Abstract
Background
Increased risk of Non Hodgkin Lymphoma (NHL) has been observed in persons occupationally exposed to benzene, but the risk among persons living near benzene release sites has not been well described.
Methods
To investigate the spatial patterns of NHL incidence and the association between NHL incidence and distance to benzene release sites, we linked and geocoded data on benzene release sites in Georgia 1988–1998 from the Environmental Protection Agency's (EPA) Toxics Release Inventory (TRI), census tract level population statistics, and NHL incidence from the Georgia Comprehensive Cancer Registry (GCCR) 1999–2008. We mapped standardized incidence ratios by census tract and performed Poisson regression on NHL and NHL subtype incidence data, using the mean distance between the tract centroids and release sites as a marker of exposure. Cluster analyses were conducted at the global, local, and focal levels.
Results
Poisson regression indicated that for every mile the average distance to benzene release sites increased, there was an expected 0.31% decrease in the risk of NHL. Similar results were observed for all NHL subtypes analyzed. Clusters of NHL were spatially associated with benzene release sites located in metropolitan areas, but not with release sites in other areas of the state.
Conclusions
NHL incidence is significantly higher in census tracts which are closer, on average, to benzene release sites. Additional studies are needed to examine spatial patterns of NHL incidence in other geographic regions and interactions between benzene and other exposures.
Keywords: Lymphoma, Non-Hodgkin, Geographic Information Systems, Benzene, Surveillance
INTRODUCTION
Since the 1970s, the incidence of non-Hodgkin lymphoma (NHL) has increased by 3–4% annually [1] driven by: modifications of the lymphoma classification system, improved techniques for diagnosis and reporting hematological malignancies, and the human immunodeficiency virus (HIV) epidemic. However, these factors account for approximately 50% of the additional cases of NHL [2, 3]. The rise of NHL cases also appears to trail expanded industrial production in the U.S., suggesting occupational chemical exposures are risk factors for NHL [4].
Among carcinogenic occupational chemicals, benzene has been consistently linked to hematological cancers [5]. Numerous studies in human populations support a link between occupational benzene exposure and NHL, although some controversy remains [6–8]. Biologically, benzene exposure produces chromosomal aberrations and genetic changes, and toxic effects of benzene exposure can occur at air levels of 1 ppm or less, suggesting that even low levels of benzene exposure can be harmful [9]. Additional research is necessary to investigate associations between benzene exposure and NHL in residential settings since urban and rural populations are exposed to benzene through release into ambient air and surface waters. The purpose of this study was to examine the relationships between the incidence of NHL at the census tract level and benzene release sites to determine whether NHL incidence varies with distance from these sites.
MATERIALS AND METHODS
We linked and geocoded data on benzene release sites in Georgia 1988–1998 from the Environmental Protection Agency's (EPA) Toxics Release Inventory (TRI), NHL incidence from the Georgia Comprehensive Cancer Registry (GCCR) 1999–2008, and population and demographic data from the 2000 U.S. Census. This study was approved by the Emory University Institutional Review Board, the Winship Cancer Institute Clinical Research Committee, and the Georgia Department of Public Health Institutional Review Board. Because individual patients were not contacted and unmasked data were handled according to Health Insurance Portability and Accountability Act of 1996 privacy standards, individual informed consent was not required for this study.
Benzene Release Site Data
The EPA requires that facilities meeting thresholds defined by Section 313 of Emergency Planning and Community Right-to-Know Act annually report their disposal or releases for listed toxic chemicals. Launched in 1987, the TRI captures release information about certain chemicals, including the quantities, media type, and geographic coordinates of the releases from sources such as manufacturing facilities, service business, and federal facilities [10]. Between 1988 and 1998, 19 facilities in Georgia reported benzene releases. Total releases, calculated as the sum of fugitive air releases, stack air releases, and surface water discharges between 1988 and 1998 for each site ranged from 52 to 3,830,097 pounds of benzene. Some release sites reported releases for only one year while others reported benzene releases for multiple years during this period.
Census Tract Population and Demographic Data
Census tracts are subdivisions of counties with an average population of 4,000 persons. At the time of the 2000 census, there were 1,618 census tracts within the state of Georgia. Population and demographic data were available for 1,616 of these tracts, and data on sex, age, and race were obtained from Summary File 1 [11]. Because accurate yearly population values were not available for each census tract, we used 2000 census data and census tract boundaries to geocoded GCCR data and calculate standardized incidence ratios (SIRs) for each census tract. We used census data on median year moved into residence (MYMI) derived from a sample of individuals residing within a census tract, to provide information on the length of time residents were in their current home and on their residential exposure to benzene release sites.
Non-Hodgkin Lymphoma Data
GCCR provided data for all 12,716 incident NHL cases among adults ≥20 years residing in Georgia at the time of diagnosis, for the period 1999–2008. We have previously examined similar population-level incidence data for NHL and Hodgkin lymphoma subtypes in the United States [12–16]. In order to standardize the NHL incidence rates from Georgia to national NHL incidence rates, SEER*Stat Version 7.05 (National Cancer Institute, Bethesda, MD) was used to access the SEER 13 Registries Database [17]. Age-sex-race-specific crude incidence rates were obtained for the period from 1999–2008 for NHL and NHL subtypes in order to standardize Georgia incidence data by age, sex, and race. Incident cases were separated into B-cell NHL (NHL-B), T-cell NHL (NHL-T), diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL) using ICD-O-3 codes based on the proposed WHO-based nested classification of malignant lymphoid neoplasms for epidemiologic research from InterLymph [18]. Cases without age, sex, or race information were excluded from all analyses. We used four major race categories (White, Black, American Indian/Alaskan Native, and Asian or Pacific Islander); cases whose race was categorized as “other” or “unknown” were excluded. Cases that were geocoded successfully were aggregated to the census tract level [19]. SIRs were calculated for each census tract by dividing the number of observed cases by expected cases.
Geographic Data and Spatial Analyses
We utilized GIS data through ArcGIS 10 (ESRI, Redlands, CA) to examine the spatial distribution of benzene release sites and SIRs by census tract. Census tract shapefiles were obtained from the U.S. Census Bureau's 2000 TIGER/Line files [20]. GIS was used to calculate the distances between the 1,616 census tract centroids to each of the 19 benzene release sites. Descriptive spatial analysis was performed using ArcGIS. Choropleth maps were created to depict the NHL and NHL subtype SIRs by census tract. Locations of benzene release sites were overlayed upon the SIR maps. Since sparse data can pose statistical problems in small area analyses, spatial smoothing was performed to allow census tracts to borrow strength from spatial neighbors and produce more stable estimates of SIRs while maintaining geographic precision. Spatial Empirical Bayes (SEB) smoothing was performed on the SIR values using GeoDa 1.01 (Luc Anselin, Tempe, AZ) [21]. We defined neighbors as census tracts with common borders or vertices and constructed choropleth maps of smoothed SIRs for NHL and NHL subtypes.
To assess spatial correlation of SIRs, we conducted global, local, and focal spatial analyses. A global measure of spatial autocorrelation, the global Moran's I, was calculated for SIR patterns of NHL and NHL subtypes using GeoDa. It is important to note that smoothing will induce additional spatial correlation, thus we expected to see higher and more statistically significant Moran's I values for the SEB-smoothed SIRs. To measure spatial autocorrelation at the local scale, a local Moran's I, also termed Local Indicators of Spatial Autocorrelation (LISA) [21], was calculated for SIR patterns of NHL and NHL subtypes using GeoDa. LISA cluster maps were created to identify the locations of any significant clusters of SIRs. Both the global and local spatial statistics based significance on 999 Monte Carlo simulations, with neighbors defined by shared boundaries and vertices [21].
We used indirectly standardized incidence rates to test for focal clustering, calculated by multiplying the crude incidence rate by the non-smoothed SIR, to test the hypothesis that there was no spatial clustering near the benzene release sites (the focus). The Lawson-Waller Score test was used to individually assess each of the 19 benzene release sites for focal clustering of NHL [21]. Each census tract was scored for the difference between the observed and expected counts of NHL, weighted by inverse distance from each census tract centroid to the focus [21]. The standard normal distribution was used to estimate upper-tail p-values in ClusterSeer 2.3 (BioMedware, Ann Arbor, MI). Because the Lawson-Waller test was conducted 19 times (once for each release site), we adjusted our significance level using the Bonferroni correction method [22].
Poisson regression models were constructed under the assumption that the number of observed incident cases for each census tract had a Poisson distribution that was dependent on the number of expected cases for that census tract, based on its age, sex, and race demographics, and the explanatory variable of mean distance from benzene release site. Median year moved into residence was also assessed as a potential confounder and/or effect modifier. The models were estimated using the maximum likelihood method. Models were fitted for each subtype of NHL, in addition to the total NHL cases model using SAS Version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Of the 12,716 incident cases of NHL in our original dataset, 11,355 cases were successfully geocoded to the census tract level. Among these, 11,323 had age, sex, and race information available and could be classified into the U.S. census race categories of “white,” “black,” “American Indian/Alaskan Native,” or “Asian or Pacific Islander.” We mapped SIRs and SEB-smoothed SIRs for NHL, NHL-B, NHL-T, DLBCL, and FL with location data for each benzene release site (Figure 1). Two census tracts were not included due to a lack of demographic data from the U.S. Census Bureau. Fourteen of the nineteen benzene release sites were in the greater metropolitan Atlanta area.
Figure 1.
In these standardized incidence ratio (SIR) maps of non-Hodgkin lymphoma (NHL) among adults in Georgia from 1999 to 2008, blue represents an SIR of 0.00 to 0.50, green represents an SIR of 0.51 to 1.00, yellow represents an SIR from 1.01 to 1.25, orange represents an SIR from 1.26 to 2.00, and red represents an SIR >2.00. Maps show (a) SIRs for NHL, (b) spatial empir- ical Bayes-smoothed SIRs for NHL, (c) SIRs for B-cell NHL (NHL-B), (d) SIRs for T-cell NHL (NHL-T), (e) SIRs for diffuse large B-cell lymphoma (DLBCL), and (f) SIRs for follicular lymphoma (FL).
Spatial Analyses
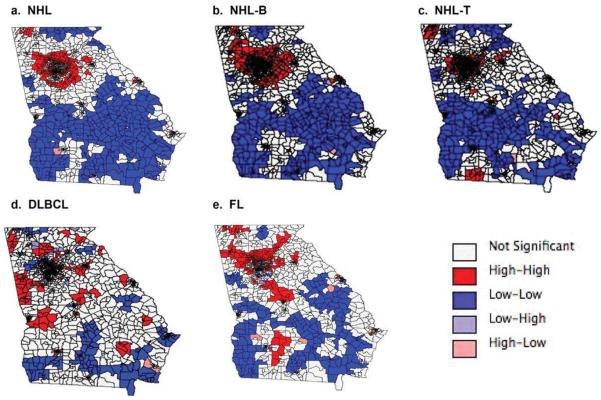
The global Moran's I values were greater than 0 and significant at the α=0.05 level for the NHL, NHL-B, NHL-T, and DLBCL raw SIRs demonstrating positive spatial autocorrelation (Table 2). This indicates that census tracts with similar incidence rates (high or low) were clustered. When spatial smoothing was performed to allow census tracts to borrow strength from spatial neighbors, there was significant positive spatial autocorrelation for the SEB-smoothed SIRs for NHL and all subtypes, which was expected due to the smoothing process inducing additional spatial correlation. LISA cluster maps of SEB-smoothed SIRs (Figure 2) show the locations of “hot-spots” (high-high clusters) and “cold-spots” (low-low clusters). High-high areas indicate significant clustering of census tracts with high SIR values surrounded by tracts that also have high SIR values while low-low areas indicate significant clustering of census tracts with low SIR values surrounded by tracts that also have low SIR values. Low-high areas indicate census tracts with low SIR values surrounded by tracts with high SIR values, and high-low areas indicate census tracts with high SIR values surrounded by tracts with low SIR values. Clustering of high SIRs appears to be located in the metro-Atlanta area for NHL and NHL subtypes, while clustering of low SIRs appears to be mostly in the southern region of the state.
Table 2.
Global measures of spatial autocorrelation
| Measure | NHL | NHL-B | NHL-T | DLBCL | FL |
|---|---|---|---|---|---|
| Nonsmoothed SIR | |||||
| Moran I | 0.2009 | 0.0628 | 0.0430 | 0.0179 | −0.0011 |
| P | .0010 | .0010 | .0020 | .1090 | .5150 |
| SEB-smoothed SIR | |||||
| Moran I | 0.8317 | 0.6377 | 0.5879 | 0.6403 | 0.5938 |
| P | .0010 | .0010 | .0010 | .0010 | .0010 |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NHL, non-Hodgkin lymphoma; NHL-B, B-cell non-Hodgkin lymphoma; NHL-T, T-cell non-Hodgkin lymphoma; SEB, Spatial Empirical Bayes; SIR, standardized incidence ratio.
Figure 2.
Local Indicators of Spatial Autocorrelation cluster maps of spatial empirical Bayes-smoothed standardized incidence ratios show (a) non-Hodgkin lymphoma (NHL), (b) B-cell NHL (NHL-B), (c) T-cell NHL (NHL-T), (d) diffuse large B-cell lymphoma (DLBCL), and (e) follicular lymphoma (FL).
For 15 of the 19 benzene release sites, the Lawson-Waller test was statistically significant at the α=0.0026 level (using Bonferroni's correction for 19 comparisons, Table 4). All benzene release sites located within the metro-Atlanta area had significant focal clustering, with greater incidence rates observed near these sites than expected. Incidence rates were lower than expected surrounding sites 6, 8, 9, and 10. Only one benzene release site associated with increased NHL incidence was located outside of the metropolitan Atlanta area; this site was located near Savannah, GA. No statistically significant Poisson coefficients emerged for the interaction term between mean distance to benzene release site and median year moved into residence (MYMI), so this variable was dropped. Additionally, comparing models with and without MYMI suggested that it was not a confounder. A 0.31% decrease in NHL risk is expected for every mile the average distance to benzene release sites increases. Results of the Poisson regression analyses are displayed in Table 3.
Table 4.
Poisson regression results
| Variable | Model 1: NHL | Model 2: NHL-B | Model 3: NHL-T | Model 4: DLBCL | Model 5: FL |
|---|---|---|---|---|---|
| Intercept | 0.1407 | 0.3453 | 0.0762 | 2.4831 | 0.1460 |
| Explanatory variables | |||||
| Mean distance from benzene release site β | −0.0031 | −0.0035 | −0.0032 | −0.0032 | −0.0020 |
| Standard error | 0.0002 | 0.0002 | 0.0006 | 0.0004 | 0.0003 |
| Waid chi-square statistic | 227.27 | 223.85 | 31.25 | 80.09 | 55.72 |
| P | < .0001 | < .0001 | < .0001 | < .0001 | < .0001 |
| Model fit | |||||
| Deviance, df = 1614 | 2599.96 | 2913.62 | 1843.55 | 2118.79 | 8964.21 |
| Deviance/df | 1.61 | 1.81 | 1.14 | 1 31 | 5,55 |
Abbreviations: df, degrees of freedom; DLBCL, diffuse large B-ceil lymphorma; FL, fbllicilar lymphoma; NHL, non-Hodgkin lymphoma; NHL-B, B-cell non-Hodgkin lymphoma; NHL-T, T-cell non-Hodgkin lymphoma.
Table 3.
Lawson-Waller Score Test for Focal Clustering
| Benzene Site | Score | P | |
|---|---|---|---|
| 1. | Metropolitan Atlanta | 13.1831 | <.0001a |
| 2. | Metropolitan Atlanta | 18.0533 | <.0001a |
| 3. | Metropolitan Atlanta | 36.3879 | <.0001a |
| 4. | Metropolitan Atlanta | 14.9471 | <.0001a |
| 5. | Metropolitan Atlanta | 15.6043 | <.0001a |
| 6. | Homerville | −23.6137 | 1.0000 |
| 7. | Metropolitan Atlanta | 15.8019 | <.0001a |
| 8. | Macon | −3.8011 | .9999 |
| 9. | Columbus | −3.4838 | .9999 |
| 10. | Augusta | −6.7270 | 1.0000 |
| 11. | Metropolitan Atlanta | 26.9324 | <.0001a |
| 12. | Metropolitan Atlanta | 20.8564 | <.0001a |
| 13. | Metropolitan Atlanta | 14.9068 | <.0001a |
| 14. | Metropolitan Atlanta | 15.6043 | <.0001a |
| 15. | Metropolitan Atlanta | 15.0695 | <.0001a |
| 16. | Savannah | 9.2829 | <.0001a |
| 17. | Metropolitan Atlanta | 29.9130 | <.0001a |
| 18. | Metropolitan Atlanta | 37.9698 | <.0001a |
| 19. | Metropolitan Atlanta | 18.4694 | <.0001a |
These p values are significant at α = .0026 (Bonferroni correction for multiple comparisons).
DISCUSSION
We examined residential proximity to benzene release sites and risk of NHL in Georgia using census tract level data. Our Poisson regression results suggest an approximate 0.3% decrease in NHL risk is expected for a given population in a census tract when increasing the average distance to a benzene release site by 1 mile. Among NHL subtypes, mean distance had the least effect on FL incidence, but cases were still significantly more likely to occur in census tracts that were closer, on average, to benzene release sites. It is unclear as to why the effect was weakest on FL incidence, but recent research on FL suggests that genetics and lifestyle are more predictive of FL than environmental factors, and that these genetic and lifestyle risk factors are more associated with FL than other NHL subtypes [23, 24]. While the overall incidence of NHL in Georgia is low (17.4 per 100,000 individuals per year), NHL was significantly higher in tracts that were closer to benzene release sites. Cluster analyses identified several hot spots and cold spots for NHL and NHL subtypes throughout the state. The metropolitan Atlanta area was almost always identified as a hot spot, while other urban areas, namely Augusta and Savannah, were sometimes implicated. Cold spots were most often located in the southern half of the state. A test of focal clustering identified significantly increased NHL incidence surrounding 15 of the 19 benzene release sites. These results suggest that focal clustering is present around benzene release sites located in the metropolitan Atlanta, but this phenomenon was not observed in other areas of the state. An alternative hypothesis that could explain the results observed from our cluster analyses is that populations living in urban areas are exposed to some unmeasured factor or an unaccounted for confounder. Other sources of benzene exposure associated with urban areas include mainstream smoke from cigarettes and auto exhaust [25]. However, neither of these have been consistently associated with an increased risk of NHL [26]. Additionally, pesticides, which have also been associated with increased risk of NHL, would more commonly be found in rural areas [27].
Strengths of this study include the use of publicly available data from EPA and the U.S. Census Bureau. Spatial smoothing of NHL incidence data addressed differences in census tract population size that could result in variance instability and spurious outliers. By standardizing the data indirectly, we eliminated the effects of age, sex, and race on NHL incidence. Our study is the first to examine the relationship between passive benzene exposure and the incidence of NHL at the state population level. One limitation of this study is the use of aggregated data, as our findings may not hold true at the individual level. Furthermore, the presence of chemical releases into the environment is not sufficient to determine personal exposure, or to calculate potential risks to human health.
A potential weakness of this study was the lack of quantitative exposures and temporal analyses. A nationwide study revealed that amounts of benzene emissions may be as much as five times higher than reported by the TRI [28]. Thus, while data on the amounts of benzene released from facilities in Georgia between 1988 and 1998 were available, these were not incorporated into the models due to concerns about reliability. However, we attempted to control for time and latency by only including benzene release sites from 1988 to 1998 and using incidence and population data from 1999 to 2008, as well as assessing MYMI as a potential confounder and effect modifier. The average value for MYMI for all census tracts used in this investigation was 1989, which is during the period for potential benzene exposure (1988–1998) and allows for a theoretical period of ten years to develop NHL as our incidence data was for the years 1999–2008. Changes in the population of Georgia between 1999 and 2008 and the use of 2000 U.S. Census data as denominators for incidence rates also may have introduced bias to this study. In addition, any unmeasured confounding variables could have been spatially correlated which could have biased our Poisson regression coefficients.
Associations between exposure to benzene and hematological effects were observed as early as the 1940s[8]. In a 35-year cohort study of rubber workers from this time period, significant decreases in white and red blood cell counts were observed among workers exposed to benzene. More recent occupational studies have shown similar associations [5,7]. In China, workers exposed to benzene for 10 or more years were 4.2 (95% CI = 1.1–15.9) times more likely to develop NHL [6]. Development of NHL has been most strongly linked to benzene exposures that occurred > 10 years before diagnosis, suggesting that benzene-related NHL may be associated with longer induction periods. Often, it is difficult to discern the specific role benzene plays in the elevation of NHL risk, because workers exposed to benzene in an occupational setting tend to be exposed to other potential carcinogens. In a systematic review of 43 case-control studies by Smith et al. that analyzed occupational benzene exposure and risk of NHL, 40 studies (93%) indicated an increase in NHL risk, and 23 studies (53%) demonstrated statistically significant associations between NHL risk and benzene exposure [29]. Additional meta-analyses have been supportive of this finding [30], while other meta-analyses have reached different conclusions [31]. For example, a recent meta-analysis by Kane and Newton found no effect of occupational benzene exposure on NHL risk, regardless of the amount or duration of benzene exposure [32]. One explanation for differences in these findings is that NHL represents a heterogeneous group of diseases and benzene exposure may be more strongly associated with some variants than others [30]. The Kane and Newton meta-analysis showed no association between occupational benzene exposure and NHL, DLBCL, and FL, whereas our study showed associations between passive benzene exposure and NHL and NHL subtypes. The latency period before NHL development also may explain why some studies failed to observe an association with NHL. Future studies can examine temporal relationships between passive benzene exposure and incidence of NHL.
Our study contributes to a growing body of literature that has identified the association between NHL and organic solvents such as benzene. Previous studies of non-occupational exposure and NHL have been limited by their reliance on subject recall [33], whereas more recent studies have used geographic approximations such as zip code or municipality centroids to conduct their analyses [34–36]. Johnson et al found an increased risk of NHL in individuals living in proximity to copper smelters and sulfite pulp mills, while Ramis et al. found an association between NHL and proximity to paper industries. Another study found an increased risk of NHL in subjects living in wards surrounding a municipal solid waste incinerator that was known to exude dioxins [37].
A recent study by Wheeler et al. used case residential histories to examine spatio-temporal clustering of NHL in Iowa, Los Angeles, Detroit, and Seattle. Despite adjusting for PCB exposure and genetic polymorphisms (known risk factors for developing NHL), spatio-temporal clusters of high NHL incidence remained unexplained [38]. Other studies have found associations between NHL and proximity to petroleum refineries and toxic industrial waste sites [39, 40]. De Roos et al. conducted a case control study of industrial facilities and NHL in 2010 that utilized geographic positioning systems to identify the exact coordinates of residence for 99% of their 1548 participants [4]. Their study was unique in its ability to determine risk estimates for individual NHL subtypes and to assess trend with increasing proximity to various industrial facilities. Although they did not study benzene exposure specifically, their study found that subjects living within 2 miles of petroleum refineries (which are known to contain benzene) for more than 10 years had a 90% increased risk of NHL (OR 1.9, 95% CI: 1.0–3.6, p-trend = 0.04). Continued improvements in the GIS analysis techniques employed to study the associations between benzene and NHL will contribute to an improved understanding of NHL etiology.
This study identified a significant association between distance to benzene release sites and NHL incidence at the census tract level. Future studies should examine this association using individual-level geographic data, temporal trends, and dose-response relationships between benzene exposure and NHL. Additional future directions for this research will evaluate other potential sources of benzene exposure, other environmental exposures, and genetic and clinical factors that may explain the higher incidence of NHL observed in certain areas. These studies investigating the etiology of NHL are critical to identify and enact public health policies that may decrease or prevent NHL.
Table 1.
Study population demographics
| Cases, N = 11,323a |
Georgia Population, N = 5,582,707b |
|||
|---|---|---|---|---|
| Variable | No. | % | No. | % |
| Age group, y | ||||
| 20–59 | 4585 | 40.5 | 4,521,891 | 81.0 |
| ≥60 | 6738 | 59.5 | 1,060,816 | 19.0 |
| Sex | ||||
| Women | 5274 | 46.6 | 2,907,559 | 52.1 |
| Men | 6049 | 53.4 | 2,675,148 | 47.9 |
| Race | ||||
| White | 8991 | 79.4 | 3,922,068 | 70.3 |
| BlacK | 2208 | 19.5 | 1,521,316 | 27.3 |
| American Indian/Alaskan | 4 | 0.0 | 15,326 | 0.3 |
| Native | ||||
| Asian or Pacific Islander | 120 | 1.1 | 123,997 | 2.2 |
| NHL subtype | ||||
| B-cell NHL | 8925 | 78.8 | ||
| Diffuse lange B-cell lymphoma | 3851 | 34.0 | ||
| Follicular lymphoma | 2171 | 19.2 | ||
| T-cell NHL | 1489 | 13.2 | ||
| Median year moved Into residence | 1989 | |||
| Mean distance fnom benzene release sites: Mean±SD, miles | 99.5±55.2 | |||
Abbreviations: NHL, non-Hodgkin lymphoma: SD: standard deviation.
Total number of NHL cases In Georgia from 1999 to 2008.
This population was based on 1616 census tracts In Georgia from the 2000 US Census.
Acknowledgments
Funding Sources: This work had no specific funding.
Footnotes
Financial Disclosures: There are no financial disclosures from any authors.
REFERENCES
- 1.Muller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84(1):1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 2.Hartge P, Devesa SS. Quantification of the impact of known risk factors on time trends in non-Hodgkin's lymphoma incidence. Cancer Res. 1992;52(19 Suppl):5566s–5569s. [PubMed] [Google Scholar]
- 3.Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60(6):393–408. doi: 10.3322/caac.20087. [DOI] [PubMed] [Google Scholar]
- 4.De Roos AJ, Davis S, Colt JS, et al. Residential proximity to industrial facilities and risk of non-Hodgkin lymphoma. Environ Res. 2010;110(1):70–8. doi: 10.1016/j.envres.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Public Health. 2010;31:133–48. doi: 10.1146/annurev.publhealth.012809.103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes RB, Yin SN, Dosemeci M, et al. Benzene and the dose-related incidence of hematologic neoplasms in China. Chinese Academy of Preventive Medicine--National Cancer Institute Benzene Study Group. J Natl Cancer Inst. 1997;89(14):1065–71. doi: 10.1093/jnci/89.14.1065. [DOI] [PubMed] [Google Scholar]
- 7.Rinsky RA, Hornung RW, Silver SR, Tseng CY. Benzene exposure and hematopoietic mortality: A long-term epidemiologic risk assessment. Am J Ind Med. 2002;42(6):474–80. doi: 10.1002/ajim.10138. [DOI] [PubMed] [Google Scholar]
- 8.Kipen HM, Cody RP, Crump KS, Allen BC, Goldstein BD. Hematologic effects of benzene: a thirty-five year longitudinal study of rubber workers. Toxicol Ind Health. 1988;4(4):411–30. doi: 10.1177/074823378800400401. [DOI] [PubMed] [Google Scholar]
- 9.Bollati V, Baccarelli A, Hou L, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67(3):876–80. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 10.Environmental Protection Agency The Toxics Release Inventory (TRI) and Factors to Consider When Using TRI Data. [cited 2012 March 22]; Available from: http://www.epa.gov/tri/triprogram/FactorsToConPDF.pdf.
- 11.U.S. Census Bureau Introduction to Census 2000 Data Products. [cited 2012 March 22]; Available from: http://www.census.gov/prod/2001pubs/mso-01icdp.pdf.
- 12.Flowers C, Fedewa S, Chen A, et al. Disparities in the early adoption of chemo-immunotherapy for diffuse large B-cell lymphoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abouyabis AN, Shenoy PJ, Lechowicz MJ, Flowers CR. Incidence and outcomes of the peripheral T-cell lymphoma subtypes in the United States. Leuk Lymphoma. 2008;49(11):2099–107. doi: 10.1080/10428190802455867. [DOI] [PubMed] [Google Scholar]
- 14.Shenoy P, Maggioncalda A, Malik N, Flowers CR. Incidence patterns and outcomes for hodgkin lymphoma patients in the United States. Adv Hematol. 2011;2011:725219. doi: 10.1155/2011/725219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shenoy P, Malik N, Sinha R, et al. Racial differences in the presentation and outcomes of chronic lymphocytic leukemia and variants in the United States. Clin Lymphoma Myeloma Leuk. 2011;11(6):498–506. doi: 10.1016/j.clml.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Shenoy PJ, Malik N, Nooka A, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. 2010 doi: 10.1002/cncr.25765. [DOI] [PubMed] [Google Scholar]
- 17.Surveillance E, End Results (SEER) Program . SEER*Stat Database: Incidence - SEER 13 Registries Research Data, Nov 2010 Sub (1973–2008) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S. [Google Scholar]
- 18.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110(2):695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ESRI Common Questions. [cited 2012 January 9]; Available from: http://www.esri.com/software/coder/common-questions.
- 20.U.S. Census Bureau Census 2000 TIGER/Line® Files: TIGER/Line Shapefiles and TIGER/Line Files. [cited 2012 January 9]; Available from: http://www.census.gov/geo/www/tiger/tiger2k/tgr2000.html.
- 21.Waller L, Gotway C. Applied Spatial Statistics for Public Health Data. Wiley; New York: 2004. [Google Scholar]
- 22.Kleinbaum D, Kupper L, Nizam A, Muller K. Applied Regression Analysis and Other Multivariable Methods. Thomson Brooks/Cole; California: 2008. [Google Scholar]
- 23.Chang CM, Wang SS, Dave BJ, et al. Risk factors for non-Hodgkin lymphoma subtypes defined by histology and t(14;18) in a population-based case-control study. Int J Cancer. 2011;129(4):938–47. doi: 10.1002/ijc.25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambinder AJ, Shenoy PJ, Malik N, Maggioncalda A, Nastoupil LJ, Flowers CR. Exploring risk factors for follicular lymphoma. Adv Hematol. 2012;2012:626035. doi: 10.1155/2012/626035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace LA. Major sources of benzene exposure. Environ Health Perspect. 1989;82:165–9. doi: 10.1289/ehp.8982165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton LM, Hartge P, Holford TR, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph) Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(4):925–33. doi: 10.1158/1055-9965.EPI-04-0693. [DOI] [PubMed] [Google Scholar]
- 27.Brauner EV, Sorensen M, Gaudreau E, et al. A prospective study of organochlorines in adipose tissue and risk of non Hodgkin lymphoma. Environmental health perspectives. 2012;120(1):105–11. doi: 10.1289/ehp.1103573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Environmental Integrity Project (EIP) and The Galveston-Houston Association for Smog Prevention (GHASP) 2004. Who's Counting? The Systematic Underreporting of Toxic Air Emissions. [Google Scholar]
- 29.Smith MT, Jones RM, Smith AH. Benzene exposure and risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):385–91. doi: 10.1158/1055-9965.EPI-06-1057. [DOI] [PubMed] [Google Scholar]
- 30.Cocco P, t'Mannetje A, Fadda D, et al. Occupational exposure to solvents and risk of lymphoma subtypes: results from the Epilymph case-control study. Occup Environ Med. 2010;67(5):341–7. doi: 10.1136/oem.2009.046839. [DOI] [PubMed] [Google Scholar]
- 31.Vlaanderen J, Lan Q, Kromhout H, Rothman N, Vermeulen R. Occupational benzene exposure and the risk of lymphoma subtypes: a meta-analysis of cohort studies incorporating three study quality dimensions. Environ Health Perspect. 2011;119(2):159–67. doi: 10.1289/ehp.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane EV, Newton R. Benzene and the risk of non-Hodgkin lymphoma: a review and meta-analysis of the literature. Cancer Epidemiol. 2010;34(1):7–12. doi: 10.1016/j.canep.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Linos A, Blair A, Gibson RW, et al. Leukemia and non-Hodgkin's lymphoma and residential proximity to industrial plants. Arch Environ Health. 1991;46(2):70–4. doi: 10.1080/00039896.1991.9937431. [DOI] [PubMed] [Google Scholar]
- 34.Ramis R, Vidal E, Garcia-Perez J, et al. Study of non-Hodgkin's lymphoma mortality associated with industrial pollution in Spain, using Poisson models. BMC Public Health. 2009;9:26. doi: 10.1186/1471-2458-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson KC, Pan S, Fry R, Mao Y. Residential proximity to industrial plants and non-Hodgkin lymphoma. Epidemiology. 2003;14(6):687–93. doi: 10.1097/01.ede.0000091600.89417.58. [DOI] [PubMed] [Google Scholar]
- 36.Ramis R, Diggle P, Boldo E, Garcia-Perez J, Fernandez-Navarro P, Lopez-Abente G. Analysis of matched geographical areas to study potential links between environmental exposure to oil refineries and non-Hodgkin lymphoma mortality in Spain. Int J Health Geogr. 2012;11:4. doi: 10.1186/1476-072X-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viel JF, Floret N, Deconinck E, Focant JF, De Pauw E, Cahn JY. Increased risk of non-Hodgkin lymphoma and serum organochlorine concentrations among neighbors of a municipal solid waste incinerator. Environ Int. 2011;37(2):449–53. doi: 10.1016/j.envint.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler D, Ward M, Waller L. Spatial-Temporal Analysis of Cancer Risk in Epidemiologic Studies with Residential Histories. Annals of the Association of American Geographers. 2012;102(5) doi: 10.1080/00045608.2012.671131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreiher J, Novack V, Barachana M, Yerushalmi R, Lugassy G, Shpilberg O. Non-Hodgkin's lymphoma and residential proximity to toxic industrial waste in southern Israel. Haematologica. 2005;90(12):1709–10. [PubMed] [Google Scholar]
- 40.Dahlgren J, Klein J, Takhar H. Cluster of Hodgkin's lymphoma in residents near a non-operational petroleum refinery. Toxicol Ind Health. 2008;24(10):683–92. doi: 10.1177/0748233708100553. [DOI] [PubMed] [Google Scholar]