Abstract
We recently published procedures describing the isolation of absolute IR spectra for all of the intermediates of the bacteriorhodopsin photocycle, and from these to obtain transitional difference spectra between consecutive intermediates (Hendler et al. Applied Spectroscopy, 65, 1029-1045 (2011)).
In that work, we concentrated mainly on proton-binding centers and the route of proton transport across the membrane. In the current communication we use isolated spectra for the Amide I, Amide II, and Amide III envelopes to obtain quantitative information on the extents of conformational change accompanying each transition in the photocycle. Our main finding is that most of the conformational changes occur in the conversion of the MF intermediate to N.
In the earlier publication, a new proton acceptor, absorbing at 1650 cm−1 was identified which appeared to accept a proton from Asp96COOH during the transformation of †BR* to L. Below, we present evidence which supports this interpretation and propose a possible role for this new component.
Introduction
We recently described new approaches that enabled us to isolate individual absolute and difference spectra for all of the bacteriorhodopsin photocycle intermediates.1 In interpreting these results, we relied heavily on a large number of previously published FTIR studies (see ref. 1 for these citations). In the current study attention is focused on the isolated spectra for the Amide I, II, and III regions.
Protein conformational changes are reflected in particular vibrational modalities of the peptide bond that are seen in three regions of the IR spectrum. The strongest of these occur in the Amide I region from 1700 cm−1 to 1600 cm−1, and emanate from C=O stretching weakly coupled with C-N stretching, and N-H in-plane bending. The next strongest signals occur in the Amide II region from 1600 cm−1 to 1500 cm−1, and reflect N-H in-plane bending weakly coupled with C-N stretching. A much weaker set of signals is seen in the Amide III region from 1350 cm−1 to 1200 cm−1. These reflect N-H in-plane bending coupled with C-N stretching and also include C-H and N-H deformation modes. Because of their strong signals, the Amide I and II regions have received the most attention in attempts to quantify conformational changes. The Amide III region has been largely ignored because of the weak signals it presents. However, there is a wealth of information that can be gathered from changes seen to occur in that region. Using our cleanly isolated absolute spectra, we examined all three regions. The one consistent finding is that the maximum extents of conformational changes occur during the transition between the MF and N intermediates in the MF path of the bacteriorhodopsin photocycle (discussed below). In addition, significant structural differences between the MF and MS intermediates were seen in all three spectral regions.
With the combined spectra of both MF and MS (as is normally done), we confirmed the consensus view on the proton path. 1 Using separate spectra for the MF and MS intermediates, we were able to distinguish important differences between the functions of the two separate pathways for MF and MS decays as defined in the parallel cycles model of Hendler at al. 1-3
The subscripts F and S refer to the faster and slower decay time constants (τ) for the intermediates. The asterisks indicate that only the photo-activated forms of bacteriorhodopsin undergo photocycles. Our time resolution does not allow the isolation of these states. The spectra we obtain are for the ground states. It was shown that protons traveling the MF path form a ΔpH, while protons traveling the MS path build a membrane potential (Δψ). 1 In the current communication, attention is focused mainly on conformational changes that accompany each transition in the photocycle.
Although, most thoroughly studied, the assignment of vibrational frequencies in the Amide I region to specific protein or peptide configurations is not an exact science. However, in the case of soluble proteins, some real progress has been made 4-7 In some instances, Amide I band frequency assignments for particular types of secondary structure, obtained from soluble proteins have been found to apply to membrane proteins. In ref. 5, equations were presented, which evaluate relative amounts of α-helix, β-sheet, β-turn and random coil, based on absorbances at a series of specified frequency ranges. In a review, Goormagtigh, et al. defined specific regions where different secondary structures tend to absorb.7 However; progress in the case of membrane proteins has lagged far behind what has been reported for soluble proteins. In the first part of the studies reported below, we have examined the Amide I, Amide II, and Amide III regions for the extents of conformational changes.
The X-ray crystallographic structure of bR at 1.55 Å resolution shows that 171 amino acid residues (69%) are in α-helices, 67 (27%) in loops and turns, and 10 (4%) form a twisted anti-parallel β-sheet on the external surface connecting helices B and C. 8 Since the predominant stable structure is α-helix, we assume that most of the changes result from changes in tilts and immediate environments rather than major changes in structure per se. Our main purpose is to quantify extents of conformational changes rather than to identify specific changes in protein structure.
In the earlier publication 1, a previously unrecognized proton-binding center (X) with a characteristic frequency at 1650 cm−1 was described. In the second part of the work presented here, we suggest a possible role for X.
Methods
All procedures for obtaining the isolated absolute and transitional difference spectra used here were previously described in detail. 1 The spectra are averages of 2200 repeats using a Step Scan protocol and fitting procedures for the kinetic constants which presented very low errors and dependencies. In this work, to obtain quantitative data on the extents of conformational change based on the isolated absolute IR spectra for each intermediate, we developed some new approaches.
The absolute IR spectra for pairs of consecutive photocycle intermediates contained within the frequency ranges of the Amide I, Amide II, and Amide III envelopes were compared to reveal directly the extents of differences.
Difference spectra between these pairs of intermediates were examined to determine all of the distinct peaks and troughs commonly seen throughout the photocycle. In this way, we identified separate components in the envelope profiles. Using the characteristic frequencies for these components, we quantified the extents of change in these components for each successive transition in the two decay paths, by averaging the ± ΔA at each central wave number with its two adjacent neighbors, and plotted these against the corresponding steps in the photocycle. The difference spectrum between MF and MS intermediates was also included in these analyses.
Results
A. Extents of conformational changes in the Amide I envelope between consecutive intermediates in the BR photocycle
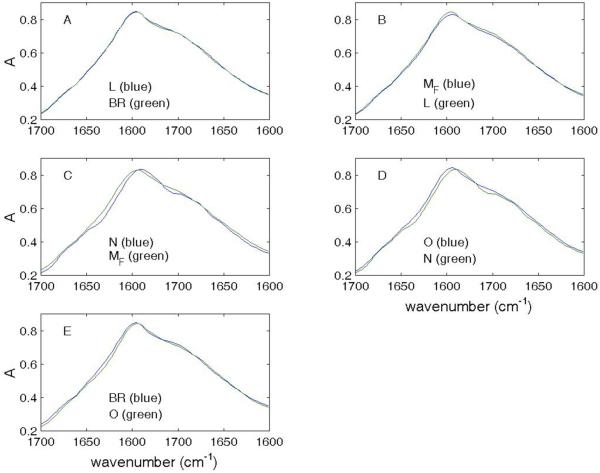
Figures 1 and 2 show absolute IR spectra for pairs of consecutive intermediates contained within the Amide I envelope. It is apparent that the most extensive changes occur in the MF → N transition (Fig. 1C) and the least between BR and the L intermediates (Fig. 1A).
Figure 1.
Absolute spectra of consecutive intermediates in MF path (Amide I).
Superimposition of absolute spectra in the Amide I region for consecutive intermediates in the MF path.
Figure 2.
Absolute spectra of consecutive intermediates in MS path and comparison of MF and MS (Amide I).
Superimposition of absolute spectra in the Amide I region for consecutive intermediates in the MS path (panels A and B). Although not directly linked in the photocycle, conformational differences between MF and MS are shown in panel C.
Figure 2 shows pairs of intermediates for the L → MS (Fig 2A) and MS → BR (Fig 2B) transitions in the MS path of the photocycle. It is clear that the extents of conformational changes are much less in the MS than the MF pathway. In addition, we show in Fig 2C, conformational differences between MF and MS.
Quantification based on amplitudes in difference spectra
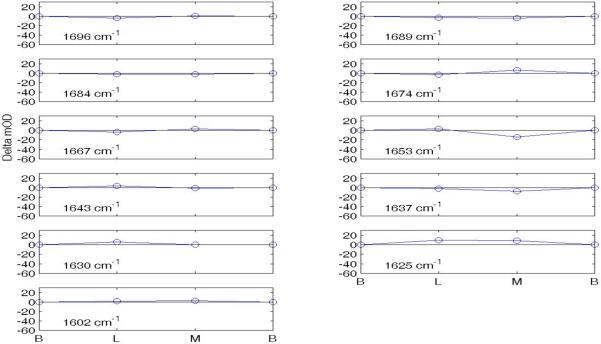
In order to assess the number of individual components contributing to the photocycle changes seen in the Amide I envelope, we looked at the difference spectra for each transition depicted in Figs. 1 and 2. These difference spectra displayed distinct peaks and troughs and are presented in the Supplementary Material (Figs S1 for the MF path and Fig. S2 for the MS path). This analysis produced 11 characteristic peaks and troughs as likely indicators of conformational changes. These are located at frequencies (cm−1) 1696, 1689, 1684, 1674, 1667, 1653, 1643, 1637, 1630, 1625, and 1602.
To quantify the accumulative extents of change, referenced to the ground state BR, in these 11 features for each successive transition in the two decay paths, we averaged the ± ΔA at each central wave number with its two adjacent neighbors. The results for the MF path are shown graphically in Fig. 3 and quantitatively in Table I. Corresponding data for the MS path are shown in Fig. 4 and Table II.
Figure 3.
Changes in mOD at 11 frequencies in MF path (Amide I).
The six points in the abscissa are for the sequential intermediates BR, L, MF, N, O, and BR in the MF pathway. The eleven graphs show the quantified changes at each characteristic frequency. The vertical green lines are drawn at intermediate N and the plotted ±ΔA amplitudes as listed in Table I.
Table I.
Amide I cumulative changes in amplitudes (mOD) at each intermediate relative to BR in the MF path
| wave number (cm−1) | BR | L | MF | N | O | BR |
|---|---|---|---|---|---|---|
| 1696 | 0 | −4.1934 | −11.3612 | −33.2623 | −17.9382 | 0 |
| 1689 | 0 | −3.3955 | −7.4821 | −15.9572 | −11.6790 | 0 |
| 1684 | 0 | −2.4939 | −5.3947 | −11.1565 | −5.5868 | 0 |
| 1674 | 0 | −3.1139 | −15.0086 | −55.8166 | −24.1135 | 0 |
| 1667 | 0 | −3.3735 | −9.6661 | −54.3496 | −25.6135 | 0 |
| 1653 | 0 | 2.5339 | 2.3903 | 21.9138 | 8.1056 | 0 |
| 1643 | 0 | 3.1271 | −10.6883 | −34.7582 | −9.3163 | 0 |
| 1637 | 0 | −1.9352 | −11.9717 | −20.4228 | −8.7701 | 0 |
| 1630 | 0 | 5.4881 | −2.5133 | 0.7591 | 5.1915 | 0 |
| 1625 | 0 | 9.2321 | 1.9501 | −7.7191 | 8.3165 | 0 |
| 1602 | 0 | 9.2321 | −5.2057 | −19.8785 | −9.1275 | 0 |
Note: The maximum changes (shaded) occurred mostly at the N intermediate.
Figure 4.
Changes in mOD at 11 frequencies in MS path (Amide I).
The four points in the abscissa are for the sequential intermediates BR, L, MS, and BR in the MS pathway. The eleven numbered graphs correspond to the numbered rows and corresponding wave numbers listed in Table II.
Table II.
Amide I cumulative changes in amplitudes (mOD) at each intermediate relative to BR in the MS path.
| wave number (cm−1) | BR | L | MS | BR |
|---|---|---|---|---|
| 1696 | 0 | −4.1934 | 0.6982 | 00 |
| 1689 | 0 | −3.3955 | −3.9117 | 0 |
| 1684 | 0 | −2.4939 | −1.7770 | 0 |
| 1674 | 0 | −3.1139 | 6.6154 | 0 |
| 1667 | 0 | −3.3735 | 2.8778 | 0 |
| 1653 | 0 | 2.5339 | −13.9023 | 0 |
| 1643 | 0 | 3.1271 | −0.8560 | 0 |
| 1637 | 0 | −1.9352 | 8.0422 | 0 |
| 1630 | 0 | 5.4881 | 0.3470 | 0 |
| 1625 | 0 | 9.2321 | 8.6072 | 0 |
| 1602 | 0 | 2.0052 | 2.7006 | 0 |
Note: The maximum changes in amplitudes are shaded.
Figure 4, using the same scaling as in Fig. 3, shows that the extents of conformational changes were far less than in the MS path than seen in the MF path. The extents of structural differences between MF and MS are listed in Table III.
Table III.
Amide I MF – MS differences
| wave number (cm−1) | delta A (mOD) |
|---|---|
| 1696 | 12.06 |
| 1689 | 3.57 |
| 1684 | 3.62 |
| 1674 | 21.62 |
| 1667 | 12.54 |
| 1653 | −16.29 |
| 1643 | 9.83 |
| 1637 | 3.93 |
| 1630 | 2.86 |
| 1625 | 6.66 |
| 1602 | 7.91 |
B. Extents of conformational changes in the Amide II envelope between consecutive intermediates in the BR photocycle
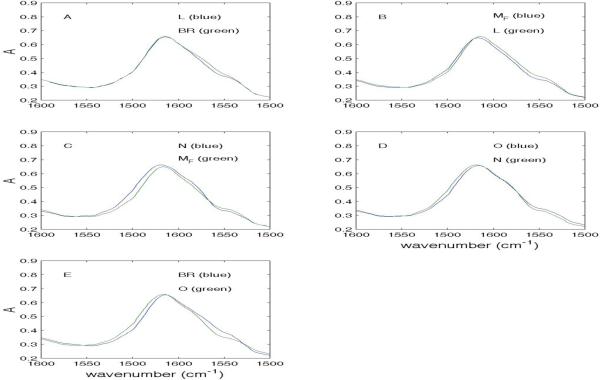
Figure 5 shows absolute IR spectra for pairs of consecutive intermediates contained within the Amide II envelope in the MF path. It is apparent that the most extensive changes occur in the MF → N transition (Fig. 5C) and the O → BR transition (Fig. 5E). Figure 6 shows the same information for steps in the MS path and the differences between MF and MS.
Figure 5.
Absolute spectra of consecutive intermediates in MF path (Amide II).
Superimposition of absolute spectra in the Amide II region for consecutive intermediates in the MF path.
Figure 6.
Absolute spectra of consecutive intermediates in MS path and comparison of MF and MS (Amide II).
Superimposition of absolute spectra in the Amide II region for consecutive intermediates in the MS path. Although not directly linked in the photocycle, conformational differences between MF and MS are shown in panel C.
The difference spectra between consecutive intermediates in the Amide II envelope for both the MF and MS paths are shown in the Supplementary material (Figs S3 and S4). Seven peaks and troughs were seen. One of these at 1557 cm−1 is for uncharged Arg82 and another for ‡C=C-SBH is at 1525 cm−1. The other five shared in more than one panel were located at frequencies (cm−1) 1585, 1545, 1534, 1515, and 1508. These changes quantified as described above for Amide I are shown graphically in Fig. 7 and quantitatively in Table IV. Corresponding data for the MS path are shown in Fig. 8 and Table V. Shading in the Tables indicates the maximum change at each wave number.
Figure 7.
Changes in mOD at 7 frequencies in MF path (Amide II).
The six points in the abscissa are for the sequential intermediates BR, L, MF, N, O, and BR in the MF pathway for the characteristic frequencies listed above. The vertical green lines are drawn at intermediate N and the plotted Δ amplitudes are listed in Table IV. The asterisks for frequencies 1557 cm−1 and 1525 cm−1 refer to the fact that the scales for the former (Arg82 (neutral)) and the latter (protonated Schiff base) are 2.5 greater than those at the other frequencies. This is also shown by the relative heights of the vertical green bars drawn at the N intermediate and which represent 80 mOD of change
Table IV.
Amide II cumulative changes in amplitudes (mOD) at each intermediate relative to BR in the MF path
| Wave number (cm−1) | BR | L | MF | N | O | BR |
|---|---|---|---|---|---|---|
| 1585 | 0 | −0.7348 | −5.7134 | −6.3741 | −4.6484 | 0 |
| 1557 | 0 | −0.6867 | 22.1833 | 81.5068 | 47.0981 | 0 |
| 1545 | 0 | 3.0732 | −10.4140 | −0.4648 | −0.0451 | 0 |
| 1534 | 0 | −10.0511 | −30.1238 | −6.4381 | −11.5702 | 0 |
| 1525 | 0 | −27.9144 | −55.1132 | −61.5929 | −46.1896 | 0 |
| 1515 | 0 | −5.4534 | −20.4997 | −34.5622 | −17.2947 | 0 |
| 1508 | 0 | 0.7532 | −4.0836 | −6.1354 | −12.0956 | 0 |
Note: The maximum changes (shaded) occurred mostly at the N intermediate.
Figure 8.
Changes in mOD at 7 frequencies in MS path (Amide II).
The four points in the abscissa are for the sequential intermediates BR, L, MS, and BR in the MS pathway. The seven numbered graphs correspond to the numbered rows and corresponding wavenumbers listed in Table V.
Table V.
Amide II cumulative changes in amplitudes (mOD) at each intermediate relative to BR in the MS path.
| wave number (cm−1) | BR | L | MS | BR |
|---|---|---|---|---|
| 1585 | 0 | −0.7348 | −0.4710 | 0 |
| 1557 | 0 | −0.6867 | 2.9445 | 0 |
| 1545 | 0 | 3.0732 | −0.4457 | 0 |
| 1534 | 0 | −10.0511 | −17.1943 | 0 |
| 1525 | 0 | −27.9144 | −27.8482 | 0 |
| 1515 | 0 | −5.4534 | −6.9755 | 0 |
| 1508 | 0 | 0.7532 | 0.4987 | 0 |
It is important to note that the large amplitude seen at 1557 cm−1, which coincides with the frequency for uncharged Arg82, is not associated with the deprotonation of Arg82+. Instead, as concluded in ref 1, it more likely signals conformational and/or environmental changes.
Cumulative changes in amplitudes (mOD) at each characteristic wave number relative to BR in the MS path are shown in Table V. The midpoint was averaged with its two neighbors. Because there is no clear consensus on a common intermediate showing a consistent maximum change, shading is not used in Table V. As was the case for Amide I, the changes in the MS path, were much smaller than seen in the MF path. Table VI shows the differences between MF and MS in the Amide II envelope.
Table VI.
Amide II MF – MS differences
| wave number (cm−1) | delta A (mOD) |
|---|---|
| 1585 | 5.24 |
| 1557 | −19.24 |
| 1545 | 9.97 |
| 1534 | 12.93 |
| 1525 | 27.26 |
| 1515 | 13.52 |
| 1508 | 4.58 |
C. Extents of conformational changes in the Amide III envelope between consecutive intermediates in the BR photocycle
Figure 9 shows absolute IR spectra for pairs of consecutive intermediates contained within the Amide III envelope in the MF path. Significant changes are seen to accompany each transition. Figure 10 shows the same information for steps in the MS path and the differences between MF and MS.
Figure 9.

Absolute spectra of consecutive intermediates in MF path (Amide III).
Superimposition of absolute spectra in the Amide III region for consecutive intermediates in the MF path.
Figure 10.

Absolute spectra of consecutive intermediates in MS path and comparison of MF and MS (Amide III).
Superimposition of absolute spectra in the Amide III region for consecutive intermediates in the MS path. Although not directly linked in the photocycle, conformational differences between MF and MS are shown in panel C.
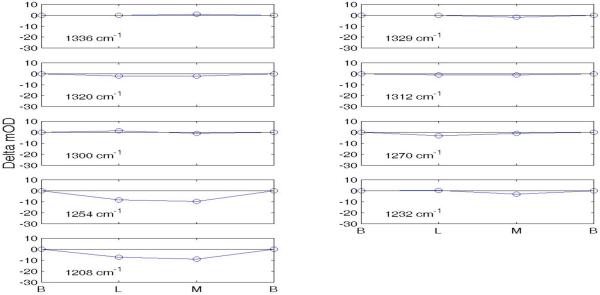
Difference spectra between consecutive intermediates in the Amide III envelope for both the MF and MS paths are shown in Supplementary material (Figs. S5 and S6). There are nine commonly shared peaks and troughs located at frequencies (cm−1) 1336, 1329, 1320 1312, 1300, 1270, 1254, 1232, and 1208. Changes in amplitude, quantified as described above for Amide I, are shown graphically in Fig. 11 and quantititatively in Table VII for the MF path. Corresponding data for the MS path are shown in Fig. 12 and Table VIII. Shading in the Tables indicates maximum change at each wavenumber.
Figure 11.
Changes in mOD at 9 frequencies in MF path (Amide III).
The six points in the abscissa are for the sequential intermediates BR, L, MF, N, O, and BR in the MF pathway. The nine numbered graphs correspond to the numbered rows and corresponding wave numbers listed in Table VII. The vertical green lines are drawn at intermediate N and the plotted ±Δ amplitudes are listed in Table VII.
Table VII.
Amide III mOD Changes in amplitudes at each intermediate relative to BR
| Wave number (cm−1) | BR | L | MF | N | O | BR |
|---|---|---|---|---|---|---|
| 1336 | 0 | 0.0451 | −1.8919 | −1.4738 | 0.0828 | 0 |
| 1329 | 0 | −0.1426 | −0.1170 | 7.0868 | 1.6295 | 0 |
| 1320 | 0 | −2.1854 | −3.7652 | −5.6338 | −1.7168 | 0 |
| 1312 | 0 | −1.2084 | −3.9793 | 1.4597 | −0.9368 | 0 |
| 1300 | 0 | 1.5113 | 0.1479 | 11.1025 | 4.5911 | 0 |
| 1270 | 0 | −3.1045 | −2.3798 | −9.0624 | −2.9957 | 0 |
| 1254 | 0 | −8.3418 | −14.7381 | −26.2879 | −15.8792 | 0 |
| 1232 | 0 | 0.4982 | 0.1410 | 2.7482 | 0.6056 | 0 |
| 1208 | 0 | −7.1067 | −9.6221 | −7.9633 | −6.1417 | 0 |
Figure 12.
Changes in mOD at 9 frequencies in MS path (Amide III).
The four points in the abscissa are for the sequential intermediates BR, L, MS, and BR in the MS pathway. Scaling is the same as in Fig. 11. The nine numbered graphs correspond to the numbered rows and corresponding wave numbers listed in Table VIII. Differences between the MF and MS intermediates are shown in Table IX.
Table VIII.
Amide III mOD Changes in amplitudes at each intermediate relative to BR
| wave number (cm−1) | BR | L | MS | BR |
|---|---|---|---|---|
| 1336 | 0 | 0.0451 | 1.0093 | 0 |
| 1329 | 0 | −0.1426 | −1.8207 | 0 |
| 1320 | 0 | −2.1854 | −2.0732 | 0 |
| 1312 | 0 | −1.2084 | −1.0505 | 0 |
| 1300 | 0 | 1.5113 | −0.8791 | 0 |
| 1270 | 0 | −3.1045 | −1.0249 | 0 |
| 1254 | 0 | −8.3418 | −9.5446 | 0 |
| 1232 | 0 | 0.4982 | −2.9973 | 0 |
| 1208 | 0 | −7.1067 | −8.7973 | 0 |
The significance of the Amide III region has been most thoroughly studied by B. R. Singh and associates, who use model proteins with secondary structures determined by X-ray crystallography and a variety of deconvolution and analytical methods to determine the number of components contained within the envelope. 9-11 The yellow, cyan, and green backgrounds in Table VII denote wave number ranges for α-helix, turn, and β-sheet conformations according to Singh and co-workers. Although, none of the model calibration standards was a membrane protein, the appearance of our spectra and number of features are quite similar.
D. Kinetics and possible role for newly identified proton-binding group (XH) with a frequency at 1650 cm−1
Figure 13 (panel A) compares the time course for Asp96COOH at 1740 cm−1 (blue) with that of XH 1650 cm−1 (green). Panel B compares the time course of XH at 1650 cm−1 (blue) with that of C=C-SBH at 1524 cm−1 (green). Panel C shows corresponding time courses for all of the intermediates.
Figure 13.

Relationship of protonations and deprotonations among AspCOOH, SBH, and X at 1650 cm−1 to each other and to the time course profiles of the photocycle intermediates.
The small magnitudes for changes of absorbance in panels A and B were scaled to match each other and, the absorbance values shown in panel C. For the green trace in panel A, a gain of 250 and an offset of 0.22 were used; for the blue trace, a gain of 100 and an offset of 0.9 were used. For the blue trace in panel B, a gain of 40 and an offset of 0 were used; for the green trace, a gain of 20 and an offset of 0.2 were used.
The vertical blue and green lines in panel A show that the time courses for Asp96COOH and XH are mirror images during the BR → L → MF stages. This finding corresponds to difference spectra which show that Asp96COOH is deprotonated during the BR → L transformation and reprotonated during the L → MF transition. 1 The reprotonation of Asp96COO– occurs during the N → O transition. Panel B shows a donor-acceptor relationship between SB and X during the conversion of BR to L.
Discussion
In a recent comprehensive review on conformational changes in the bR photoycle, from findings based on electron and X-ray crystallographic analyses, Hirai and Subramiam reviewed a large number of publications (cited therein) from many laboratories. 12 The main conclusion was that no consensus had been reached. Specifically, they found the structures for the same intermediate reported by multiple research groups are not only significantly different, but are also not on the same trajectory of conformational change of the protein. We are not surprised by this analysis and conclusion for many reasons.
Because of the overlap of all intermediates at any point in time of the photocycle there is always a mixture of several intermediates (see Fig. 13C above).
Trapping procedures based on rapid freezing, and the use of mutants, or varying conditions such as pH or temperatures result in different photocycles where different, unquantified degrees of contamination are present.
Hydrophobic binding is considerably weakened at low temperatures.
There is no way to ensure that any of these procedures can lead to isolated intermediates relevant to any one kind of photocycle, nor that studies pursued in any particular laboratory should produce the same results as obtained in any other laboratory. Even if it were possible to reproducibly obtain unique structures for the intermediates by the methods used, what is the relevance of structures obtained and analyzed as described above to the real photocycle that occurs in a fully hydrated membrane at ambient temperatures?
As reported here, new approaches, based on our recent isolation of IR absolute spectra for all of the intermediates 1, revealed that the extents of change are most pronounced during the MF → N transition and reversed during the N → O → BR transitions. What needs to be done next is to define precisely the specific conformational changes that have occurred and how they relate to the formation of the electrochemical potential that drives ATP synthesis. To this end, we have been developing instrumentation and procedures for obtaining isolated X-ray diffraction maps for each intermediate, using the same mathematical deconvolution methods described in this and the preceding publication. 1 An early indication of our present findings was that IR markers in the Amide I and Amide II regions steadily increased from the ground state (BR) to the N intermediate. 13 Using discrete sampling techniques to minimize cross contamination of overlapping peaks, Lórenz-Fonfrίa et al. reported that most of the structural changes during the photocycle accompany the formation of the N intermediate. 14 We also found evidence of significant structural differences between MF and MS. In this respect, we confirm the electron crystallographic findings of Subramaniam et al. 15
Possible role for the newly discovered proton-binding and release component X at 1650 cm−1
We have recently reported, using isolated absolute spectra, that a proton lost by Asp96COOH in the BR → L transformation is bound by a component whose protonated form absorbs at 1650 cm−1. It was also found that in the L → MF transition, the proton is transferred back to Asp96COO –. The time profiles in Fig. 13 (above) document this sequence of events. These findings raise some important questions.
What is the identity of X?
What is its function?
Why is a proton transferred from Asp96 to X in the BR → L transformation returned back to Asp96 in the L → MF transition?
Questions 2 and 3 are related. We do not believe, and do not propose, that X should be inserted into the proton flow pathway that has been established, and which our work confirms, when we use the combined form of MF and MS. The parallel cycles model which led to the isolated absolute IR spectra, presents two separate pathways for the decays of MF and MS back to their respective ground states. One additional question which we have not addressed is how the MS pathway obtains a proton from the external cytoplasmic surface? In the pathway using either undivided M or in the MF pathway, the external proton is acquired by Asp96COO – via an aqueous channel during the N → O transition. However, the N and O intermediates are not present in the MS pathway.
The model we present below is one that offers a possible answer to all of the questions posed above. At this point, the explanation is only a supported speculation to be further evaluated by future work.
In the MF pathway, a cytoplasmic proton has access to Asp96 in the N → O transition, and then is subsequently transferred to SB in the MF → N transition, but, neither of these transitions is present in the MS pathway. If, the only available route for supplying an external proton to the photocycle is in the N → O transition, then this proton must be funneled to the MS pathway, as needed. With X in contact both with Asp96 and SB, the scheme below can operate.
 |
There are still the same two separate pathways, and X serves as a channel and proton buffer for both. The function of X could be as a conduit and regulator for the distribution of protons between the two pathways as needed for the buildup of ΔpH and Δψ. 1 As to the identity of X, its characteristic frequency at 1650 cm−1 suggests that it could be one of the other arginines (not Arg82) present in bR.
On the differences between the isolated MF and MS spectra
In our previous paper based on isolated absolute IR spectra of the intermediates, we noted a difference in proton distribution between Asp85 and SB in the MF and MS intermediates (Fig. 8 of ref. 1), and stated that we would present an interpretation after further consideration, which we now present.
The M intermediate is formed in the L → M transition where a proton is transferred from C=C-SBH‡ to Asp85. Previous analyses of the photocycle were based only the combined M spectrum. If the proton is completely transferred, there should be no C=C-SBH in M. In the parallel cycles model, there are two separate ground states, two separate L's and two separate M's. Since there is still only one proton transferred per bR molecule, a statistical fraction goes through each path. Previously, it was shown that the two forms of M are functionally different 1, and in the present communication they are seen to differ in structure. Therefore, the pK's of Asp85COOH and C=C-SBH in MF and MS need not be identical. The distribution of H+ between Asp85 and SB is determined by the pK's for SB in MF and MS vis-à-vis the pK for Asp85. The earlier publication showed that Asp85 becomes more protonated in the L → MF transition than in the L → MS transition (compare Fig. 11A to 11D). 1 More protonated Asp85 means less protonated SB. This suggests that the MS formed has less Asp85COOH and more C=C-SBH than MF. The higher content of Asp85COOH in MF than MS is clearly shown by the peak at 1763 cm−1 in the MF – MS difference spectrum (Fig. 8 of ref. 1). This is consistent with and can explain the trough at 1524 cm−1 in the MF – MS difference spectrum.
Supplementary Material
Table IX.
shows the differences between MF and MS in the Amide III envelope.
| Table IX Amide III MF – MS differences | |
|---|---|
| wave number (cm−1) | delta A (mOD) |
| 1336 | 2.90 |
| 1329 | −1.70 |
| 1320 | 1.69 |
| 1312 | 2.93 |
| 1300 | −1.03 |
| 1270 | 1.35 |
| 1254 | 5.19 |
| 1232 | −3.14 |
| 1208 | 0.82 |
Acknowledgements
The authors thank Dr. Ira W. Levin for many helpful discussions and suggestions.
This research was supported in part by the Intramural Research Program of the NIH, National Heart Lung and Blood Institute, National Institute of Biomedical Imaging and Bioengineering, and the Center for Information Technology.
Certain commercial equipment, instruments, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institutes of Health or the National Institutes of Standards and Technology, or that the materials and equipment are necessarily the best available for the purpose
Footnotes
(BR*): photon-activated BR (authentic spectrum not isolated here); BR: ground state of photocycle ; bR: the protein.
C=C-SBH: protonated Schiff base
C=C-SBH: protonated Schiff base
Bibliography
- 1.Hendler RW, Meuse CW, Braiman MS, Smith PD, Kakareka JW. Infrared and Visible Absolute and Difference Spectra of Bacteriorhodopsin Photocycle Intermediates. Appl. Spectrosc. 2011;65(9):1029–1045. doi: 10.1366/11-06302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendler RW. An Apparent General Solution for the Kinetic Models of the Bacteriorhodopsin Photocycles. J. Phys. Chem. B. 2005;109:16515–16528. doi: 10.1021/jp052733h. [DOI] [PubMed] [Google Scholar]
- 3.Hendler RW, Shrager RI, Bose S. Theory and Procedures for Finding a Correct Kinetic Model for the Bacteriorhodopsin Photocycle. J. Phys, Chem. B. 2001;105:3319–3328. doi: 10.1021/jp002362z. [DOI] [PubMed] [Google Scholar]
- 4.Barth A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta. 2007;1767:1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Goormaghtigh E, Gasper R, Bérnard A, Goldztein A, Raussens V. Protein secondary structure content in solution, films and tissues: Redundancy and complementarity of the information content in circular dichroism, transmission and ATR FTIR spectra. Biochim. Biophys. Acta. 2009;1794:1332–1343. doi: 10.1016/j.bbapap.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Goormagthtigh E, Roysschaert J-M, Raussens V. Evaluation of the Information Content in Infrared Spectra for Protein Secondary Structure Determination. Biophys. J. 2006;90(8):2946–2957. doi: 10.1529/biophysj.105.072017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goormaghtigh E, Cabiaux V, Ruusschaert J-M. Determination of Soluble and Membrane Protein Structure by Fourier Transform Infrared Spectroscopy III. Secondary Structures. In: Herwig JH, Gregory BR, editors. Subcellular Biochemistry. Vol. 23. Plenum Press; New York, NY: 1994. pp. 405–450. [DOI] [PubMed] [Google Scholar]
- 8.Luecke H, Schobert B, Richter HT, Cartailler JP, Lanyi JK. Structure of Bacteriorhodopsin at 1.55 Å Resolution. J. Mol. Biol. 1999;299:899–911. doi: 10.1006/jmbi.1999.3027. [DOI] [PubMed] [Google Scholar]
- 9.F-N Fu D, DeOliveira B, Trumble WR, Sarkar HK, Singh BR. Secondary Structure Estimation of Proteins Using the Amide III Region of Fourier Transform Infrared Spectroscopy: Application to Analyze Calcium-Binding-Induced Structural Changes in Calsequestrin. Appl. Spectrosc. 1994;48(11):1432–1441. [Google Scholar]
- 10.Cai S, Singh BR. Identification of β-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys. Chem. 1999;80:7–20. doi: 10.1016/s0301-4622(99)00060-5. [DOI] [PubMed] [Google Scholar]
- 11.Cai S, Singh BR. A distinct Utility of the Amide III Infrared Band for Secondary Structure Estimation of Aqueous Protein Solutions Using Partial Leat Squares Methods. Biochem. 2004;43:2541–2549. doi: 10.1021/bi030149y. [DOI] [PubMed] [Google Scholar]
- 12.Hirai T, Subramaniam S. Protein Conformational Changes in the Bacteriorhodopsin Photocycle: Comparison of Findings from Electron and X-Ray Crystallographic Analyses. PloS One. 2009;4(6):e5769. doi: 10.1371/journal.pone.0005769. doi:10.1371/journal.pone.0005769 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braiman MS, Bousche O, Rothschild KE. Protein dynamics in the bacteriorhodopsin photocycle: Submillisecond Fourier transform infrared spectra in the L, M, and N photointermediates. Proc. Nat. Acad. Sci. U. S. A. 1991;88:2388–2392. doi: 10.1073/pnas.88.6.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lórenz-Fonfrίa VE, Kandori H, Padrós E. Probing Specific Molecular Peocesses and Intermediates by Time-resolved Fourier Transform Infrared Spectroscopy: Application to the Bacteriorhodopsin Photocycle. J. Phys. Chem. B. 2011;115:7972–7985. doi: 10.1021/jp201739w. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam S, Lindahl M, Bullough P, Faruqi AR, Tottor J, Oesterhelt D, Brown L, Lanyi J, Henderson R. Protein Conformational Changes in the Bacteriorhodopsin Photocycle. J. Mol. Biol. 1999;287:145–161. doi: 10.1006/jmbi.1999.2589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.