Figure 1.
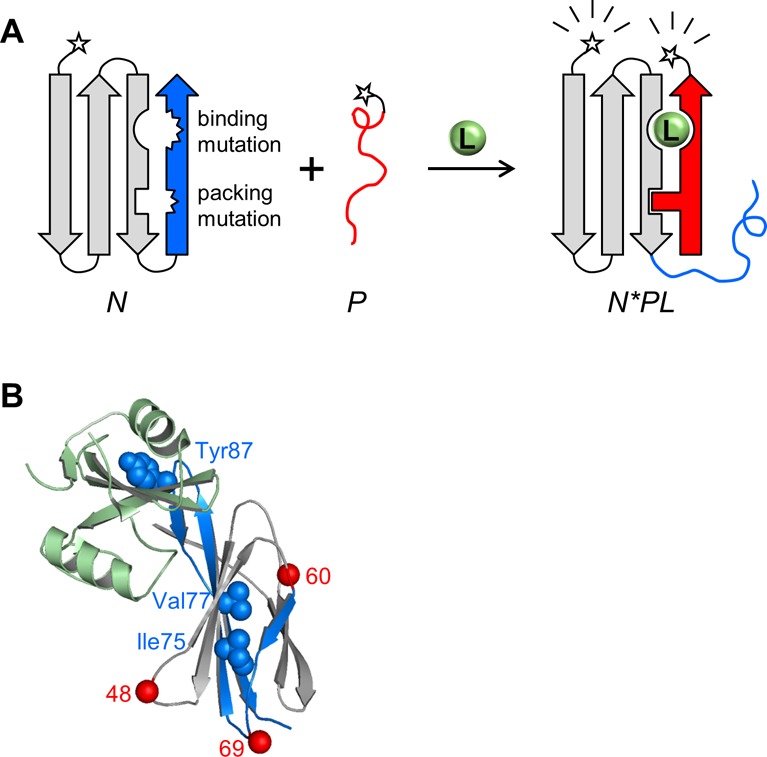
Schematic of FREX and X-ray structure of the FN3-HA4/SH2 complex. (A) An N- or C-terminal segment (blue) of an arbitrary binding protein N (gray) is chosen such that it contains at least one critical ligand binding residue. The blue segment is duplicated to generate peptide P (red). FRET donor and acceptor groups (stars) are attached to N and P at either terminus. The ligand binding residue is mutated in the blue sequence, along with a residue at the packing interface between the blue and gray regions. The resulting protein (N) is destabilized but still folded; consequently, the binary complex of N and P (N*P) does not form to a significant extent. Only in the presence of a ligand (L) do the blue and red segments exchange to generate the ternary complex (N*PL). Formation of N*PL is driven by the restoration of binding and packing interactions, supplied by the WT residues at those positions in P. (B) FN3-HA4 is shown with the starting positions of P48, P60, and P69 indicated by red spheres. Blue/gray color coding is the same as that in panel A; blue denotes the P60 segment. Side chains of binding (Tyr87) and packing (Ile75/Val77) residues are represented by blue spheres. SH2 is colored light green.
