Abstract
There is a heightened interest and concern among scientists, clinicians, and regulatory agencies as well as the general public, regarding the effects of environmental endocrine-disrupting chemicals (EDCs). In this review, we identify the main epigenetic mechanisms and describe key ovarian processes that are vulnerable to the epigenetic actions of EDCs. We also provide an overview of the human epidemiological evidence documenting the detrimental effects of several common environmental EDCs on female reproduction. We then focus on experimental evidence demonstrating the epigenetic effects of these EDCs in the ovary and female reproductive system, with an emphasis on methoxychlor, an organochlorine pesticide. We conclude the review by describing several critical issues in studying epigenetic effects of EDCs in the ovary, including transgenerational epigenetic effects.
Keywords: DNA methylation, histone modifications, non-coding RNA, epigenetics, ovary, female fertility, endocrine disruptors
1. Introduction
Endocrine-disrupting chemicals (EDCs) are synthetic or natural compounds in the environment that can interfere with endocrine functions. The EDCs in the environment can be categorized as pesticides, plasticizers, industrial side products, pharmaceuticals, flame-retardants, phytoestrogens, or heavy metals such as cadmium (Diamanti-Kandarakis et al., 2009; Iavicoli et al., 2009; Kortenkamp, 2011).
2. Developmental exposures to EDCs and the Barker hypothesis
Exposure to EDCs during development is a bigger concern than exposure during adulthood. The health consequences of developmental exposure are mostly permanent or long-lasting while the individuals who are exposed during adulthood usually regain their normal physiological functions after the withdrawal of chemicals. The long-lasting effects of EDCs can be viewed from the perspective of developmental origins of health and diseases (DoHaD) concept, often called the Barker hypothesis. This concept was first developed based on the observation that in utero exposure to poor nutrition can lead to various adult disorders, including metabolic and cardiovascular diseases (Barker, 1995). The same concept applies to EDC exposure during the critical window(s) of development, which can alter the programming of the female reproductive organs, including the ovary, and lead to infertility. Abnormal developmental programming occurs when the adverse environmental conditions alter the epigenetic mechanisms which modify gene expression patterns (Heijmans et al., 2009).
3. Epigenetic mechanisms
Epigenetics is the study of mitotically and meiotically heritable changes in the gene function (i.e., gene expression) without changing the DNA sequence. Three common epigenetic mechanisms are DNA methylation, post-translational modifications of histone proteins (histone modifications), and non-coding RNA (ncRNA) (Jirtle and Skinner 2007), and they collectively make up the major components of the epigenome. Exposure to EDCs can alter these three epigenetic mechanisms in the ovary and various female reproductive organs, leading to gene expression changes (Zama and Uzumcu, 2009; Bredfeldt et al., 2010; Luense et al., 2011).
3.1. DNA methylation
Methylation of DNA occurs at cytosine residues in CpG dinucleotides and plays a role in genomic imprinting, suppression of retrotransposons, and X-chromosome inactivation, as well as gene expression regulation (Ehrlich, 2003). CpGs are underrepresented in the genome, except in segments of DNA called CpG islands (CGIs), where the CG content is markedly increased and is associated with the regulatory sequences of the genes (Turner, 2009). Methylation of DNA can interfere with transcription factor binding, leading to a reduction of gene expression. In the absence of DNA methylation, transcription factors can bind to the regulatory sequences leading to upregulation of gene expression (Yagi and Koshland, 1981; Ehrlich, 2003). The DNA methylation alterations in CpGs outside CGIs are now also considered to be important in gene regulation (Suzuki and Bird, 2008), necessitating more genome-wide methylation studies.
3. 2. Histone modifications
Histones are the protein components of the nucleosome, through which genomic DNA is packaged in eukaryotes. Post-translational modifications of histones at lysine, arginine, serine, and threonine amino acids play significant roles in both structural and functional states of the chromatin (Hirose et al., 1985; Turner, 2009). These modifications include acetylation of lysine, methylation of lysine and arginine, and phosphorylation of serine and threonine. Whereas acetylation of lysine residues is associated with the relaxation of chromatin, allowing access to transcription factors and active transcription, deacetylation leads to compaction of the chromatin, preventing access of transcription factors and silencing the loci. Similarly, methylation or phosphorylation can be associated with gene activation or gene silencing, depending on the position of the amino acid modified (see (Sharma et al., 2005; Yoo and Jones, 2006; Turner, 2009) for more details).
3.3. Non-coding RNA
The importance of ncRNAs in biological processes has been recently recognized (Chang et al., 2006). Non-coding RNAs are transcripts without a clear open reading frame; therefore they do not code proteins, but regulate the expression of other genes in cis and trans manners. The ncRNAs vary in size from microRNAs, or miRNAs (15-21 nucleotides, nt), and small RNAs (100-200 nt) to large RNAs (>200 nt) and are involved in vital functions such as X-chromosome inactivation, genomic imprinting, transposon and virus silencing, and developmental patterning and differentiation (reviewed in (Costa, 2008; Brosnan and Voinnet, 2009)).
The above-mentioned three epigenetic mechanisms are known to influence each other’s roles in cellular development, differentiation and function (Li, 2002; Costa, 2008; Namihira et al., 2008). During establishment of the epigenetic marks, histone modifications lead to DNA methylation, whereas in maintenance or inheritance of the marks, DNA methylation plays a primary role. In addition, ncRNAs, histone modifications and DNA methylation work cooperatively in various processes, including X-chromosome inactivation (Avner and Heard, 2001) and genomic imprinting (Bartolomei, 2009; Brosnan and Voinnet, 2009).
4. Major epigenetic modifications in the mammalian life cycle
The epigenome, especially DNA methylation patterns, is stably maintained in somatic cells. However, major epigenetic programming occurs during two stages of development: (1) in the preimplantation embryo, and (2) in primordial germ cells (PGCs) in the bipotential gonad (Reik et al., 2001). In the preimplantation embryo, a genome-wide epigenetic reprogramming occurs through which the epigenetic marks, except on imprinted genes, are eliminated (Santos and Dean, 2004); the zygote gains its totipotency; and embryonic cell lineage-specific methylation occurs, including in the PGCs. After the PGCs migrate from the extragonadal sites to the genital ridge, the DNA methylation patterns of the imprinted genes in the PGCs are also erased in both sexes (Figure 1) (Hajkova et al., 2002), and reestablished in a sex-specific manner. In males, the remethylation starts on embryonic day (E) 14 and is mostly completed by E16-17 (Ueda et al., 2000). In contrast, the female germ cell remethylation is initiated during postnatal day (PND) 1 through PND5 and continues throughout oocyte growth until the pre-antral follicle stage in mice (Obata and Kono, 2002).
Figure 1. The stages of gonadal development in females (see the text for details).
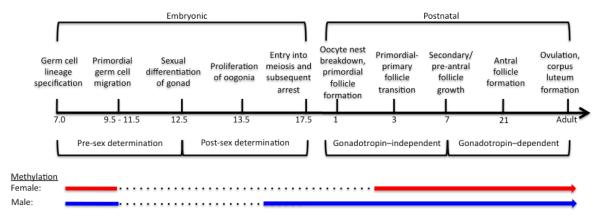
One of the processes that are particularly vulnerable to the epigenetic effects of endocrine-disrupting chemicals is epigenetic reprogramming (i.e., DNA demethylation and remethylation) in primordial germ cells (PGCs). During the genome-wide epigenetic programming in the preimplantation embryo, the DNA methylation patterns in both male and female genomes are eliminated (not shown), except in imprinted genes (color solid lines) whose methylation patterns are maintained, including in PGCs until their migration to the gonad. After the migration, the patterns are erased (dotted lines) and reestablished (color solid lines) in a sex-specific manner. The timeline shown is primarily based on the events in mice. Adapted from (Zama and Uzumcu, 2010).
The two stages that are described above are likely to be the most vulnerable to the epigenetic effects of EDCs. If EDCs interfere with DNA methylation during the epigenetic reprogramming in the preimplantation embryo, all three embryonic germ layers can be affected, and therefore all tissues of the organism (Dolinoy et al., 2007). If EDCs alter epigenetic reprogramming (i.e., imprinted genes) of the germline, subsequent generations can be affected (Stouder and Paoloni-Giacobino, 2011). Although the effects that occur during the erasure can influence both sexes, the effect during remethylation can be sex-specific.
5. Ovarian development and function
In addition to effects that can occur during the two major epigenetic modifications described above, EDCs can have ovary-specific effects if the exposure takes place during the critical developmental processes in the ovary. In addition, the cyclic processes that occur in adult ovary throughout the female’s reproductive life which are closely regulated by local and endocrine factors, including estradiol (E2), progesterone (P4), and androgens, can be affected by EDCs that display estrogenic, antiestrogenic, and antiandrogenic actions (Zama and Uzumcu, 2010). The specific ovarian processes are discussed using a timeline that applies to rodent species (Figure 1). We focused on processes that are likely to be affected by EDCs, rather than describing the normal ovarian development and function in detail, which can be found in recent reviews (Edson et al., 2009; Gougeon, 2010).
5.1. Oocyte nest breakdown and primordial follicle formation
Oocyte nest breakdown that leads to the formation of primordial follicles is mediated by oocyte apoptosis starting at E16.5 in mice (Pepling and Spradling, 2001). By PND3-4, the primordial follicles are formed. Each primordial follicle consists of an oocyte at the center surrounded by a single layer of squamous pre-granulosa cells. In vitro and in vivo experiments have shown that the steroids P4 and E2 inhibit primordial follicle formation by inhibiting oocyte nest breakdown, leading to multiovular follicles (MOF; presence of more than one oocyte within a follicle) (Kezele and Skinner, 2003; Chen et al., 2007).
5.2. Primordial-to-primary follicle transition
The transition from primordial to primary follicles, which is also known as “initial recruitment” (McGee and Hsueh, 2000), ensures a steady supply of follicles for further growth and ovulation. Once the primordial follicle is recruited, the oocyte undergoes growth and maturation to gain competence for fertilization, and the pre-GCs differentiate into GCs by growing in size and becoming cuboidal and they proliferate. Most of the growing follicles (99.9%) are destined to become atretic and the rest will ovulate after completing the following follicular stages: primary, secondary; pre-, early-, mid-, and late-antral; and pre-ovulatory (see (Armenti et al., 2008) for a detailed description of follicular stages).
Both E2 and P4 also have inhibitory roles in the initial recruitment, and in the ovaries that are exposed to E2 and P4 in vitro, the transition is inhibited (Kezele and Skinner, 2003). In addition, when newborn rats are injected with estradiol benzoate, their ovaries have more primordial follicles and fewer growing follicles (Ikeda et al., 2001). In contrast, cytochrome P450 aromatase knockout (ArKO) mice, which lack endogenous E2, have fewer primordial and primary follicles (Britt et al., 2004). These results show that whereas E2 is needed for early events in folliculogenesis, excessive E2 inhibits these events. In contrast, androgens stimulate the primordial-to-primary follicle transition but inhibit follicle growth beyond the primary stage (Yang and Fortune, 2006; Yang et al. 2009).
5.3. Pre-antral and early-antral follicular growth
Follicular growth during primary and secondary stages is regulated by local factors. Starting with the secondary follicle stage, follicles begin to gain receptors for gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Sokka and Huhtaniemi, 1990). Around this time, follicles also gain a second somatic cell type, thecal cells. The follicular progression becomes dependent on gonadotropins during the pre-antral stage (Kumar et al., 1997). Granulosa and thecal cells cooperate in the production of E2 under the regulation of FSH and LH, respectively (Magoffin, 2005). Increasing E2 concentrations cause negative feedback at the hypothalamus and pituitary levels, thereby reducing the secretion of gonadotropins.
Estradiol plays stimulatory a role in later stages of folliculogenesis. This role is the clearest in transgenic mouse strains, in which ERβ is globally deleted via homologous recombination (Carpenter and Korach, 2006) or ERα is deleted in thecal cells (Lee et al., 2009), the primary cell type that expresses ERα. Both mouse strains show inhibition in follicular maturation and ovulation. In contrast, while androgens are stimulatory during the initial stages of folliculogenesis, excess androgen is inhibitory and its receptor is actively down-regulated at the later stages of folliculogenesis (Tetsuka et al., 1995).
5.4. Follicular selection, maturation, and ovulation
To complete the full course of folliculogenesis and ovulation, one follicle within the recruited cohort is selected in monoovulators whereas multiple follicles are selected in polyovulators. The selection process is under complex regulation, which is primarily determined by the elevated gonadotropin responsiveness of the selected follicle(s) despite decreasing gonadotropin concentrations. A whole host of local growth factors, such as insulin-like growth factors (IGFs), play significant roles throughout folliculogenesis including the final maturation (reviewed in (Edson et al., 2009; Sirotkin, 2011). At the preovulatory stage, elevated E2 exerts positive feedback at the hypothalamus, leading to the preovulatory LH surge. During this stage, GCs also gain responsiveness to LH, which initiates numerous gene expression alterations that prepare the follicle for ovulation (Richards and Pangas, 2010). The oocyte is released during the ovulation along with surrounding cumulus cells – a special subpopulation of GCs that are immediately adjacent to oocyte. The remaining cells of the follicle form the corpus luteum (CL), which is the source of the P4 that prepares the reproductive tract for the pregnancy. The formation, function, and demise of the CL (in the case of continuous reproductive cycle) or maintenance of CL (in case of pregnancy) are closely regulated events (Stocco et al., 2007), which are affected by EDCs.
Although the ovary plays a central role in female reproduction, its function is closely regulated by the hypothalamus and pituitary. The EDCs can act at the levels of the hypothalamus and pituitary as well as the ovary. However, in this review, we focus on the direct effects of select EDCs in the ovary.
6. Epidemiological evidence linking EDCs to female reproductive disorders
Epidemiological studies strongly suggest that EDCs affect both male and female reproduction. One of the earliest reports related to the decline in sperm parameters, such as reduced sperm count and seminal volume in men during the previous 50 years pointed out the harmful effects of EDCs (Carlsen et al., 1992). This time period closely corresponds to the time of the widespread use of synthetic EDCs. Although this 1992 report is controversial (Fisch et al., 1996; Younglai et al., 1998), there is agreement regarding a geographical region-dependent decline in sperm parameters (Swan et al., 2003), which supports an environmental etiology. For example, several studies have shown that there are clear differences between male fertility parameters of men in Denmark and Finland. These studies show that semen quality parameters are lower while the incidence of testicular cancer, hypospadias, and cryptorchidism is higher in males living in Denmark, including the Denmark-born offspring of immigrants, as compared to males from Finland (Boisen et al., 2004; Jorgensen et al., 2006; Myrup et al., 2008). The human data have been supported by findings from laboratory animals (Gray et al., 2001) and wildlife species (Guillette and Gunderson, 2001). The pathologies in males are collectively named as testicular dysgenesis syndrome (TDS), which has been extensively reviewed (Toppari et al., 2010).
Females also have EDC-originated fertility problems, leading to ovarian dysgenesis syndrome (ODS) (Buck Louis et al., 2011). Although it is not as clearly defined or well accepted as TDS, the use of the term ODS is gaining support (Fowler et al., 2012). The impaired fecundity rate in the U.S. increased from 11 to 15% between 1982 and 2002 (Guzick and Swan, 2006). Although various other confounding factors such as lifestyle changes can contribute to this decline, the role of EDCs are also strongly considered due to supporting studies. The incidence of female reproductive disorders, such as ovarian cancer, is on the rise in a region-dependent manner (reviewed in (Buck Louis et al., 2011)). Furthermore, numerous studies suggest a strong link between exposure to EDCs either during the adult life or during development and increased female reproductive disorders.
6.1. Diethylstilbestrol (DES)
The most convincing human evidence that estrogenic EDC exposure during development can permanently affect female reproduction comes from reports of the effects of DES, a nonsteroidal synthetic estrogen. From the 1940s to the 1970s, DES was prescribed at doses of 5–150 mg⁄ day to prevent miscarriages (Smith and Gabbe 1999). Numerous abnormalities in the reproductive, cardiovascular, and immune systems have since been reported in both male and female offspring of women treated with DES, and similar effects have been demonstrated in animal models (see (Newbold, 2004) for specific examples). Some of these effects, such as irregular cylicity or ovarian cancer, are being observed in the granddaughters of DES-treated women as well (Blatt et al., 2003; Titus-Ernstoff et al., 2006). In light of this multigenerational aspect, whether epigenetic mechanisms are involved is a significant question (Newbold et al., 1998).
6.2. Bisphenol A (BPA)
Bisphenol A is a plasticizer used in the manufacture of polycarbonate plastics and epoxy resins, and its total worldwide production exceeds 6 million tons per year. Exposure to BPA may occur through various materials that humans use daily, such as food and drink cans, baby bottles, and carbonless paper (reviewed in (Zama and Uzumcu, 2010)). As a result, 95% of adults who were tested had detectable levels of BPA in their urine (Calafat et al., 2008). Urine BPA levels of women undergoing infertility treatment are negatively correlated with the number and quality of eggs retrieved, and with serum E2 levels (Mok-Lin et al. 2010; Fujimoto et al., 2011).
6.3. Genistein
Genistein is a well-studied isoflavonoid phytoestrogen derived from soy. Phytoestrogens were originally shown to have endocrine-disrupting potential in domestic animal species: Newborn lambs born to ewes fed clover, a rich source for phytoestrogens, had reproductive abnormalities (reviewed in (Jefferson, 2010)). Soy products are popular because they are lactose-free substitutes for dairy products and are considered to be protective against breast cancer risks (Peeters et al., 2003). However, a major cause for concern is that babies who are fed soy formula consume on average of 6-9 mg/kg body weight, which is many-fold higher than levels approved by the FDA for adults for cardio-protective purposes (Setchell et al., 1998; Johns et al., 2003). It is alarming to know that early life exposure to soy formula is associated with a greater risk of uterine fibroids in adulthood (D’Aloisio et al., 2010).
6.4. Phthalates
Phthalates are used as plasticizers in polyvinyl chlorides, personal care products, some flooring, car products, medical devices, and insect repellent. Although phthalates have been mostly studied for their effects on male reproduction, there is accumulating evidence that they adversely affect female reproduction as well. Di-(2-ethylhexyl) phthalate and its metabolite mono-(2-ethylhexyl) phthalate (MEHP) are the most commonly studied phthalates (reviewed in (Craig et al., 2011)). There is increasing evidence that exposure to phthalates may affect reproductive health in humans (Jurewicz and Hanke, 2011). The levels of phthalates, especially MEHP, in the urine of women undergoing infertility treatments correlate with significantly higher risk of implantation failure (Ehrlich et al., 2010).
6.5. Methoxychlor (MXC)
Methoxychlor (MXC) is a well-studied organochlorine pesticide used as a replacement for dichlorodiphenyltrichloroethane (DDT). It is an estrogenic compound that demonstrates low-affinity binding for estrogen receptors (Cummings, 1997). The major MXC metabolites, 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) and mono-OH MXC, can function as estrogenic, anti-estrogenic, or anti-androgenic compounds (Gaido et al., 2000), and therefore it is used as a model compound (Uzumcu and Zachow, 2007). Epidemiological studies have shown that there is a strong association between developmental exposure to organochlorine pesticides and subsequent female fertility problems (reviewed in (Zama and Uzumcu 2010)). In addition, in a study that examined pesticide-use data and the breast cancer incidence rates in Hispanic females in California, the amount of MXC used was positively associated with increased breast cancer incidence (Mills and Yang, 2006).
7. Experimental evidence from in vivo studies regarding the effects of EDCs in the ovary
Characteristic sets of defects in the ovary are the hallmark of EDC exposure in rodent models. For example, mice injected as little as a single dose of 10 μg/kg DES on E15 and examined at 7 months of age had numerous atretic follicles, and no corpora lutea (an indicator of ovulation) (Wordinger and Highman, 1984). Other studies in rodents of varying doses of DES administered either in utero (E9-16) or neonatally (PND1-5) demonstrated similar defects by adulthood (Haney et al., 1984; Tenenbaum and Forsberg, 1985). Estrous cyclicity was completely disrupted and high levels of testosterone were found. A vacuolated interstitial tissue with lipid droplet inclusions and hemorrhagic cysts were also observed. A recent study proposes that while increased lipid droplet are caused by impaired steroidogenesis due to suppressed LH levels, the hemorrhagic follicles are results of direct effects of DES in the ovary (Kakuta et al., 2012). Furthermore, there was a dose-dependent reduction in the number of litters as well as the number of oocytes ovulated after stimulation with exogenous gonadotropins (McLachlan et al., 1982) with such oocytes when used in IVF showing lower levels of fertilizability, suggesting reduced oocyte quality (Wordinger and Derrenbacker, 1989; Iguchi et al., 1991). Most striking of all, an excessive number of MOFs is found in adult ovaries; such a finding is considered to be an indicator of reduced reproductive lifespan (Iguchi and Takasugi, 1986; Iguchi et al., 1990). The above-mentioned phenotype is mostly recapitulated by exposure to other EDCs, including BPA (Howdeshell et al., 1999; Markey et al., 2005) and genistein (Jefferson et al., 2005; Chen et al., 2007). Phthalates also can decrease E2 production, prolong the estrous cycle, and cause anovulation (Davis et al., 1994; Lovekamp and Davis, 2001); however, effects of phthalates on the ovarian follicular composition are not known.
Such estrogenic actions of these EDCs are mediated via the ER signaling pathway (Kuiper et al., 1998; Drummond and Fuller, 2010; Patisaul and Adewale, 2009). Recent studies have shown that MOFs induced by neonatal exposure to 3 μg/kg DES is mediated by ERβ and not ERα (Kirigaya et al., 2009). DES exposure was shown to reduce oocyte apoptosis (potentially suppressing oocyte nest breakdown) via ERβ signaling mechanisms. Furthermore, it was hypothesized that alterations in the germ cell–to–somatic cell ratio may affect the invasion of pregranulosa cells and basement membrane remodeling during primordial follicle formation (Kim et al., 2009). In contrast to ERβ signaling mechanisms involved in mediating ovarian effects, Couse and colleagues reported that ERα is essential for the mediation of DES effects in the uterus: αERKO female mice exhibited a complete resistance to the effects of DES while βERKO mice did not (Couse and Korach, 2004). Interestingly, in utero treatment of βERKO females with low doses of BPA did not enhance the oocyte meiotic defects usually caused by BPA, suggesting that the compound could act via the ERβ signaling pathway (Susiarjo et al., 2007). Furthermore, microarray-based gene expression analysis in the fetal ovary (E12-14.5) after low-dose BPA exposure revealed that mitotic cell-cycle genes are significantly down-regulated, potentially leading to accelerated meiotic entry of the oogonia and maybe eventually leading to a reduced-size oocyte pool after birth (Lawson et al., 2011). Another recent study in rats showed that BPA reduces the pool of primordial follicles by stimulating initial recruitment after a PND1-7 exposure (Rodriguez et al., 2010). Interestingly, Arase and coworkers reported an increase in estrogen production potentially through increase in Cyp19a1 and Cyp11a1 expression, in the mouse urogenital sinus specifically in the mesenchymal component after BPA exposure between E9 and E15 (Arase et al., 2011). Thus BPA has a double-edged effect on mitosis and meiosis as well as pre- and postnatal steroidogenesis.
8. Epigenetic mechanisms associated with the female reproductive system
Although there are now well-documented studies of the physiological and morphological effects of EDCs on the ovary, the early research providing evidence of an epigenetic component of EDC actions was predominantly on the uterus. For example, it is well known that DES caused T-shaped uteri and clear cell adenocarcinoma of the uterus, cervix, and vagina in women whose mothers were exposed to DES during pregnancy (Herbst et al., 1971). In the numerous animal studies validating these human reports, developmentally DES-treated mice manifest malformations of the uterus, squamous metaplasia of the luminar and glandular epithelium, endometrial hyperplasia and leiomyomas, and oviductal proliferative lesions (McLachlan et al., 1980; Kitajewski and Sassoon, 2000). Ovariectomized animals when supplemented with E2 are able to respond by a transient increase in gene expression and concomitant uterine proliferation and growth. When such a stimulus was removed, the uterus returned to its unstimulated state. However, when DES or E2 is administered during neonatal development, expression of immediate early genes such as c-fos, c-jun, and c-myc as well as lactoferrin and EGF are upregulated even into adulthood (Nelson et al., 1994; Falck and Forsberg, 1996). This was associated with hypomethylation of the promoter region of the lactoferrin gene in the adult uterus (Li et al., 1997). However, if animals were exposed for the same interval during adulthood, no such methylation or expression defects were observed, indicating the importance of developmental exposure. Subsequently, it was also found that exon 4 of the c-fos gene was extensively hypomethylated while the promoter region and intron 1 were unaffected, thereby potentially allowing for the upregulation of c-fos expression (Li et al., 2003). Furthermore, recent work by Block and coworkers has shown that DES disrupts the regionalization of expression of Hoxa-9 and Hoxa-10 and the homeotic anterior transformations associated with hypermethylation in the promoter and intron 1 regions of Hoxa-10 gene (Block et al., 2000). Additionally, ERα induction is necessary for activation of estrogen-responsive gene expression in the uterus, including that of the lactoferrin and c-fos genes (Nelson et al., 1994; Falck and Forsberg, 1996). Since many of the above genes are downstream of ER signaling, which is involved in direct actions of EDCs, it is imperative to thoroughly examine the potential role of epigenetic mechanisms in the regulation of ER expression after EDC exposure. In addition, PI3K/Akt signaling downstream of membrane-associated ER signaling caused reduction in trimethylation of the histone H3K27 in response to E2 and DES exposures. More interestingly, activation of this non-genomic signaling caused reprogramming of the uterine gene expression profile (Bredfeldt et al., 2010). Another example is that of the investigation by Tang and colleagues (Tang et al., 2008), wherein it was demonstrated that neonatal DES/genistein exposure reduced DNA methylation and increased gene expression of nucleosomal binding protein 1 in adult uteri. These studies highlighted the age-dependent aspect of epigenetic reprogramming and also its interaction with steroid hormones.
9. Epigenetic effects of methoxychlor in the ovary
Early studies that exposed females to MXC for 6-10 weeks during both in utero and early postnatal development periods, show that exposed females have acceleration of the vaginal opening (sign of puberty), acceleration of the onset of the first estrus, irregular cycles with persistent vaginal estrus, reduced pregnancy rate and litter size despite apparent mating, and early reproductive senescence (Gray et al., 1989; Chapin et al., 1997; You et al., 2002). Serum E2 and P4 levels were altered with increased FSH levels (Chapin et al., 1997). The effects on the ovary were dramatic, with both folliculogenesis and ovulation being inhibited. In our more recent study, female rats were treated for 12 days during fetal and neonatal development (E19-PND7) with 100 mg/kg/day MXC, higher than the dose normally encountered by humans or animals on a daily basis, but similar to doses used in the above studies. The exposed females displayed similar abnormalities in reproductive parameters and in ovarian morphology by adulthood despite the fact that the treatment period was much shorter (Armenti et al., 2008). A close examination of follicle composition showed that developmental MXC treatment did not affect the total number of follicles or follicles at primary and secondary stages in adult females. However, the number of pre-antral and early antral follicles was increased and the number of CL was reduced, with numerous cystic follicles. Immunohistochemical staining and quantification of expression patterns of important regulators of ovarian functions revealed that while LHR, CYP11A1, and CYP19A1 levels were reduced, levels of anti-Mullerian hormone and androgen receptor were increased, and levels of StAR and ERα were unchanged (Armenti et al., 2008). Especially noteworthy was that ERβ level was unchanged in primary and secondary follicles, yet decreased dramatically in peri-antral stage follicles, which are very responsive to gonadotropins (unpublished observations). These data suggest that hormone-responsive follicles are most affected by EDC exposure.
Epigenetic analyses using bisulfite-sequencing PCR and methylation-specific PCR showed that MXC caused hypermethylation in multiple CpGs in two CGIs in ERβ promoter sequences (Zama and Uzumcu, 2009). Further analysis has shown that the DNA methylation levels in the promoter regions of these genes were unchanged in neonatal ovaries (PND7) immediately after the exposure (Zama and Uzumcu, unpublished). These data demonstrate the age-dependence and hormone responsiveness of the epigenetic changes, which has also been shown in other tissues (e.g., uterus) with other compounds (e.g., DES, genistein) (Tang et al., 2008). The global DNA methylation analysis using methylation-sensitive arbitrarily primed PCR (AP-PCR) showed that there were multiple loci that were hypermethylated in MXC-treated ovaries (Zama and Uzumcu, 2009). The majority of candidates were those encoding transcription factors or ribosomal proteins. Our most recent studies have involved genome-wide methylation analyses. Since assays such as AP-PCR depend on the availability of restriction enzyme sites, they may not capture all possible methylation events. Furthermore, since DNA methylation is dynamic and reversible (Bhutani et al., 2011), we hypothesized that genome-wide methylation analyses at both ages would be highly informative. Therefore, we examined ovaries: (1) immediately after exposure, at PND7; and (2) after animals reached adulthood, at PND60. The methylated DNA immunoprecipitation protocol (MeDIP), which employs an antibody against the 5-methyl cytosine moiety, was conducted followed by hybridization to microarrays that included probes spanning the promoter regions as well as the CpG islands of all RefSeq genes in the rat genome. This was in an effort to examine not only individual gene methylation patterns but also potential gene networks that were exhibiting altered methylation patterns in response to EDC exposure, immediately after exposure as well as in adulthood, long after the exposure ends. We have now found that multiple loci belonging to critical ovarian signaling pathways (e.g., PTEN signaling, IGF signaling) were hypermethylated and their gene expression was suppressed. These pathways are essential for early folliculogenesis as well as follicular maturation and ovulation.
10. Conclusions and future studies
Several aspects should be considered when the effects of EDCs in the ovary are evaluated. The ovary is the major source of the hormones that are mimicked by EDCs. The same hormones that the ovary produces also regulate the ovarian functions. Thus, the effects of EDCs in the ovary can be twofold. In addition, the ovary of a reproductive-aged adult consists of follicles in various developmental stages as well as corpora lutea. Therefore, both developing and adult ovaries require involvement of numerous factors that are spatio-temporally regulated. It is likely that these factors are regulated by various epigenetic mechanisms, including DNA methylation (Vanselow et al., 2010), histone modifications (LaVoie, 2005), and ncRNAs (Carletti et al., 2010; Torley et al., 2011; da Silveira et al., 2012).
One of the most pressing issues in the field of epigenetics and EDCs is the transgenerational (TG) epigenetic effects. All epigenetic actions in the ovary have the potential to affect the subsequent generation(s) via the female germ cells. The effects that are transmitted through epigenetic mechanisms to an unexposed generation are described as epigenetic TG inheritance (Youngson and Whitelaw, 2008). There have been reports in males that EDCs can cause TG epigenetic effects in experimental animals (Anway et al., 2005; Guerrero-Bosagna et al., 2010). Since then, there have been additional reports concerning the effects of EDCs in males (Salian et al., 2009; Stouder and Paoloni-Giacobino, 2011). Although the described effects are also transmitted by the female germline, the impacts are less obvious. The potential reasons for the less prominent effects are not known but can be related to the timing of the treatment and the nature of the chemicals. Importantly, there are some reports in humans regarding TG effects of the environment (for examples, see Section 6.1 and (Titus-Ernstoff et al., 2006).
Additional key questions or challenges related to studying the epigenetic effects of EDCs in the ovary include the following:
Follicular stage–specific effects in the ovary: Tissues in the body are composed of different cell types differentiations of which are believed to be primarily due to epigenetic modifications specific to a given cell type. Therefore, the epigenetic analysis in a whole tissue consisting of various cell types is considered to be problematic. An additional complexity in the postnatal ovary is the presence of the follicles at different stages of their development. When epigenetic analysis in the ovary is being considered, not only should different cell types be examined individually but also these cell types should be separated based on the follicle stage from which they are obtained.
EDC mixture effects: Most studies examining the effects of EDCs in the ovary as well as in other organs are conducted with single chemicals. However, individuals are normally exposed to mixture of these chemicals in daily life. Therefore, studies that are examining the effects of mixtures with various approaches have been intensifying. These studies in other organs have shown that when chemicals are combined their impact increases synergistically—much more dramatically than additive effects of individual compounds (Jacobsen et al., 2010; Rider et al., 2010). The mixture studies further show the complexity of the potential harmful effects of the EDCs as mixtures (Kortenkamp et al., 2007).
Epigenome-wide effects: The genome is relatively more resistant to the effects of environmental factors that individuals are likely to encounter daily, such as EDCs. In contrast, the epigenome can be vulnerable to such effects. In addition, as compared to the primary DNA sequence, the epigenome is much complex and dynamic. Furthermore, the importance of epigenome in the developmental origins of health and diseases is widely accepted. Therefore, a comprehensive understanding of the effects the EDCs requires studying not only the methylome but also whole epigenome (including histone modifications and ncRNAs, especially miRNA). Such a broader and more precise understanding of epigenetic effects of EDCs will enable the development of effective prevention and therapy.
Acknowledgments
The authors thank Dr. Kathy Manger for her assistance in the preparation of this manuscript. The research results from our laboratory described in this review supported in part by NIEHS grants ES013854 and ES017059 and the NIEHS-sponsored UMDNJ Center for Environmental Exposures and Disease grant NIEHS ES005022. Dr. Elif Oruc, visiting scholar from Cukurova University, Turkey, is supported in part by TUBITAK-BIDEB International Postdoctoral Research Scholarship Program.
Footnotes
Conflicts of interest None to report.
References
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase S, Ishii K, Igarashi K, Aisaki K, Yoshio Y, Matsushima A, Shimohigashi Y, Arima K, Kanno J, Sugimura Y. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biol Reprod. 2011;84:734–742. doi: 10.1095/biolreprod.110.087502. [DOI] [PubMed] [Google Scholar]
- Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avner P, Heard E. X-chromosome inactivation: counting, choice and initiation. Nat Rev Genet. 2001;2:59–67. doi: 10.1038/35047580. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc Biol Sci. 1995;262:37–43. doi: 10.1098/rspb.1995.0173. [DOI] [PubMed] [Google Scholar]
- Bartolomei MS. Genomic imprinting: employing and avoiding epigenetic processes. Genes Dev. 2009;23:2124–2133. doi: 10.1101/gad.1841409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146:866–872. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt J, Van Le L, Weiner T, Sailer S. Ovarian carcinoma in an adolescent with transgenerational exposure to diethylstilbestrol. J Pediatr Hematol Oncol. 2003;25:635–636. doi: 10.1097/00043426-200308000-00009. [DOI] [PubMed] [Google Scholar]
- Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. Faseb J. 2000;14:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- Boisen KA, Kaleva M, Main KM, Virtanen HE, Haavisto AM, Schmidt IM, Chellakooty M, Damgaard IN, Mau C, Reunanen M, Skakkebaek NE, Toppari J. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363:1264–1269. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod. 2004;71:1712–1723. doi: 10.1095/biolreprod.104.028175. [DOI] [PubMed] [Google Scholar]
- Brosnan CA, Voinnet O. The long and the short of noncoding RNAs. Curr Opin Cell Biol. 2009;21:416–425. doi: 10.1016/j.ceb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Cooney MA, Peterson CM. The ovarian dysgenesis syndrome. J Dev Orig Health Dis. 2011;2:25–35. [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod. 2010;83:286–295. doi: 10.1095/biolreprod.109.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. British Medical Journal. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KD, Korach KS. Potential biological functions emerging from the different estrogen receptors. Ann N Y Acad Sci. 2006;1092:361–373. doi: 10.1196/annals.1365.033. [DOI] [PubMed] [Google Scholar]
- Chang SC, Tucker T, Thorogood NP, Brown CJ. Mechanisms of X-chromosome inactivation. Front Biosci. 2006;11:852–866. doi: 10.2741/1842. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Harris MW, Davis BJ, Ward SM, Wilson RE, Mauney MA, Lockhart AC, Smialowicz RJ, Moser VC, Burka LT, Collins BJ. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 1997;40:138–157. doi: 10.1006/faat.1997.2381. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17. doi: 10.1016/j.gene.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor-alpha mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology. 2004;205:55–63. doi: 10.1016/j.tox.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142:633–646. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- D’Aloisio AA, Baird DD, DeRoo LA, Sandler DP. Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the sister study. Environ Health Perspect. 2010;118:375–381. doi: 10.1289/ehp.0901423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-Secreted Vesicles in Equine Ovarian Follicular Fluid Contain miRNAs and Proteins: A Possible New Form of Cell Communication Within the Ovarian Follicle. Biol Reprod. 2012;86:71, 1–10. doi: 10.1095/biolreprod.111.093252. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128:216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A, Fuller P. The importance of ER{beta} signalling in ovarian function. J Endocrinol. 2010;205:15–23. doi: 10.1677/JOE-09-0379. [DOI] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem. 2003;88:899–910. doi: 10.1002/jcb.10464. [DOI] [PubMed] [Google Scholar]
- Ehrlich SR, Meeker JD, Williams PL, Wright D, Petrozza JC, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with increased risk of implantation among women, undergoing IVF. Fertil Steril. 2010;94:S73. [Google Scholar]
- Falck L, Forsberg JG. Immunohistochemical studies on the expression and estrogen dependency of EGF and its receptor and C-fos proto-oncogene in the uterus and vagina of normal and neonatally estrogen-treated mice. Anat Rec. 1996;245:459–471. doi: 10.1002/(SICI)1097-0185(199607)245:3<459::AID-AR2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–1014. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Bellingham M, Sinclair KD, Evans NP, Pocar P, Fischer B, Schaedlich K, Schmidt JS, Amezaga MR, Bhattacharya S, Rhind SM, O’Shaughnessy PJ. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol Cell Endocrinol. 2012;355:231–239. doi: 10.1016/j.mce.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril. 2011;95:1816–1819. doi: 10.1016/j.fertnstert.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58:852–858. [PubMed] [Google Scholar]
- Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol (Paris) 2010;71:132–143. doi: 10.1016/j.ando.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr., Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol. 1989;12:92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One. 2010;5:pii:e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ, Jr., Gunderson MP. Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction. 2001;122:857–864. doi: 10.1530/rep.0.1220857. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Swan S. The decline of infertility: apparent or real? Fertil Steril. 2006;86:524–526. doi: 10.1016/j.fertnstert.2006.05.027. discussion 534. [DOI] [PubMed] [Google Scholar]
- Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- Haney AF, Newbold RR, McLachlan JA. Prenatal diethylstilbestrol exposure in the mouse: effects on ovarian histology and steroidogenesis in vitro. Biol Reprod. 1984;30:471–478. doi: 10.1095/biolreprod30.2.471. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: Archive of the prenatal environment. Epigenetics. 2009;4:526–531. doi: 10.4161/epi.4.8.10265. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Hirose M, Sarui K, Sunagawa A. Correlation between nuclear histone acetylation and casein messenger RNA induction in the mammary gland. J Biochem. 1985;97:781–789. doi: 10.1093/oxfordjournals.jbchem.a135118. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev. 2009;12:206–223. doi: 10.1080/10937400902902062. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Kamiya K, Uesugi Y, Sayama K, Takasugi N. In vitro fertilization of oocytes from polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. In Vivo. 1991;5:359–363. [PubMed] [Google Scholar]
- Iguchi T, Takasugi N. Polyovular follicles in the ovary of immature mice exposed prenatally to diethylstilbestrol. Anat Embryol (Berl) 1986;175:53–55. doi: 10.1007/BF00315455. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Neonatal estrogen exposure inhibits steroidogenesis in the developing rat ovary. Dev Dyn. 2001;221:443–453. doi: 10.1002/dvdy.1162. [DOI] [PubMed] [Google Scholar]
- Jacobsen PR, Christiansen S, Boberg J, Nellemann C, Hass U. Combined exposure to endocrine disrupting pesticides impairs parturition, causes pup mortality and affects sexual differentiation in rats. Int J Androl. 2010;33:434–442. doi: 10.1111/j.1365-2605.2009.01046.x. [DOI] [PubMed] [Google Scholar]
- Jefferson WN. Adult ovarian function can be affected by high levels of soy. J Nutr. 2010;140:2322S–2325S. doi: 10.3945/jn.110.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns P, Dowlati L, Wargo W. Determination of isoflavones in ready-to-feed soy-based infant formula. J AOAC Int. 2003;86:72–78. [PubMed] [Google Scholar]
- Jorgensen N, Asklund C, Carlsen E, Skakkebaek NE. Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int J Androl. 2006;29:54–61. doi: 10.1111/j.1365-2605.2005.00635.x. discussion 105-108. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W. Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int J Occup Med Environ Health. 2011;24:115–141. doi: 10.2478/s13382-011-0022-2. [DOI] [PubMed] [Google Scholar]
- Kakuta H, Tanaka M, Chambon P, Watanabe H, Iguchi T, Sato T. Involvement of gonadotropins in the induction of hypertrophy-hyperplasia in the interstitial tissues of ovaries in neonatally diethylstilbestrol-treated mice. Reprod Toxicol. 2012;33:35–44. doi: 10.1016/j.reprotox.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Kim H, Nakajima T, Hayashi S, Chambon P, Watanabe H, Iguchi T, Sato T. Effects of diethylstilbestrol on programmed oocyte death and induction of polyovular follicles in neonatal mouse ovaries. Biol Reprod. 2009;81:1002–1009. doi: 10.1095/biolreprod.108.070599. [DOI] [PubMed] [Google Scholar]
- Kirigaya A, Kim H, Hayashi S, Chambon P, Watanabe H, Lguchi T, Sato T. Involvement of estrogen receptor beta in the induction of polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. Zoolog Sci. 2009;26:704–712. doi: 10.2108/zsj.26.704. [DOI] [PubMed] [Google Scholar]
- Kitajewski J, Sassoon D. The emergence of molecular gynecology: homeobox and Wnt genes in the female reproductive tract. Bioessays. 2000;22:902–910. doi: 10.1002/1521-1878(200010)22:10<902::AID-BIES5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. Are cadmium and other heavy metal compounds acting as endocrine disrupters? Met Ions Life Sci. 2011;8:305–317. doi: 10.1039/9781849732116-00305. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Faust M, Scholze M, Backhaus T. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(Suppl 1):106–114. doi: 10.1289/ehp.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- LaVoie HA. Epigenetic control of ovarian function: the emerging role of histone modifications. Mol Cell Endocrinol. 2005;243:12–18. doi: 10.1016/j.mce.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T, Hunt PA. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol Reprod. 2011;84:79–86. doi: 10.1095/biolreprod.110.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kang DW, Hudgins-Spivey S, Krust A, Lee EY, Koo Y, Cheon Y, Gye MC, Chambon P, Ko C. Theca-specific estrogen receptor-alpha knockout mice lose fertility prematurely. Endocrinology. 2009;150:3855–3862. doi: 10.1210/en.2008-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–673. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinog. 2003;38:78–84. doi: 10.1002/mc.10147. [DOI] [PubMed] [Google Scholar]
- Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, McLachlan JA, Negishi M. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57:4356–4359. [PubMed] [Google Scholar]
- Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172:217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- Luense LJ, Veiga-Lopez A, Padmanabhan V, Christenson LK. Developmental programming: gestational testosterone treatment alters fetal ovarian gene expression. Endocrinology. 2011;152:4974–4983. doi: 10.1210/en.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37:1344–1349. doi: 10.1016/j.biocel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Shah HC, Hogan MD, Dixon RL. Reduced fertility in female mice exposed transplacentally to diethylstilbestrol (DES) Fertil Steril. 1982;38:364–371. doi: 10.1016/s0015-0282(16)46520-9. [DOI] [PubMed] [Google Scholar]
- Mills PK, Yang R. Regression analysis of pesticide use and breast cancer incidence in California Latinas. J Environ Health. 2006;68:15–22. quiz 43-14. [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrup C, Westergaard T, Schnack T, Oudin A, Ritz C, Wohlfahrt J, Melbye M. Testicular cancer risk in first- and second-generation immigrants to Denmark. J Natl Cancer Inst. 2008;100:41–47. doi: 10.1093/jnci/djm276. [DOI] [PubMed] [Google Scholar]
- Namihira M, Kohyama J, Abematsu M, Nakashima K. Epigenetic mechanisms regulating fate specification of neural stem cells. Philos Trans R Soc Lond B Biol Sci. 2008;363:2099–2109. doi: 10.1098/rstb.2008.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KG, Sakai Y, Eitzman B, Steed T, McLachlan J. Exposure to diethylstilbestrol during a critical developmental period of the mouse reproductive tract leads to persistent induction of two estrogen-regulated genes. Cell Growth Differ. 1994;5:595–606. [PubMed] [Google Scholar]
- Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19:1655–1663. doi: 10.1093/carcin/19.9.1655. [DOI] [PubMed] [Google Scholar]
- Obata Y, Kono T. Maternal primary imprinting is established at a specific time for each gene throughout oocyte growth. J Biol Chem. 2002;277:5285–5289. doi: 10.1074/jbc.M108586200. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters PH, Keinan-Boker L, van der Schouw YT, Grobbee DE. Phytoestrogens and breast cancer risk. Review of the epidemiological evidence. Breast Cancer Res Treat. 2003;77:171–183. doi: 10.1023/a:1021381101632. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CV, Furr JR, Wilson VS, Gray LE., Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 2010;33:443–462. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez HA, Santambrosio N, Santamaria CG, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol. 2010;30:550–557. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci. 2009;85:11–18. doi: 10.1016/j.lfs.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Santos F, Dean W. Epigenetic reprogramming during early development in mammals. Reproduction. 2004;127:643–651. doi: 10.1530/rep.1.00221. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol. 2005;19:1740–1751. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- Sirotkin AV. Growth factors controlling ovarian functions. J Cell Physiol. 2011;226:2222–2225. doi: 10.1002/jcp.22588. [DOI] [PubMed] [Google Scholar]
- Smith OW, Gabbe SG. Diethylstilbestrol in the prevention and treatment of complications of pregnancy. 1948. Am J Obstet Gynecol. 1999;181:1570–1571. doi: 10.1016/s0002-9378(99)70409-6. [DOI] [PubMed] [Google Scholar]
- Sokka T, Huhtaniemi I. Ontogeny of gonadotrophin receptors and gonadotrophin-stimulated cyclic AMP production in the neonatal rat ovary. J Endocrinol. 1990;127:297–303. doi: 10.1677/joe.0.1270297. [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- Stouder C, Paoloni-Giacobino A. Specific transgenerational imprinting effects of the endocrine disruptor methoxychlor on male gametes. Reproduction. 2011;141:207–216. doi: 10.1530/REP-10-0400. [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, Redmon JB, Wang C, Overstreet JW. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5931. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum A, Forsberg JG. Structural and functional changes in ovaries from adult mice treated with diethylstilboestrol in the neonatal period. J Reprod Fertil. 1985;73:465–477. doi: 10.1530/jrf.0.0730465. [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Whitelaw PF, Bremner WJ, Millar MR, Smyth CD, Hillier SG. Developmental regulation of androgen receptor in rat ovary. J Endocrinol. 1995;145:535–543. doi: 10.1677/joe.0.1450535. [DOI] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Wise LA, Palmer J, Hyer M, Kaufman R, Adam E, Strohsnitter W, Noller K, Herbst AL, Gibson-Chambers J, Hartge P, Hoover RN. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES) Int J Epidemiol. 2006;35:862–868. doi: 10.1093/ije/dyl106. [DOI] [PubMed] [Google Scholar]
- Toppari J, Virtanen HE, Main KM, Skakkebaek NE. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol Teratol. 2010;88:910–919. doi: 10.1002/bdra.20707. [DOI] [PubMed] [Google Scholar]
- Torley KJ, da Silveira JC, Smith P, Anthony RV, Veeramachaneni DN, Winger QA, Bouma GJ. Expression of miRNAs in ovine fetal gonads: potential role in gonadal differentiation. Reprod Biol Endocrinol. 2011;9:2. doi: 10.1186/1477-7827-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM. Epigenetic responses to environmental change and their evolutionary implications. Philos Trans R Soc Lond B Biol Sci. 2009;364:3403–3418. doi: 10.1098/rstb.2009.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Abe K, Miura A, Yuzuriha M, Zubair M, Noguchi M, Niwa K, Kawase Y, Kono T, Matsuda Y, Fujimoto H, Shibata H, Hayashizaki Y, Sasaki H. The paternal methylation imprint of the mouse H19 locus is acquired in the gonocyte stage during foetal testis development. Genes Cells. 2000;5:649–659. doi: 10.1046/j.1365-2443.2000.00351.x. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Zachow R. Developmental exposure to environmental endocrine disruptors: consequences within the ovary and on female reproductive function. Reprod Toxicol. 2007;23:337–352. doi: 10.1016/j.reprotox.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanselow J, Spitschak M, Nimz M, Furbass R. DNA methylation is not involved in preovulatory down-regulation of CYP11A1, HSD3B1, and CYP19A1 in bovine follicles but may have a role in permanent silencing of CYP19A1 in large granulosa lutein cells. Biol Reprod. 2010;82:289–298. doi: 10.1095/biolreprod.109.079251. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Derrenbacker J. In utero exposure of mice to diethylstilbestrol alters neonatal ovarian follicle growth and development. Acta Anat (Basel) 1989;134:312–318. doi: 10.1159/000146708. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Highman B. Histology and ultrastructure of the adult mouse ovary following a single prenatal exposure to diethylstilbestrol. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:241–253. doi: 10.1007/BF02889867. [DOI] [PubMed] [Google Scholar]
- Yagi M, Koshland ME. Expression of the J chain gene during B cell differentiation is inversely correlated with DNA methylation. Proc Natl Acad Sci U S A. 1981;78:4907–4911. doi: 10.1073/pnas.78.8.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zhang CP, Li L, Huang L, Ji SY, Lu CL, Fan CH, Cai H, Ren Y, Hu ZY, Gao F, Liu YX. Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology. 2009;151:774–782. doi: 10.1210/en.2009-0751. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biol Reprod. 2006;75:924–932. doi: 10.1095/biolreprod.106.051813. [DOI] [PubMed] [Google Scholar]
- Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- You L, Casanova M, Bartolucci EJ, Fryczynski MW, Dorman DC, Everitt JI, Gaido KW, Ross SM, Heck Hd H. Combined effects of dietary phytoestrogen and synthetic endocrine-active compound on reproductive development in Sprague-Dawley rats: genistein and methoxychlor. Toxicol Sci. 2002;66:91–104. doi: 10.1093/toxsci/66.1.91. [DOI] [PubMed] [Google Scholar]
- Younglai EV, Collins JA, Foster WG. Canadian semen quality: an analysis of sperm density among eleven academic fertility centers. Fertil Steril. 1998;70:76–80. doi: 10.1016/s0015-0282(98)00118-6. [DOI] [PubMed] [Google Scholar]
- Youngson NA, Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–257. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]
- Zama AM, Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009;150:4681–4691. doi: 10.1210/en.2009-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zama AM, Uzumcu M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: An ovarian perspective. Front Neuroendocrinol. 2010;31:420–439. doi: 10.1016/j.yfrne.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


