariate Cox-regression analyses of factors predicting mortality revealed that IPI score 2 (Hazard Ratio [HR] 1.58, p=0.03), IPI score 3 (HR 2.53, p<0.001), IPI score 4-5 (HR 4.37, p<0.001), and presence of B-symptoms (HR 1.48, p=0.002) predicted for worse survival. Table III provides the HRs for individual IPI components. Patients with Medicare also had statistically lower survival rates compared to patients with private insurance (HR 1.4, p=0.04). Patients who received R-CHOP had improved survival compared with those who received CHOP as first line of treatment (HR 0.61, p<0.001). Black race did not predict for poorer survival (HR 1.23, p=0.15). The observed survival rates did not differ significantly between black and white patients (5-year OS 62% vs. 66%, p=0.15). In the multiple variable Cox-regression analyses of factors predicting mortality, IPI score 3 (HR 2.51, p<0.001) and IPI score 4-5 (HR 3.37, p<0.001) predicted worse survival while first-line treatment with R-CHOP predicted better survival as compared to CHOP (HR 0.58, p=0.002).
Table III. Cox regression models of predictors of mortality in DLBCL patients.
| Factors | Univariate model | Multiple variable model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hazard Rate | 95% CI | Hazard Rate | 95% CI | |||
| Age, years | ||||||
| ≤60 | Reference | |||||
| >60 | 1.58* | 1.25 | 2.00 | 1.53* | 1.17 | 2.00 |
| Gender | ||||||
| Female | Reference | |||||
| Male | 1.07 | 0.85 | 1.35 | 1.23 | 0.97 | 1.57 |
| Race | ||||||
| White | Reference | |||||
| Black | 1.23 | 0.93 | 1.64 | 1.22 | 0.90 | 1.67 |
| Other | 0.81 | 0.38 | 1.73 | 1.07 | 0.49 | 2.32 |
| ECOG performance status | ||||||
| 0-1 | Reference | |||||
| ≥2 | 3.45* | 2.68 | 4.45 | 2.62* | 1.97 | 3.49 |
| LDH level | ||||||
| Normal | Reference | |||||
| >ULN | 2.13* | 1.56 | 2.91 | 1.59* | 1.15 | 2.21 |
| Disease stage | ||||||
| I/II | Reference | |||||
| III/IV | 1.51* | 1.19 | 1.93 | 1.20 | 0.91 | 1.59 |
| No. of extranodal sites | ||||||
| 0-1 | Reference | |||||
| ≥2 | 1.33 | 1.00 | 1.78 | 0.92 | 0.66 | 1.28 |
| B-symptoms | ||||||
| Absent | Reference | |||||
| Present | 1.49* | 1.15 | 1.92 | 1.20 | 0.92 | 1.58 |
| Insurance Type | ||||||
| Private +/- M/M | Reference | |||||
| Medicaid | 1.29 | 0.87 | 1.93 | 1.36 | 0.88 | 2.09 |
| Medicare +/- Medicaid | 1.41* | 1.01 | 1.96 | 0.95 | 0.66 | 1.36 |
| Uninsured | 0.45 | 0.14 | 1.40 | 0.51 | 0.16 | 1.64 |
| Employed | ||||||
| No | Reference | |||||
| Yes | 0.93 | 0.66 | 1.31 | 1.07 | 0.74 | 1.54 |
| Diagnosis Era | ||||||
| 1992-1997 | Reference | |||||
| 1998-2002 | 1.46* | 1.08 | 1.97 | 1.62* | 1.17 | 2.26 |
| 2003-2010 | 0.95 | 0.67 | 1.34 | 1.47 | 0.93 | 2.34 |
| 1981-1991 | 0.59 | 0.26 | 1.30 | 0.47 | 0.20 | 1.09 |
| First line of treatment | ||||||
| CHOP | Reference | |||||
| R-CHOP | 0.61* | 0.47 | 0.81 | 0.66* | 0.46 | 0.94 |
| R +/- Other | 1.21 | 0.78 | 1.89 | 1.09 | 0.65 | 1.82 |
| Other | 0.88 | 0.61 | 1.27 | 0.89 | 0.60 | 1.33 |
| Family Hist. of Lymphoma | ||||||
| Absent | Reference | |||||
| Present | 0.72 | 0.43 | 1.22 | 0.79 | 0.46 | 1.35 |
Indicates statistical significance
Abbreviations: CI, Confidence Interval; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; ULN, upper limit of normal; M/M, Medicare/Medicaid; R-CHOP, rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
In multiple variable model Cox regression models, adjusted for year of diagnosis, among patients who received CHOP, black race (HR 1.80, p=0.01, Figure 3A), male gender (HR 1.55, p=0.02), presentation with B-symptoms (HR 1.61, p=0.02), IPI score 3 (HR 2.38, p<0.001), and IPI score 4-5 (HR 3.47, p<0.001) each were independently associated with significantly inferior survival (Table IV). In similar multiple variable model Cox regression models involving patients who received R-CHOP, patients who presented with IPI score 3 (HR 2.28, p=0.01), IPI score 4-5 (HR 3.43, p<0.001), and those insured by Medicaid had poorer survival (HR 2.54, p=0.01, Table IV). Black and white patients who received R-CHOP had similar outcomes (5-year OS 79% vs. 70%, p=0.79, Figure 3B).
Figure 3. Kaplan Meier Survival Curves of DLBCL Patients.
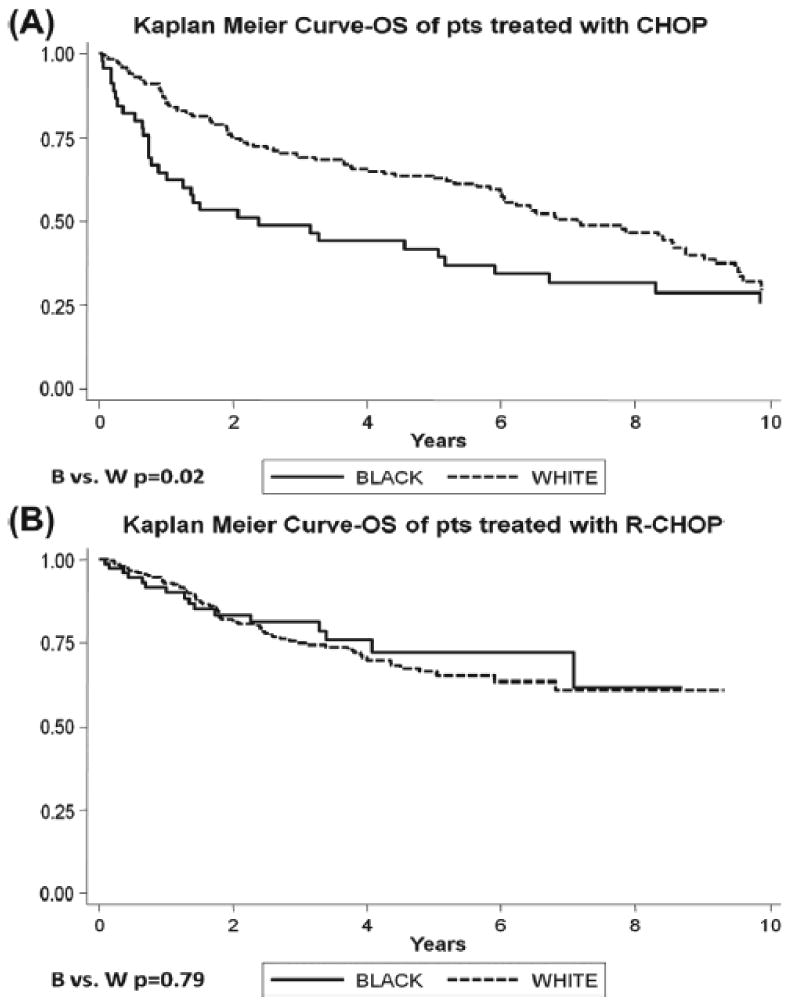
(A) Survival curves of patients receiving CHOP by race. (B) Survival curves of patients receiving R-CHOP by race.
Table IV. Cox regression models of predictors of mortality in DLBCL patients treated with R-CHOP and CHOP.
| R-CHOP | CHOP | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate model | Multiple variable model | Univariate model | Multiple variable model | |||||
|
|
|
|
|
|||||
| Factors | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Gender | ||||||||
| Female | Reference | |||||||
| Male | 0.86 | 0.57-1.31 | 0.88 | 0.57-1.36 | 1.22 | 0.87-1.72 | 1.55 | 1.07-2.24 |
| Race | ||||||||
| White | Reference | |||||||
| Black | 0.93 | 0.56-1.59 | 0.83 | 0.46-1.48 | 1.58* | 1.07-2.33 | 1.80* | 1.15-2.82 |
| Other | 2.42 | 0.97-6.02 | 1.28 | 0.47-3.84 | 0.26 | 0.04-1.85 | 0.27 | 0.04-2.13 |
| IPI | ||||||||
| 0-1 | Reference | |||||||
| 2 | 1.60 | 0.78-3.26 | 1.61 | 0.78-3.32 | 1.29 | 0.71-2.36 | 1.37 | 0.73-2.57 |
| 3 | 2.53* | 1.29-5.00 | 2.48* | 1.22-5.04 | 2.14* | 1.25-3.65 | 2.38* | 1.34-4.24 |
| 4-5 | 4.18* | 2.12-8.25 | 3.43* | 1.56-7.54 | 3.84* | 2.20-6.70 | 3.47* | 1.92-6.26 |
| B-symptoms | ||||||||
| Absent | Reference | |||||||
| Present | 1.06 | 0.67-1.69 | 0.85 | 0.57-1.58 | 1.90* | 1.31-2.76 | 1.61* | 1.08-2.39 |
| Insurance Type | ||||||||
| Private +/- M/M | Reference | |||||||
| Medicaid | 2.52* | 1.35-4.72 | 2.54* | 1.24-5.20 | 1.21 | 0.66-2.19 | 0.72 | 0.37-1.41 |
| Medicare +/- Medicaid | 1.69 | 0.97-2.94 | 1.61 | 0.88-2.95 | 1.74* | 1.05-2.87 | 1.29* | 0.76-2.21 |
| Uninsured | 0.27 | 0.04-1.98 | 0.35 | 0.05-2.58 | 1.33 | 0.33-5.46 | 0.78 | 0.16-3.82 |
| Employed | ||||||||
| No | Reference | |||||||
| Yes | 0.82 | 0.49-1.38 | 1.24 | 0.69-2.21 | 1.08 | 0.61-1.9 | 1.29 | 0.69-2.37 |
| Diagnosis Era | ||||||||
| 1992-1997 | Reference | |||||||
| 1998-2002 | 2.18 | 0.28-16.69 | 2.77 | 0.34-22.83 | 1.83* | 1.27-2.63 | 2.12* | 1.39-3.21 |
| 2003-2010 | 1.86 | 0.24-14.38 | 2.11 | 0.25-17.69 | 0.51 | 0.12-2.12 | 0.73 | 0.17-3.13 |
| 1981-1991 | No observations | 0.52 | 0.16-1.68 | 0.62 | 0.19-2.08 | |||
| Family History Lymphoma | ||||||||
| Absent | Reference | |||||||
| Present | 0.81 | 0.39-1.68 | 0.79 | 0.37-1.69 | 0.76 | 0.33-1.72 | 0.81 | 0.34-1.94 |
Indicates statistical significance
Abbreviations: R-CHOP, rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP); HR, Hazard ratio; CI, Confidence Interval; IPI, International Prognostic Index; M/M, Medicare/Medicaid.
Discussion
In this academic center-based retrospective study in the Southeastern United States, we found that black patients comprised 20.5% of the study population, compared with census data that indicate that blacks comprise 26.2% of the population of Alabama and 30.5% of the populations of Georgia,[26] consistent with prior findings of a lower relative incidence of lymphoma in the black population. Although the younger age and diagnosis of all patients in the study likely reflects a referral bias of younger patients to these academic medical centers, we found that black patients were diagnosed at a significantly younger age compared to white patients, and more commonly presented with an elevated LDH level, B-symptoms, and ECOG-PS ≥2. These findings are consistent with our previous SEER study that showed that black patients were younger compared to white patients, but were more likely to present with B-symptoms and advanced stage.[1] In the current study, although there were no racial differences in stage or IPI score at diagnosis, a greater proportion of black patients presented with high risk age-adjusted IPI scores. Among patients treated with CHOP, multivariable risk models indicated that black race remained a poor prognostic factor even independent of IPI factors.
One of the limitations of this study is the lack of central pathology review across the two institutions; however, central pathology review did occur at each center individually for a selection of cases from 2001-2010 (43% Emory cases and 100% UAB cases), and we did find a high degree of concordance in the histological diagnosis for the cases where inter-institutional central review did occur.[27] We also acknowledge that the World Health Organization (WHO) classification of lymphomas changed over the time period of the study and other classifications were previously used; all cases were identified and selected using the 2008 WHO system.[11,28,29] Missing data in the earlier years of analysis precluded us from utilizing additional measures of socioeconomic status like education, household income, and occupation, and from examining disease-specific survival. Other limitations of this retrospective analysis include missing or incomplete data on staging (8%), race (7%), and treatment (2%). We also had slightly different time intervals for data collection across the two institutions, but the central findings regarding racial differences in age of onset, poor risk features for black patients , and similar observed survival among black and white patients was true for cohort studies involving each site separately.[30,31] Primary hypotheses were pre-specified before the analysis was planned, and additional findings are considered provocative and hypothesis generating due to the risk of type I error.
Interestingly, twice as many white patients (8%) as black patients (3%) had a positive family history of lymphoma (p=0.048). This is a novel finding, and may explain to some extent the lower relative incidence of DLBCL in the African American population and will have to be explored in further studies. Although a single susceptibility gene for DLBCL has not been identified, evidence suggests that genetic predisposition underlies the etiology of DLBCL for some patients.[32-34] For example, individuals with a first-degree relative with leukemia, NHL, Hodgkin lymphoma, or any hematological malignancy have higher risks of DLBCL.[32] Focused case-control studies are needed to determine whether differences in underlying genetic predisposition or exposures exist among black and white patients with DLBCL which might explain the differences in lymphoma relative incidence. Our study was limited by the relatively smaller number of black patients as is the case with nearly all US-based and European lymphoma population studies that predominantly have examined white patients.
Other studies have suggested that there are racial and socioeconomic differences in the use of lymphoma therapies in the United States.[12,35,36] Wang and colleagues performed a retrospective analysis of 13,321 patients from the linked SEER-Medicare dataset that were diagnosed with NHL between 1992 and 1999 using administrative billing codes to determine treatment patterns. While African Americans (n=533) were less likely to receive lymphoma therapy (OR 0.68) and had inferior all-cause and NHL-specific 5-year survival when compared to Caucasians, these differences were best explained by differences in socioeconomic status.[12] In an overlapping study of linked SEER-Medicare data for NHL patients diagnosed from 1995 through 2002 including 14,831 patients with DLBCL (84% white and 4.8% black),[36] black patients were significantly less likely than Whites to be treated within 90 days of a NHL diagnosis and less likely to receive rituximab for DLBCL (OR 0.71). In contrast, in our study which had complete, direct ascertainment of treatment based on clinical and pharmacy data, there were no racial differences in the use of R-CHOP. While the time period under study was quite long, rates of adoption of R-CHOP use were similar for black and white patients, perhaps related to the facts that this was cohort managed at academic institutions and was predominantly insured. Despite the finding that there were racial differences in the types of insurance coverage, there were no significant racial differences in first line treatment selection. The surprising finding that patients with no healthcare insurance coverage were more likely to receive R-CHOP compared to those with private insurance may relate to the use of R-CHOP as an inpatient regimen in the earlier years of adoption for this subgroup, other biases toward treatment of uninsured patients with additional drugs in combination regimens, or unexplained factors. A similar observation has been made among Medicare patients within a “poverty” income category in the referenced SEER-Medicare analysis, and also may relate to the existence of special rituximab access programs for such patients at that time.[36]
We have confirmed that the addition of rituximab to CHOP chemotherapy significantly improved survival for patients with DLBCL, which has been described earlier in randomized clinical trials[7-9,37] and in a population-based study in Canada.[38] Despite adjustment for year of diagnosis, insurance status, and age, among patients who received first line CHOP therapy, IPI score 4-5 and black race were the strongest predictors of increased mortality. However, such findings need to be interpreted with caution since these patients were treated over a wide time span, relatively few black patients were treated with CHOP, and other variations in the processes of care and random variation could have influenced outcomes. Whether racial differences in DLBCL survival relate to treatment, tumor response, lymphoma biology or some combination is remains unclear, but our analyses suggest that when modern lymphoma therapy with R-CHOP is administered there are no racial differences in survival. A similar observation has been noted in previous studies of other adverse prognostic factors, most notably for Bcl-2 expression, and suggests that the addition of rituximab may abrogate the adverse prognostic impact of race.[39] This also raises the question as to whether there are racial differences in the frequency of known biological subtypes of DLBCL with variable susceptibility to rituximab.[40,41]
Gene expression analyses of DLBCL have identified at least two biologically distinct and prognostically meaningful molecular subgroups of DLBCL.[42] Ethnic/racial differences in the frequency of activated B-cell like and germinal center B-cell-like subtypes have been identified in studies of DLBCL patients in Malaysia, Japan, Turkey, China, Germany, and North America,[43-47] but have not been compared in black and white populations. There also may be ethnic differences in polymorphism of FcγRIIIA and FcγRIIA, but there remains debate as to the effect this may have on response to R-CHOP and treatment outcomes for patients with DLBCL.[48,49] Although both genetic and non-genetic factors are likely important factors involved in ethnic differences in cancer outcomes, data acute lymphoblastic leukemia suggests that there can be a biological basis for disparities in treatment outcomes that can be overcome by treatment modification.[50] Additional data on DLBCL subtype and other molecular predictors of response are needed in future studies to improve our understanding of the relationship between race and treatment outcomes in DLBCL particularly when the same treatment is administered.
Our data confirm that when modern standard of care therapy is applied equally to patients with DLBCL, similar outcomes occur for black and white patients. Developing interventions to address the broader disparities in treatment selection and treatment outcomes identified in claims-based cohort studies requires improved understanding of the context in which cancer treatments are selected for patients with DLBCL.[51,52] As distinct biological subgroups of DLBCL associated with differences in outcomes have been described,[42,44] it is critical that we improve our understanding of racial differences in disease biology and epidemiology to address cancer disparities. Based on our analysis, such studies are now planned.
Acknowledgments
This work was supported by Dr. Flowers' Georgia Cancer Coalition Distinguished Scientist Award and American Society of Hematology Amos Medical Faculty Development Award and Dr. Foran's University of Alabama at Birmingham Health Services Foundation Scholar of Excellence Award. Research reported in this publication was also supported by the National Cancer Institute of the National Institutes of Health under Dr. Flowers' Award Number R21CA158686. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors appreciate the support of Alison Maggioncalda, Neha Malik, and Dionne Duc in the process of data collection.
Footnotes
Declaration of Interest: Flowers: Consultancy (paid) - Spectrum, Celgene, Optum Rx, Seattle Genetics; Consultancy (unpaid) – Genentech/Biogen-Idec/Roche, Millenium/Takeda; Research Funding – Spectrum, Novartis, Millenium/Takeda, Gilead; Payment for development of educational presentations – Clinical Care Options, Educational Concepts.
None of the other authors have any conflict of interest to disclose
References
- 1.Shenoy PJ, Malik N, Nooka A, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. 2011;117:2530–2540. doi: 10.1002/cncr.25765. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60:393–408. doi: 10.3322/caac.20087. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RI, Miller TP, O'Connor OA. Diffuse aggressive lymphoma. Hematology (Am Soc Hematol Educ Program) 2004:221–236. doi: 10.1182/asheducation-2004.1.221. [DOI] [PubMed] [Google Scholar]
- 5.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 6.McKelvey EM, Gottlieb JA, Wilson HE, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976;38:1484–1493. doi: 10.1002/1097-0142(197610)38:4<1484::aid-cncr2820380407>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 8.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 9.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 10.Ghafoor A, Jemal A, Cokkinides V, et al. Cancer Statistics for African Americans. CA Cancer J Clin. 2002;52:326–341. doi: 10.3322/canjclin.52.6.326. [DOI] [PubMed] [Google Scholar]
- 11.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Burau KD, Fang S, Wang H, Du XL. Ethnic variations in diagnosis, treatment, socioeconomic status, and survival in a large population-based cohort of elderly patients with non-Hodgkin lymphoma. Cancer. 2008;113:3231–3241. doi: 10.1002/cncr.23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flowers CR, Fedewa S, Chen A, Lipscomb J, Brawley O, Ward E. Disparities in the Use of Chemo-Immunotherapy for Diffuse Large B-Cell Lymphoma in the United States. Blood (ASH Annual Meeting Abstracts) 2009;114 Abstract#897. [Google Scholar]
- 14.Foran JM, McClure LA, Clarke CA, Keegan THM. Impact of Socioeconomic Status & Race/Ethnicity On Survival in Diffuse Large B-Cell Lymphoma (DLBCL): A Population-Based Study. ASH Annual Meeting Abstracts. 2009;114:1954. [Google Scholar]
- 15.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 16.Clarke CA, Undurraga DM, Harasty PJ, Glaser SL, Morton LM, Holly EA. Changes in cancer registry coding for lymphoma subtypes: reliability over time and relevance for surveillance and study. Cancer Epidemiol Biomarkers Prev. 2006;15:630–638. doi: 10.1158/1055-9965.EPI-05-0549. [DOI] [PubMed] [Google Scholar]
- 17.Graiser M, Moore SG, Victor R, et al. Development of query strategies to identify a histologic lymphoma subtype in a large linked database system. Cancer Inform. 2007;3:149–158. [PMC free article] [PubMed] [Google Scholar]
- 18.Huang T, Shenoy PJ, Sinha R, Graiser M, Bumpers KW, Flowers CR. Development of the Lymphoma Enterprise Architecture Database: a caBIG Silver level compliant system. Cancer Inform. 2009;8:45–64. doi: 10.4137/cin.s940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009:523–531. doi: 10.1182/asheducation-2009.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 21.Shipp MA, Harrington DP, Klatt MM, et al. Identification of major prognostic subgroups of patients with large-cell lymphoma treated with m-BACOD or M-BACOD. Ann Intern Med. 1986;104:757–765. doi: 10.7326/0003-4819-104-6-757. [DOI] [PubMed] [Google Scholar]
- 22.Itoh K, Ohtsu T, Wakita H, et al. Dose-escalation study of CHOP with or without prophylactic G-CSF in aggressive non-Hodgkin's lymphoma. Ann Oncol. 2000;11:1241–1247. doi: 10.1023/a:1008361513544. [DOI] [PubMed] [Google Scholar]
- 23.[Internet]. Rockville, MD. Food and Drug Administration; 1997 - [cited Date Cited Year Cited]| Available rom: URL
- 24.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 25.Coiffier B, Lepage E, Herbrecht R, et al. Mabthera (Rituximab) plus CHOP is superior to CHOP alone in elderly patients with diffuse large-B-cell lymphoma (DLCL): Interim results of a randomized GELA trial. Blood. 2000;96:223A. Abstract #950. [Google Scholar]
- 26.[Internet] . U.S. Census Bureau;- [cited Date Cited Year Cited]| Available rom: URL
- 27.Chastain EC, Fisher KE, Bumpers K, et al. Racial Differences in Prognostic Biomarkers of Diffuse Large B-Cell Lymphoma. United States & Canadian Academy of Pathology Annual Meeting. 2012 Abstract #1377. [Google Scholar]
- 28.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner JJ, Morton LM, Linet MS, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116:e90–98. doi: 10.1182/blood-2010-06-289561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenoy PJ, Bumpers K, King N, et al. Black/White Differences in the Treatment and Outcomes of Diffuse Large B Cell Lymphoma: A Matched Cohort Analysis. ASH Annual Meeting Abstracts. 2009;114:1392. [Google Scholar]
- 31.The A, Li Y, Reddy V, Davis R, Baird M, Foran J. A Comparative Study of Diffuse Large B-Cell Lymphoma (DLBCL) between African Americans and Caucasians: Single-Center Experience at the University of Alabama at Birmingham (UAB) Blood (ASH Annual Meeting Abstracts) 2007;110:4430. [Google Scholar]
- 32.Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;109:3479–3488. doi: 10.1182/blood-2006-06-031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SS, Cozen W, Cerhan JR, et al. Immune mechanisms in non-Hodgkin lymphoma: joint effects of the TNF G308A and IL10 T3575A polymorphisms with non-Hodgkin lymphoma risk factors. Cancer Res. 2007;67:5042–5054. doi: 10.1158/0008-5472.CAN-06-4752. [DOI] [PubMed] [Google Scholar]
- 34.Morton LM, Purdue MP, Zheng T, et al. Risk of non-Hodgkin lymphoma associated with germline variation in genes that regulate the cell cycle, apoptosis, and lymphocyte development. Cancer Epidemiol Biomarkers Prev. 2009;18:1259–1270. doi: 10.1158/1055-9965.EPI-08-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nabhan C, Morawa E, Bitran JD, et al. Patterns of Care in Follicular Lymphoma (FL): Are Minorities Being Treated Differently? Report from the National LymphoCare Study (NLCS) ASH Annual Meeting Abstracts. 2007;110:367. [Google Scholar]
- 36.Vance KT, Kilgore ML, Yun H, Gary LC, Foran JM. Race and Non-Hodgkin's Lymphoma: Adverse Impact of Race and Treatment Delays on Survival. A SEER-Medicare Population Study (1995 2003) Blood (ASH Annual Meeting Abstracts) 2007;110:3578. [Google Scholar]
- 37.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 38.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 39.Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2—associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–4284. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 40.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcÎ3 RIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 41.Fu K, Weisenburger DD, Choi WW, et al. Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma. J Clin Oncol. 2008;26:4587–4594. doi: 10.1200/JCO.2007.15.9277. [DOI] [PubMed] [Google Scholar]
- 42.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 43.Alacacioglu I, Ozcan MA, Ozkal S, et al. Prognostic significance of immunohistochemical classification of diffuse large B-cell lymphoma. Hematology. 2009;14:84–89. doi: 10.1179/102453309X385205. [DOI] [PubMed] [Google Scholar]
- 44.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15:5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peh SC, Gan GG, Lee LK, Eow GI. Clinical relevance of CD10, BCL-6 and multiple myeloma-1 expression in diffuse large B-cell lymphomas in Malaysia. Pathol Int. 2008;58:572–579. doi: 10.1111/j.1440-1827.2008.02273.x. [DOI] [PubMed] [Google Scholar]
- 46.Shiozawa E, Yamochi-Onizuka T, Takimoto M, Ota H. The GCB subtype of diffuse large B-cell lymphoma is less frequent in Asian countries. Leuk Res. 2007;31:1579–1583. doi: 10.1016/j.leukres.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Xia ZG, Xu ZZ, Zhao WL, et al. The prognostic value of immunohistochemical subtyping in Chinese patients with de novo diffuse large B-cell lymphoma undergoing CHOP or R-CHOP treatment. Annals of Hematology. 2010;89:171–177. doi: 10.1007/s00277-009-0799-2. [DOI] [PubMed] [Google Scholar]
- 48.Kim DH, Du Jung H, Kim JG, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;108:2720–2725. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 49.Mitrovic Z, Aurer I, Radman I, Ajdukovic R, Sertic J, Labar B. FCgammaRIIIA and FCgammaRIIA polymorphisms are not associated with response to rituximab and CHOP in patients with diffuse large B-cell lymphoma. Haematologica. 2007;92:998–999. doi: 10.3324/haematol.10327. [DOI] [PubMed] [Google Scholar]
- 50.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43:237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flowers CR, Armitage JO. A decade of progress in lymphoma: advances and continuing challenges. Clin Lymphoma Myeloma Leuk. 2010;10:414–423. doi: 10.3816/CLML.2010.n.086. [DOI] [PubMed] [Google Scholar]
- 52.Flowers CR, Glover R, Lonial S, Brawley OW. Racial Differences in the Incidence and Outcomes for Patients with Hematological Malignancies. Current Problems in Cancer. 2007;31:182–201. doi: 10.1016/j.currproblcancer.2007.01.005. [DOI] [PubMed] [Google Scholar]


