Abstract
OBJECTIVE
We sought to identify hospital characteristics associated with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) carriage among inpatients.
DESIGN
Prospective cohort study.
SETTING
Orange County, California.
PARTICIPANTS
Thirty hospitals in a single county.
METHODS
We collected clinical MRSA isolates from inpatients in 30 of 31 hospitals in Orange County, California, from October 2008 through April 2010. We characterized isolates by spa typing to identify CA-MRSA strains. Using California’s mandatory hospitalization data set, we identified hospital-level predictors of CA-MRSA isolation.
RESULTS
CA-MRSA strains represented 1,033 (46%) of 2,246 of MRSA isolates. By hospital, the median percentage of CA-MRSA isolates was 46% (range, 14%–81%). In multivariate models, CA-MRSA isolation was associated with smaller hospitals (odds ratio [OR], 0.97, or 3% decreased odds of CA-MRSA isolation per 1,000 annual admissions; P < .001), hospitals with more Medicaid-insured patients (OR, 1.2; P = .002), and hospitals with more patients with low comorbidity scores (OR, 1.3; P < .001). Results were similar when restricted to isolates from patients with hospital-onset infection.
CONCLUSIONS
Among 30 hospitals, CA-MRSA comprised nearly half of MRSA isolates. There was substantial variability in CA-MRSA penetration across hospitals, with more CA-MRSA in smaller hospitals with healthier but socially disadvantaged patient populations. Additional research is needed to determine whether infection control strategies can be successful in targeting CA-MRSA influx.
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) was first identified in the 1990s among healthy children and adults without healthcare exposure or traditional risk factors for MRSA.1-3 In the past 2 decades, CA-MRSA prevalence has steadily increased in the community and has become especially common in settings such as gyms, the military, day care facilities, and schools and among high-risk populations, such as injection drug users and men who have sex with men.4-6 CA-MRSA is genetically distinct from healthcare-associated strains of MRSA (HA-MRSA), is typically susceptible to more classes of antibiotics, and has historically caused less invasive disease, such as skin and soft-tissue infections. However, there are increasing reports of CA-MRSA causing hospital-onset disease in individual hospitals, which raises the possibility that CA-MRSA strains may become widely endemic in hospitals in the near future.7-9
In the United States, the majority of CA-MRSA isolates consist of the pulsed-field gel electrophoresis (PFGE) clone USA300 (analogous to spa type t008, multilocus sequence type ST8). As USA300 emerges in healthcare settings, there is concern that this and other CA-MRSA strains may cause more severe disease than community or HA-MRSA strains in hospitalized patients. The ability of USA300 to cause severe disease may be attributable to several distinguishing traits; USA300 carries a unique arginine catabolic element that may confer increased fitness and expresses higher levels of virulence factors, such as Panton-Valentine leukocidin and alphatoxin.10,11 In hospitals, USA300 has proven able to cause invasive illness, including bloodstream infections,9 and case reports have newly identified USA300 as a cause of rapidly progressive conditions, such as necrotizing pneumonia and fasciitis.12,13 Furthermore, there is evidence that USA300 may be more transmissible because of its greater fitness or higher growth rate.14,15 The potential for CA-MRSA strains to cause more severe disease or lead to increased transmission makes the penetration of CA-MRSA into healthcare settings of potential concern, although the few existing comparative studies have yet to confirm increased severity of infection due to CA-MRSA.16,17
CA-MRSA penetration into hospitals has not been explored systematically or among a large number of hospitals. It is currently unknown whether CA-MRSA is widely endemic, partly because of inconsistent definitions of CA-MRSA based on onset of infection or antibiotic susceptibility profile. Endemic CA-MRSA may lead to additional increases in MRSA burden among hospitals, particularly if CA-MRSA proves to be more transmissible because of greater fitness. Understanding factors associated with CA-MRSA penetration into hospitals may inform infection control strategies targeting community sources. We collected MRSA isolates from a county network of hospitals and identified hospital characteristics associated with CA-MRSA isolation among inpatients.
METHODS
We conducted a prospective collection of clinical MRSA isolates from 30 of 31 hospitals in Orange County, California, from October 2008 to April 2010. The remaining hospital was small and did not participate, because it was closing. This study was approved by the institutional review board of the University of California Regents. Because patient identifying information was not collected, we did not obtain informed consent from patients who provided MRSA samples.
Isolate Collection
Clinical (nonscreening) isolates of MRSA from unique patients were collected from hospital microbiology laboratories. Hospitals were instructed to collect nonblood MRSA isolates from consecutive unique patients up to a total of 100 or for a duration of 12 months, whichever came first. Additionally, we collected up to 20 blood-derived isolates per month from each hospital. Isolates from patients not admitted to the hospital (eg, emergency department patients) were excluded from the study, as were screening isolates. Samples were batched and delivered using soy agar slants to the Orange County Public Health Laboratory. For repeated confirmation of MRSA, isolates were plated on selective media for MRSA (BD CHROMagar). MRSA strains were stored at −65°C in 15% glycerol Brucella broth.
Specimen Data and Hospital Characteristics
We collected the hospital day for onset of infection for each isolate. We defined hospital onset (HO) as isolation 3 or more days after hospital admission and community onset (CO) as isolation less than 3 days after hospital admission. We also collected the specimen source (blood, wounds, sputum, urine, or other). Hospital characteristics were obtained from a mandatory California hospital discharge data set18 and included annual admissions, hospital type (acute care vs long-term acute care facility and pediatric vs adult), and the percentage of patients at each hospital with the following characteristics: male sex, non-White race, Hispanic ethnicity, Medicaid insurance, Medicare insurance, and Romano comorbidity score19 and its components (acute myocardial infarction, peripheral vascular disease, stroke, dementia, chronic obstructive pulmonary disease, rheumatologic disease, ulcer, paralysis, renal disease, AIDS, diabetes, liver disease, and cancer). Romano score was dichotomized around the median value (high Romano score is greater than 3).
Laboratory Methods and Molecular Typing
All strains were shipped to Imperial College London in the United Kingdom and stored at −80°C for staphylococcal protein A (spa) typing; spa typing was performed on all isolates and analyzed as described previously.20 Isolates with spa type t008 were considered CA-MRSA. Multilocus sequence typing (MLST) and PFGE were performed on a randomly sampled subset of the isolates (n = 288) to confirm spa assignments for MRSA strain types, according to methods described previously.21 We randomly selected 1 isolate per hospital for the 10 most common spa types and 1 isolate per remaining spa types.
Analysis
We calculated both the prevalence of MRSA and CA-MRSA per 10,000 admissions and the percentage of isolates corresponding to CA-MRSA from each hospital. We determined how often CA-MRSA and HA-MRSA strains were CO versus HO. We tested the association of hospital-level characteristics described above with individual carriage of CA-MRSA. Variables with P < .1 from bivariate models entered a multivariate individual-level generalized estimating equation (GEE) model, clustered by hospital, and were retained at α = 0.05. We repeated our analyses, stratifying by HO versus CO of infection.
RESULTS
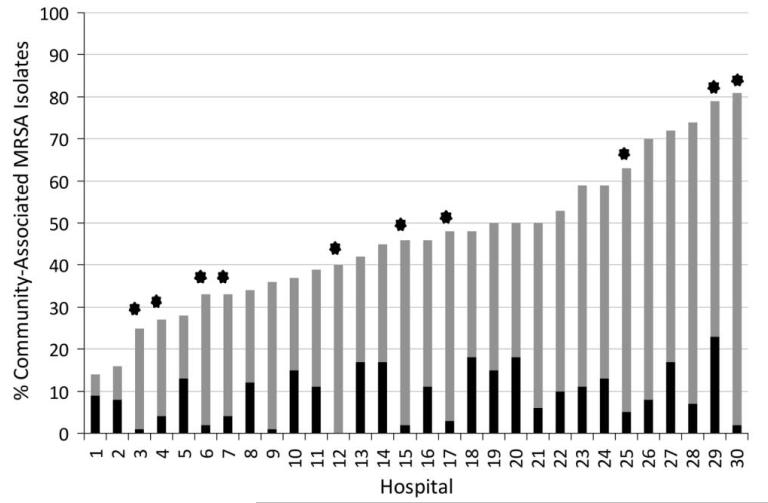
Facility characteristics are listed in Table 1. We collected 2,246 isolates from 30 hospitals during the time period October 2008 to April 2010; a median of 74 isolates were collected from each hospital (range, 4–143). Thirteen hospitals reached the goal of 100 collected isolates within 12 months (median period, 9 months (range, 6–11.5 months); the remaining smaller hospitals collected a median of 38 isolates (range, 4–91 isolates). The median prevalence of total MRSA and CA-MRSA across OC hospitals were 106 isolates per 10,000 admissions (range, 35–571) and 50 isolates per 10,000 admissions (range, 14–178), respectively. Overall, 46% of isolates (1,033 of 2,246) were spa type t008 (CA-MRSA). The median percentage of CA-MRSA isolates per hospital was 46% (range 14%–81%; Figure 1). The 2 other most common spa types were t002, which comprised 15% of isolates (347 of 2,246), and t242, which comprised 21% of isolates (478 of 2,246). Among isolates with spa type t008, all 32 isolates tested by PFGE were USA300. Both t002 and t242 spa types correspond to USA100. Among CA-MRSA isolates, 66% were isolated from wounds (668 of 1,019 with data on specimen source), 14% from the respiratory system (144 of 1,019; sputum, bronchial lavage, and tracheal aspirate), 9% from other sources (93 of 1,019), 8% from blood (81 of 1,019), and 3% from urine (33 of 1,019). Of HA-MRSA isolates, there were 26% from wounds (214 of 824), 39% from respiratory sources (324 of 824), 10% from other sources (83 of 824), 9% from blood (77 of 824), and 15% from urine (125 of 824). CA-MRSA was the most common source of community-onset isolates (51%; 751 of 1,485) but also represented more than a third of hospital-onset isolates (37%; 282 of 761; Table 2).
TABLE 1.
Characteristics of 30 Hospitals in Orange County, California, 2009
| Hospital characteristic | All hospitals (n = 30) |
Acute care only (n = 25) |
Pediatric only (n = 3) |
LTACa only (n = 5) |
|---|---|---|---|---|
| Annual no. of admissions, median (range) | 6,920 (70–33,080) | 11,140 (943–33,080) | 2,178 (70–12,496) | 592 (70–1,162) |
| Length of stay, median days (range) | 3 (2–64) | 3 (2–4) | 2 (2–64) | 24 (11–64) |
| Hospital demographic characteristic | ||||
| Age, years | ||||
| <18 | 1 (0–99) | <1 (0–100) | 97 (93–99) | 0 (0–93) |
| 18–34b | 16 (1–38) | 17 (7–38) | 3 (<31 to 7) | 1 (1–2) |
| 35–49 | 18 (6–41) | 18 (12–22) | NA | 41 (6–41) |
| 50–64 | 20 (13–33) | 20 (15–29) | NA | 18 (13–33) |
| 65–84 | 34 (7–59) | 32 (20–42) | NA | 54 (7–59) |
| ≥85 | 15 (0–24) | 14 (5–23) | NA | 23 (0–24) |
| Male sex | 43 (33–64) | 41 (33–59) | 59 (54–64) | 53 (41–64) |
| Race | ||||
| White | 86 (53–96) | 86 (53–96) | 57 (23–57) | 79 (73–80) |
| Black | 2 (1–7) | 2 (1–7) | 2 (2–7) | 4 (1–7) |
| Asian | 14 (0–42) | 15 (0–42) | 6 (0–8) | 13 (0–15) |
| Native American | 0 (0–1) | 0 (0–1) | 0 | 0 (0–1) |
| Other or unknown | 11 (1–14) | 10 (1–11) | 3 (1–14) | 12 (5–14) |
| Hispanic ethnicity | 18 (3–65) | 19 (3–65) | 28 (21–65) | 10 (4–21) |
| Insurance carrier | ||||
| Medicare insurance | 45 (26–82) | 41 (26–61) | NA | 82 (71–94) |
| Medicaid insurance | 13 (2–73) | 15 (2–73) | 39 (14–73) | 4 (2–14) |
| Private insurance | 27 (0–94) | 28 (3–56) | 56 (25–86) | 15 (1–86) |
| Hospital case mix | ||||
| Acute myocardial infarction | 9 (0–17) | 9 (0–13) | 0 (0) | 12 (0–17) |
| Congestive heart failure | 16 (0–43) | 16 (0–26) | 0 (0–2) | 36 (0–43) |
| Peripheral vascular disease | 6 (0–18) | 6 (0–9) | 0 (0) | 16 (0–18) |
| Stroke | 10 (1–36) | 9 (1–14) | 2 (1–36) | 20 (17–36) |
| Dementia | 3 (0–22) | 3 (0–10) | 0 (0) | 11 (0–22) |
| COPD/asthma | 18 (0–47) | 18 (14–27) | 15 (0–15) | 35 (0–47) |
| Rheumatologic disease | 2 (0–7) | 2 (0–5) | 0 (0) | 4 (0–7) |
| Ulcer | 2 (0–4) | 3 (0–5) | 0 (0) | 2 (0–4) |
| Paralysis | 2 (0–36) | 2 (0–6) | 2 (0–36) | 6 (5–36) |
| Renal disease | 12 (0–34) | 12 (0–17) | 0 (0–1) | 29 (0–34) |
| AIDS | 0 (0–3) | 0 (0–3) | 0 (0) | 0 (0–1) |
| Diabetes | 23 (0–43) | 23 (1–34) | 0.7 (0–1.8) | 43 (0–48) |
| Liver disease | 3 (0–7) | 3 (0–7) | 0.4 (0–0.5) | 3 (0–5) |
| Cancer | 7 (0–17) | 7 (0–17) | 0.2 (0–6) | 10 (0–12) |
| Current surgery | 25 (0–47) | 28 (8–47) | 20 (0–24) | 4 (0–10) |
| High Romano scorec | 26 (0–66) | 26 (0–38) | <1 (0–3) | 58 (0–66) |
NOTE. Data are median % of all hospitalized patients (range), unless otherwise indicated. COPD, chronic obstructive pulmonarydisease; LTAC, long-term acute care; NA, not applicable.
One hospital is both a pediatric hospital and an LTAC facility.
Median and range values for ages over 18 years and Medicare insurance do not include dedicated pediatric hospitals.
Romano score was dichotomized into high (≥3) versus low scores.
FIGURE 1.
Percentage of all isolates that were community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA; gray) and percentage of isolates that were CA-MRSA and associated with hospital-onset infection (black), by hospital. Hospitals that collected fewer than 50 isolates are marked with a star; fewer than 20 isolates were collected from hospitals 3, 4, 6, 7, and 30.
TABLE 2.
Distribution of Community-Associated (CA) versus Healthcare-Associated(HA) Methicillin-Resistant Staphylococcus aureus(MRSA) Strain Types from 30 Hospitals, by Isolate Collection Date
| Variable | No. (%) of community- onset infection isolates |
No. (%) of hospital- onset infection isolates |
||
|---|---|---|---|---|
| All sources | Blood only | All sources | Blood only | |
| CA-MRSA | ||||
| t008 spa type | 751 (51) | 62 (39) | 282 (37) | 19 (37) |
| Other CA-MRSA spa typesa | 114 (8) | 20 (12) | 47 (6) | 4 (8) |
| All CA-MRSA spa types | 865 (58) | 82 (51) | 329 (43) | 23 (44) |
| HA-MRSA | ||||
| t002 spa types | 192 (13) | 22 (14) | 155 (20) | 8 (15) |
| t242 spa types | 293 (20) | 36 (22) | 185 (24) | 11 (21) |
| Other HA-MRSA spa types | 83 (6) | 12 (7) | 71 (9) | 7 (13) |
| All HA-MRSA spa types | 568 (38) | 70 (44) | 411 (54) | 26 (50) |
| Other strainsb | 39 (4) | 7 (5) | 19 (3) | 3 (6) |
NOTE. Community-onset infection isolates were defined as those isolated on or before the second day of hospitalization, and hospital-onset infection isolates were defined as those isolated after the second day of hospitalization.
For CA-MRSA, other spa types were also multilocus sequence type ST8; for HA-MRSA,these other spa types were also multilocus sequence type ST5.
These strains were not related to ST5 or ST8.
In bivariate tests (Table 3), CA-MRSA isolation was associated (P < .1) with the following hospital characteristics: lower annual admissions, pediatric hospitals, proprietary (for profit) hospitals, and a higher percentage of patients with non-White race, Hispanic ethnicity, Medicaid insurance, acute myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, chronic obstructive pulmonary disease, rheumatologic disease, peptic ulcer, renal disease, AIDS, cancer, and high Romano score. CA-MRSA carriage was not associated with long-term acute care facilities or the percentage of patients with male sex, diabetes, dementia, paralysis, liver disease, or surgery during the current admission. Results for continuous variables are expressed per 10% increase. For hospital-onset isolates only, annual admissions and proprietary hospitals were not significant. For community-onset isolates only, male sex was significantly associated, whereas the percentage of patients with acute myocardial infarction, congestive heart failure, chronic obstructive pulmonary disease, peptic ulcer, or renal disease were not.
TABLE 3.
Bivariate Analysis of Hospital Characteristics Associated with Community-Associated Methicillin-Resistant Staphylococcus aureus(MRSA) Isolation (spa type t008)
| All isolates |
Hospital-onset infection isolates |
Community-onset infection isolates |
||||
|---|---|---|---|---|---|---|
| Hospital characteristic | OR | P | OR | P | OR | P |
| Annual admissions (per 1,000 admissions) | 0.9 | .03 | 0.9 | .5 | 0.9 | <.001 |
| Long-term acute care facility | 0.6 | .4 | 0.4 | <.001 | - | - |
| Pediatric hospital | 3.2 | <.001 | 5.2 | .001 | 2.6 | <.001 |
| Nonprofit hospital | 0.6 | .04 | 0.7 | .2 | 0.6 | .03 |
| Demographic characteristic, % of all patients in that hospital | ||||||
| Male sex | 1.3 | .3 | 1.2 | .5 | 1.4 | .06 |
| Non-White race | 1.2 | <.001 | 1.2 | <.001 | 1.2 | <.001 |
| Hispanic ethnicity | 1.3 | <.001 | 1.2 | .01 | 1.2 | <.001 |
| Medicaid insurance | 1.3 | <.001 | 1.4 | <.001 | 1.2 | <.001 |
| Case mix, % of all patients in that hospital | ||||||
| Acute myocardial infarction | 0.5 | .06 | 0.3 | .002 | 0.7 | .4 |
| Congestive heart failure | 0.7 | .004 | 0.6 | .001 | 0.8 | .2 |
| Peripheral vascular disease | 0.2 | <.001 | 0.3 | <.001 | 0.3 | .002 |
| Stroke | 0.4 | .001 | 0.4 | .005 | 0.5 | .08 |
| Dementia | 0.6 | .3 | 0.5 | .2 | 0.9 | .9 |
| COPD/asthma | 0.7 | <.001 | 0.7 | <.001 | 0.6 | .1 |
| Rheumatologic disease | 0.0 | <.001 | 0.0 | <.001 | 0.0 | <.001 |
| Peptic ulcer | 0.2 | .08 | 0.0 | .04 | 0.4 | .3 |
| Paralysis | 0.5 | .3 | 0.5 | .3 | 1.4 | .8 |
| Renal disease | 0.6 | .002 | 0.6 | .002 | 0.6 | .1 |
| AIDS | 0.1 | .1 | 0.0 | .08 | 0.2 | .2 |
| Diabetes | 0.9 | .4 | 0.8 | .07 | 1.1 | .7 |
| Liver disease | 0.5 | .6 | 0.2 | .2 | 0.9 | .9 |
| Cancer | 0.4 | .004 | 0.4 | .007 | 0.4 | .008 |
| Current surgery | 0.9 | .7 | 0.9 | .8 | 0.8 | .1 |
| High Romano score | 0.7 | <.001 | 0.7 | <.001 | 0.7 | .01 |
NOTE. Community-onset infection isolates were defined as those isolated on or before the second day of hospitalization, and hospital-onset infection isolates were defined as those isolated after the second day of hospitalization. The following variables are continuous and expressed per 10% increase: the percentage of patients with male sex, non-White race, Hispanic ethnicity, Medicaid insurance, current surgery, high Romano score, or any of the listed comorbidities. COPD, chronic obstructive pulmonary disease; OR, odds ratio.
In multivariate models (Table 4), CA-MRSA isolation was associated with smaller hospitals (odds ratio [OR], 0.97, or 3% decreased odds of CA-MRSA isolation per 1,000 annual admissions; P < .001), hospitals with more Medicaid-insured patients (OR, 1.2, or 20% increase in odds of CA-MRSA isolation per 10% increase in Medicaid-insured patients; P = .002), and those with a high proportion of patients with a low Romano comorbidity score (OR, 1.3, or 30% increase in odds of CA-MRSA isolation per 10% increase in patients with low score; P < .001). The Romano score was a stronger predictor of CA-MRSA isolation than any individual component. Results were similar when restricted to hospital-onset isolates only.
TABLE 4. Multivariate Generalized Estimating Equations Model Analysis of Hospital Characteristics Associated with Community-Associated MRSA Isolation (spa type t008).
| All isolates |
Hospital-onset infection isolates |
Community-onset infection isolates |
||||
|---|---|---|---|---|---|---|
| Hospital characteristic | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Annual admissions (per 1,000 patients) | 0.97 (0.96–0.98) | <001 | 0.98 (0.96–1.0) | .045 | 0.97 (0.95–0.98) | <001 |
| Percentage Medicaid insured patients | 1.2 (1.1–1.3) | .002 | 1.2 (1.1–1.4) | .002 | 1.2 (1.1–1.3) | .001 |
| Percentage patients with low Romano scorea | 1.3 (1.1–1.5) | <001 | 1.3 (1.1–1.6) | .004 | 1.1 (0.9–1.4) | .5 |
NOTE. CI, confidence interval; OR, odds ratio.
Romano score ranges from 0 to 6; a score less than 3 was considered low.
DISCUSSION
We found that nearly half of MRSA isolates collected in a single county were CA-MRSA. CA-MRSA was also the dominant strain in 9 of the 30 hospitals. There was substantial variation in CA-MRSA prevalence across hospitals, from less than one-fifth to over three-fourths of collected strains. Notably, our participating facilities included a range of community hospitals, academic centers, long-term care facilities, and dedicated pediatric hospitals.
CA-MRSA isolation was associated with several hospital-level characteristics, including smaller size. This finding may be explained by smaller hospitals being more likely to admit patients directly from the community, whereas larger hospitals are often academic or referral centers that receive patients transferred for a higher level of care.22 Penetration of CA-MRSA into the healthcare system may thus begin with these smaller community hospitals and then spread to larger centers as patients are referred for tertiary care. CA-MRSA isolation was also associated with hospitals serving patients with lower comorbidity scores, which is consistent with the admission of patients directly from the community who may be relatively healthy compared with those from hospitals. Finally, CA-MRSA was associated with hospitals with more patients insured by Medicaid. This may indicate that CA-MRSA carriage is linked to lower socioeconomic status, which has been found in earlier studies and may be the result of crowded living environments and fewer opportunities for personal hygiene.23,24 All multivariate model results remained significant when we included only HO isolates. Thus, smaller community hospitals, which serve patients who are generally healthier, may be initial points of entry into the healthcare system for CA-MRSA. Understanding which hospitals are at risk for high CA-MRSA burden may facilitate targeted interventions.
CA-MRSA penetration into hospitals may require new infection control strategies. There is some evidence that CA-MRSA strains, particularly USA300, may cause more severe disease than other strains in the hospital setting, including necrotizing fasciitis and pneumonia.10 CA-MRSA strains may also be more transmissible than other strains because of their higher growth rate and enhanced fitness, which may lead to a higher overall MRSA burden.11-15 Earlier studies have demonstrated that nasal colonization with CA-MRSA is not a necessary precursor for infection,4 which suggests that nasal decolonization efforts may have a limited effect on preventing infection or transmission. In addition, community-based transmission of CA-MRSA commonly occurs via direct skin-to-skin contact.4,25 This direct transmission may simply be more relevant in the community setting, where skin-to-skin contact as a result of sports or family interaction is more likely to occur, than among hospital inpatients. Alternately, it may reflect the greater propensity of CA-MRSA to inhabit or infect the skin compared with HA-MRSA.1-3,25 Environmental contamination has also been identified as a major route of CA-MRSA transmission,4,26-28 particularly among household contacts.29 Although environmental contamination has been shown to increase the risk of HA-MRSA in acute care settings as well,30-32 this transmission route may be more important for CA-MRSA. In particular, extranasal colonization with CA-MRSA is common, especially in the axilla, groin, and rectum,4 which may result in greater shedding and environmental contamination. Strategies to reduce CA-MRSA burden may also need to target community sources or focus on high-risk facilities.
This study has several limitations. We did not account for variable culturing practices among hospitals or physicians, which may impact the number or type of MRSA isolates collected. Our collection did not include screening cultures and thus did not capture the large number of patients who carry MRSA in the nares. However, California did not begin mandatory admission screening for MRSA until 2009 (midway through our strain collection period), and thus inclusion of screening cultures would have resulted in disproportionate numbers of MRSA isolates from hospitals where screening had already begun. We did not analyze individual-level characteristics, but our goal was to identify hospital-level characteristics associated with CA-MRSA isolation. Because infection control practices are usually implemented at the hospital level, identifying hospitals that are at risk for CA-MRSA may be helpful for future interventions by infection control programs.
We found that CA-MRSA burden was substantial in a large California county and varied considerably across hospitals. CA-MRSA appears to be widely endemic in hospitals, and USA300 and other strains may be more transmissible or cause more severe disease than HA-MRSA strains. Reducing CA-MRSA burden in hospitals may require additional infection control interventions targeted at high-risk facilities.
ACKNOWLEDGMENTS
We thank the participating hospitals.
Financial support. This work was supported by the Department of Medicine at the University of California, Irvine.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
REFERENCES
- 1.Gorak EJ, Yamada SM, Brown JD. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin Infect Dis. 1999;29:797–800. doi: 10.1086/520437. [DOI] [PubMed] [Google Scholar]
- 2.Adcock PM, Pastor P, Medley F, et al. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998;178:577–580. doi: 10.1086/517478. [DOI] [PubMed] [Google Scholar]
- 3.Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. MMWR Morb Mortal Wkly Rep. 1999;48(32):707–710. [PubMed] [Google Scholar]
- 4.Miller LG, Diep BA. Colonization, fomites, and virulence: re-thinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(5):752–760. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 5.Rachkam DM, Ray SM, Franks AS, et al. Community-associated methicillin-resistant Staphylococcus aureus nasal carriage in a college student athlete population. Clin J Sport Med. 2010;20(3):185–188. doi: 10.1097/JSM.0b013e3181dba80d. [DOI] [PubMed] [Google Scholar]
- 6.Cooke FJ, Gkrania-Klotsas E, Howard JC, et al. Clinical, molecular, and epidemiological description of a cluster of community-associated methicillin-resistant Staphylococcus aureus isolates from injecting drug users with bacteraemia. Clin Microbiol Infect. 2010;16(7):921–926. doi: 10.1111/j.1469-0691.2009.02969.x. [DOI] [PubMed] [Google Scholar]
- 7.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of healthcare-associated blood stream infections. Clin Infect Dis. 2006;42(5):647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 8.Maree CL, Daum RS, Boyle-Vavra S, et al. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis. 2007;13(2):236–242. doi: 10.3201/eid1302.060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otter JA, French GL. Community-associated methicillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated infection. J Hosp Infect. 2011;79(3):189–193. doi: 10.1016/j.jhin.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 11.Diep BA, Stone GG, Basuino L, et al. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2008;197(11):1523–1530. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- 12.Shilo N, Quach C. Pulmonary infections and community associated methicillin-resistant Staphylococcus aureus: a dangerous mix? Paediatr Respir Rev. 2011;12(3):182–189. doi: 10.1016/j.prrv.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Lo WT, Wang CC. Panton-valentine leukocidin in the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Pediatr Neonatol. 2011;52(2):59–65. doi: 10.1016/j.pedneo.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Thurlow LR, Joshi GS, Richardson AR. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) FEMS Immunol Med Microbiol. 2012;65(1):5–22. doi: 10.1111/j.1574-695X.2012.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto T, Nishiyama A, Takano T, et al. Community acquired methicillin-resistant Staphylococcus aureus: community transmission, pathogenesis, and drug resistance. J Infect Chemother. 2010;16(4):225–254. doi: 10.1007/s10156-010-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lessa FC, Ray SM, Dumyati G, et al. Impact of USA300 methicillin-resistant Staphylococcus aureus on clinical outcomes of patients with pneumonia or central line-associated bloodstream infections. Clin Infect Dis. 2012;55(2):232–241. doi: 10.1093/cid/cis408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hota B, Lyles R, Rim J, et al. Predictors of clinical virulence in community-onset methicillin-resistant Staphylococcus aureus infections: the importance of USA300 and pneumonia. Clin Infect Dis. 2011;53(8):757–765. doi: 10.1093/cid/cir472. [DOI] [PubMed] [Google Scholar]
- 18.Office of Statewide Health Planning and Development [Accessed June 6, 2011];Patient discharge data public data set. 2009 http://www.oshpd.ca.gov/HID/DataFlow/HospMain.html.
- 19.Leibson CL, Needleman J, Buerhaus P, et al. Identifying in-hospital venous thromboembolism (VTE): a comparison of claims-based approaches with the Rochester Epidemiology Project VTE cohort. Med Care. 2008;46:127–132. doi: 10.1097/MLR.0b013e3181589b92. [DOI] [PubMed] [Google Scholar]
- 20.Hudson LO, Murphy CR, Spratt BG, et al. Differences in MRSA strains isolated from pediatric and adult patients from hospitals in a large county in California. J Clin Microbiol. 2012;50(3):573–579. doi: 10.1128/JCM.05336-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enright MC, Day NP, Davies CE, et al. Multi-locus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–1015. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BY, McGlone SM, Song Y, et al. Social network analysis of patient sharing among hospitals in Orange County, California. Am J Public Health. 2011;101(4):707–713. doi: 10.2105/AJPH.2010.202754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: implications for patients and practitioners. Am J Clin Dermatol. 2007;8(5):259–270. doi: 10.2165/00128071-200708050-00001. [DOI] [PubMed] [Google Scholar]
- 24.McMullen KM, Warren DK, Woeltje KF. The changing susceptibilities of methicillin-resistant Staphylococcus aureus at a Midwestern hospital: the emergence of “community-associated” MRSA. Am J Infect Control. 2009;37(6):454–457. doi: 10.1016/j.ajic.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 25.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;22(3):616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai R, Pannaraj PS, Agopian J, et al. Survival and transmission of community-associated methicillin-resistant Staphylococcus aureus from fomites. Am J Infect Control. 2011;39(3):219–225. doi: 10.1016/j.ajic.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Stanforth B, Krause A, Starkey C, et al. Prevalence of community-associated methicillin-resistant Staphylococcus aureus in high school wrestling environments. J Environ Health. 2010;72(6):12–16. [PubMed] [Google Scholar]
- 28.Scott E, Duty S, McCue K. A critical evaluation of methicillin-resistant Staphylococcus aureus and other bacteria of medical interest on commonly touched household surfaces in relation to household demographics. Am J Infect Control. 2009;37(6):447–453. doi: 10.1016/j.ajic.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Miller LG, Eells SJ, Taylor AR, et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis. 2012;54(11):1523–1525. doi: 10.1093/cid/cis213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman ER, Platt R, Bass R, et al. Impact of an environmental cleaning intervention on the presence of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on surfaces in intensive care units. Infect Control Hosp Epidemiol. 2008;29:593–599. doi: 10.1086/588566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyce JM, Potter-Bynoe G, Chenevert C, et al. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997;18:622–627. [PubMed] [Google Scholar]
- 32.Dancer SJ. Importance of the environment in methicillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect Dis. 2008;8(2):101–113. doi: 10.1016/S1473-3099(07)70241-4. [DOI] [PubMed] [Google Scholar]