Abstract
Breast cancer (BC) is the most common cancer affecting women in the United States and metastatic breast cancer is the leading cause of death. The role estradiol plays in ER-positive BC is well-documented, but the way it contributes to ER-negative BC remains unclear. In the present study, we utilized an experimental model of BC metastasis into lung by injecting ER-negative murine 4T1 cells into mice via the lateral tail vein. A 56 % metastasis occurrence rate following the injection of 5 × 103 cells was observed, thus this cell number was selected to study the potential stimulatory effect of estradiol on ER-negative BC metastasis. Female ovariectomized mice were randomized into estradiol and control groups with 16 mice per group, and estradiol pellets were implanted subcutaneously in the estradiol group. Results demonstrated that estradiol accelerated BC metastasis as indicated by bioluminescent imaging. In addition, estradiol enhanced metastatic tumor colony formation and increased the size of tumor nodules in the lungs, which were due, in part, to the increase in proliferative cells in the metastatic tumors. In vitro, estradiol increased the motility and invasion of 4T1 cells, and the stimulatory effect on cell motility was not blocked by ICI 182, 780, confirming that ER was not involved in the process. Results from the present study suggest that estradiol plays a role in ER-negative BC metastasis in whole animals.
Keywords: ER-negative breast cancer, Metastasis, Estradiol, 4T1 cells
Introduction
Breast cancer (BC) is the most common cancer affecting women in the United States, with 124 cases per 100,000 women in 2001–2005 [1]. Metastatic BC (Stage IV) is the leading cause of death in BC patients [2, 3]. Cancer metastasis is a multistep process, in which cancer cells first proliferate at a primary site, invade the basement membrane and stroma of the primary organ, followed by intravasation into the circulatory system. Cancer cells are then transported through the blood stream to a secondary site, extravasate to enter the site and finally form a metastatic lesion [4]. While Stage I BC is manageable with surgical interventions, endocrine treatment and chemotherapy, approximately 30 % of patients relapse within 5 years with metastatic tumors [5, 6]. Current treatment of BC metastasis is largely palliative, such as alleviating pain and improving patients’ quality of life. Efficacious treatment still remains an ongoing therapeutic challenge.
Prolonged exposure to 17β-estradiol (estradiol), due to, for example, early menarche, late menopause, or postmenopausal hormone replacement therapy, is associated with a greater risk of developing BC [7–10]. The status of estrogen receptor (ER), currently relying only on the measurement of ERa, is essential for designing endocrine treatment strategies for the initial assessment of BC, because endocrine treatment is most effective when BC cells express ERα [11, 12]. The second subtype of ER, ERβ, has a similar protein structure as ERα [13]. Many cell-based studies suggest that ERβ acts as a negative modulator of ERα action [13]. ERβ influences endogenous ERα activities in ER-positive cells by decreasing ERα transcriptional activity [14, 15] and by increasing estradiol-induced ERα degradation [16, 17]. It appears that ERα expression is high and ERβ expression is low in early BCs, whereas the expression of both receptors declines in more invasive cancer [13, 18–20], which suggests ERβ might be a tumor suppressor. In general, the protein expression of ERα in BCs correlates with low tumor grade and negative lymph node status (no metastasis) [20–22]. While ER-positive tumors are less invasive and respond well to endocrine therapy [23], ER-negative BC is less responsive to endocrine treatment and presents with a more aggressive progression [24, 25]. However, the correlation of ERβ expression with invasiveness is less clear. Some researchers have suggested that ERβ can be a therapeutic target in ERα-negative BCs [12, 26, 27]. However, cohort size and numbers of independent studies are small to date [12] and further investigations are needed.
The most widely accepted theory on how estradiol influences ER-positive BC is that estradiol stimulates cell proliferation by acting through ER, which can provide a selective advantage for mutated phenotypes. The growth of cells harboring mutations is further promoted by estradiol until cancer results [28]. Estradiol-dependent activation of ER involves two pathways, either classical (genomic) or non-classical (non-genomic). The classical pathway is a ligand-activated pathway that regulates gene transcription through direct binding to DNA and/or interaction with specific co-activators or co-repressors, whereas the non-classical pathway involves rapid activation of various protein-kinase cascades mediated through membrane-associated ER [29, 30]. Although how estradiol influences ER-positive BC is well-documented, the role estradiol plays in ER-negative BC remains unclear. There is evidence showing that, regardless of ER status, ovariectomy decreases long-term risk of BC recurrence in pre-menopausal women, which clearly indicates the involvement of estradiol in ER-negative BC [31, 32]. Thus, even though endocrine treatment is less effective in treating ER-negative BC, it does not necessarily indicate that estradiol has no role in ER-negative BC. Recent studies have shown that estradiol may affect ER-negative BC through a systemic effect on the host compartment, not by directly acting on tumor cells [33, 34]. Furthermore, it is possible that estradiol acts directly on cancer cells via non-ER-mediated pathways. In the present study, we tested our hypothesis that estradiol alters tumor metastasis by using ER-negative murine mammary cancer 4T1 cells.
Materials and methods
Materials
Murine 4T1 cells engineered with firefly-luciferase were provided by Dr. David Piwnica-Worms from Washington University (St. Louis, MO). Dulbecco’s Modified Eagle’s Medium (DMEM) was obtained from the Cell Media Facility, University of Illinois at Urbana-Champaign (Urbana, IL). Heat inactivated fetal bovine serum (HI-FBS) was purchased from Atlanta Biological (Lawrenceville, VA). Penicillin/streptomycin and trypsin/EDTA were purchased from Invitrogen (Carlsbad, CA). Matrigel™ matrix and Biocoat™ Matrigel™ invasion chambers were obtained from BD Biosciences (Bedford, MA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and 17β-estradiol were obtained from Sigma-Aldrich Inc. (St. Louis, MO). D-luciferin potassium was purchased from Regis Technologies (Morton Grove, IL). The AIN-93G semi-purified diet with corn oil instead of soybean oil was purchased from Research Diets (Brunswick, NJ).
Animals
Female Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were ovariectomized (OVX) at 3 weeks of age by the vender and arrived at the animal facility one week later. Animals were housed separately in a controlled environment and had free access to food and water. The AIN-93G semi-purified diet was selected because it has been established to meet all nutritional requirements of mice [35]. Instead of soybean oil, corn oil was used in the diet to eliminate any additional components of soy (phytoestrogens) being added into the diet. Artificial light was provided on a 12 h light/dark schedule. Experimental procedures and animal care was carried out under animal protocols approved by the Institutional Animal Care and Uses Committee (IACUC) of the University of Illinois at Urbana-Champaign.
Cell culture
Murine 4T1 cells originated in 1983 from a single mouse mammary tumor [36, 37] and lack the expression of ER [34, 38]. The cells, when inoculated into mice, can metastasize to other organs in a way similar to what is observed in naturally occurring BC in humans [39–41]. Another advantage of using 4T1 cells was that this model allowed us to use immunocompetent syngeneic mice that have a fully functioning immune system. 4T1 cells engineered with firefly-luciferase were maintained in DMEM supplemented with 10 % HI-FBS, 100 unit/ml penicillin, 100 µg/ml streptomycin at 37 °C in an incubator with 5 % CO2 humidified air. Cells were harvested at 80 % confluence with EDTA/trypsin, counted and re-suspended for following experiments.
MTT assay
Murine 4T1 cells (2 × 103 per well) were plated into 96-well plates and incubated for 24 h in phenol red-free culture media supplemented with 10 % charcoal-striped BCS. Cells were then washed with PBS and cultured with 0, 1, 10 or 100 nM estradiol for 24, 48 and 72 h. MTT (5 mg/ml) was added to each well and optical density was measured at 570 nm with an EL800 Universal plate reader (Bio-Tek Instruments Inc., Winooski, VT). Each treatment was duplicated in 8 wells and repeated three times.
Wound-healing assay [42, 43]
Murine 4T1 cells (105 per well) were plated into 12-well plates and allowed to form a confluent monolayer. Two parallel scratches were made on the confluent cell monolayer in each well with a P1000 pipette tip. Cells were then cultured with 0, 1, 10 or 100 nM estradiol alone, or 1 nM estradiol with an ER antagonist, ICI 182,780 (1 µM) in SF medium. Three different positions on each scratch were marked and photographed immediately after scratching and again 24 h later. The unoccupied area between the two edges of the scratch was measured and the migration distance was calculated using the following formula: (measured areatime 0–measured areatime 24)/a fixed width of 2336 pixels for all graphs. Experiments were performed in triplicate and repeated three times. Data were normalized to the untreated control cells.
Invasion chamber assay [42, 43]
Murine 4T1 cells (105 per insert) were seeded into the Matrigel™ -coated cell culture insert (8 µm) and treated with or without estradiol (1 nM) in SF culture medium. The inserts were placed on a companion 24-well plate filled with SF culture medium. After 24 h, cells on the upper surface of the membrane in the insert were removed with a cotton swab and cells on the lower surface were fixed with cold methanol and stained with hematoxylin. The membrane was then cut from the insert housing, mounted onto a micro-slide and observed under an AxioSkop 40 microscope (Carl Zeiss, Thornwood, NY) with 50× magnification. Each membrane was visually divided into 9 fields and the invaded cells in each field were counted using ImageJ (NIH, Bethesda, MD). Experiments were performed in duplicate and repeated twice. Data were normalized to the untreated control cells.
Western blot
The protein expression of ER was determined using Western blot analysis. Briefly, 4T1 cells were lysed and protein concentration was quantified using the BCA assay (Thermo Fisher Scientific, Rockford, IL). Cell lysates with equal amount of total proteins (40 µg) were separated by SDS-PAGE, and then were transferred to BioTrance™ NT nitrocellulose blotting membranes (Pall Corporation, Pensacola, FL). Membranes were incubated with a primary anti-ER antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in a blocking buffer (LI-COR Bioscience, Lincoln, NE) at 4 °C overnight, washed with PBST, and then incubated with a secondary antibody at room temperature for 1 h. Bands were visualized using Odyssey Infrared Imaging System (LI-COR Bioscience, Lincoln, NE).
Optimization of cell amount to be injected via tail vein (TV)
To determine the optimal cell amount for TV injection, female OVX Balb/c mice were randomly divided into TV1, TV2 and TV3 groups, with 13–16 animals per group. Each mouse was placed in a restrainer and the tail was warmed in a 42 °C water bath for 3 min for vasodilation. Murine 4T1 cells were suspended in 100 µl sterile PBS and injected via the lateral TV. Animals in the TV1, TV2 or TV3 group received 5 × 103, 5 × 104 or 2 × 105 4T1 cells, respectively. Animals in individual groups were sacrificed at the same time when more than three mice in this group showed physiological abnormalities, such as shortness of breath and paralysis. Study duration was then recorded. Mice with bioluminescent signals detected at the end of the study were counted and the metastasis occurrence rate was presented as the percentage of mice with bioluminescent signals in all mice for each experiment group.
Animal study design
Female OVX Balb/c mice were randomly divided into control and estradiol groups with 16 mice per group. An estradiol pellet (1 mg estradiol: 19 mg cholesterol) was subcutaneously implanted on the interscapular region of each animal in the estradiol group as described previously [44, 45]. All animals received 5 × 103 4T1 cells from the lateral TV 4 days after estradiol implantation.
Bioluminescence imaging (BLI)
BLI was conducted twice a week to monitor the progression of metastasis using a custom made imaging system (Stanford Photonics, Palo Alto, CA) with a dual microchannel plate ICCD camera. Each mouse was intraperitoneally injected with freshly-made D-luciferin potassium (0.15 g/kg body weight) 3 min prior to imaging and then anesthetized with isoflurane/oxygen gas for BLI. A grey-scale image of the mouse was first recorded with dimmed light. Photon emission was then integrated for 3 min using the imaging software Piper Control (Stanford Photonics, Palo Alto, CA) and visualized in pseudo-color. To localize bioluminescent signals that indicated luciferase-engineered 4T1 tumors, grey-scale images of mouse body and bioluminescent signals of metastatic tumors were merged using Image J (NIH) and Photoshop Elements (Adobe, San Jose, CA). Half saturation time (ST1/2) was determined by calculating the half time for bioluminescent signals emitted from metastatic tumors to reach signal saturation.
Hematoxylin and eosin (H&E) staining
At the end of the study, lungs from each mouse were excised, fixed in 10 % formalin and embedded in paraffin for sectioning. Sectioned lung tissues (5 µm) were stained with H&E and observed under an AxioSkop 40 microscope. Metastatic tumor colonies were counted and tumor area was measured as described [46, 47]. Tumor area was presented as percentage in whole lung tissue area per section. All slides were measured in a blind fashion without the knowledge of treatment groups.
Expression of Ki-67
Proliferative cells in metastatic tumors in lungs was determined using immunohistochemical analysis [48]. Briefly, sectioned lung tissues were incubated with a primary Ki-67 antibody (Pharmingen, San Diego, CA) at 4 °C overnight, and then with a biotinylated anti-mouse secondary antibody (Vector Laboratories Inc. Burlingame, CA) for 30 min at room temperature. Positive cells were visualized as brown by staining with 3, 3′-diaminobenzi-dine tetrahydrachloride (DAB), and negative cells were visualized as blue by staining with hematoxylin. Both positive and negative tumor cells were counted in a given field. Around ten metastatic lung tumors from four animals in each group were evaluated. Data were presented as the percentage of proliferative cells in a given field of tumor cells.
Statistical analysis
All statistical data were analyzed using either SAS™ 9.2 (SAS Institute Inc., Cary, NC) or OriginPro® 8.5 (Origin Lab Corporation, Northampton, MA) program. Data from wound healing, invasion chamber and Ki-67 expression tests were analyzed using the Student’s t test. Data from half saturation time was analyzed using the Mann-Whitney test. Count data from H&E staining were analyzed using the PROC GENMOD of SAS. P < 0.05 was considered indicative of significant differences between groups.
Results
Estradiol stimulated the motility and invasion of 4T1 cells in vitro
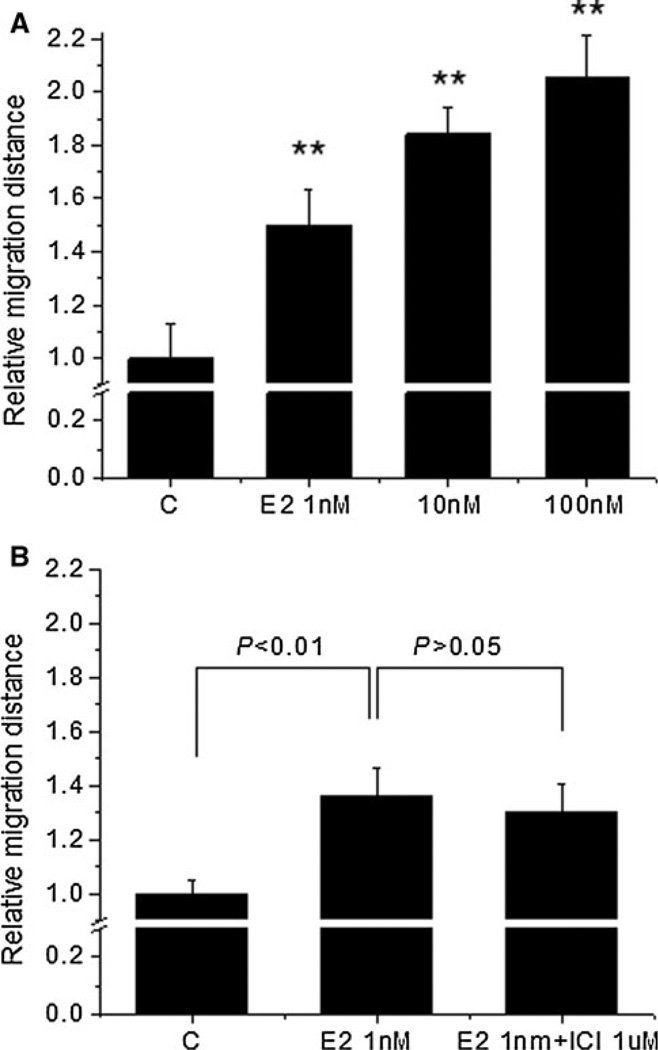
To examine the effect of estradiol on the motility of 4T1 cells, we utilized a wound-healing assay. Estradiol at 1 nM significantly increased the distance that 4T1 cells moved to 1.5-fold over the untreated cells (P < 0.01), 10 nM estradiol increased the distance to 1.8-fold (P < 0.01) while 100 nM estradiol increased the distance to 2.1-fold (P < 0.01) over the untreated cells, indicating that estradiol increased the motility of 4T1 cells in the tested concentration range (Fig. 1a).
Fig. 1.
Estradiol increased the motility of 4T1 cells in vitro via non-ER-mediated pathways. Wounded scratches were made on the confluent 4T1 cell monolayer and the migrated distance between the two edges of the scratch was calculated to indicate cell motility. a Estradiol (E2) at the indicated doses increased the motility of 4T1 cells, b The stimulatory effect of E2 (1 nM) on the motility of 4T1 cells was not blocked by the pure ER antagonist ICI 182,780 (ICI) at 1 µM. Data (mean ± SEM) are normalized to the untreated control cells (C) and are representative of 3 independent experiments.** Indicates significant difference at the level of 0.01 when compared to the untreated cells
We examined the expression of ER in 4T1 cells using Western blot and the results showed that the protein expression of ER in 4T1 cells was negative (data not shown). To confirm whether the stimulatory effect of estradiol on cell motility was mediated through ER, cells were treated with 1 nM estradiol in combination with a pure ER antagonist ICI 182,780 (1 µM). Figure 1b shows that the difference in migration distance between estradiol-treated cells and estradiol/ICI 182,780-treated cells was not significant. These results further confirmed that the stimulatory effect of estradiol on the motility of 4T1 cells was associated with non-ER-mediated pathways.
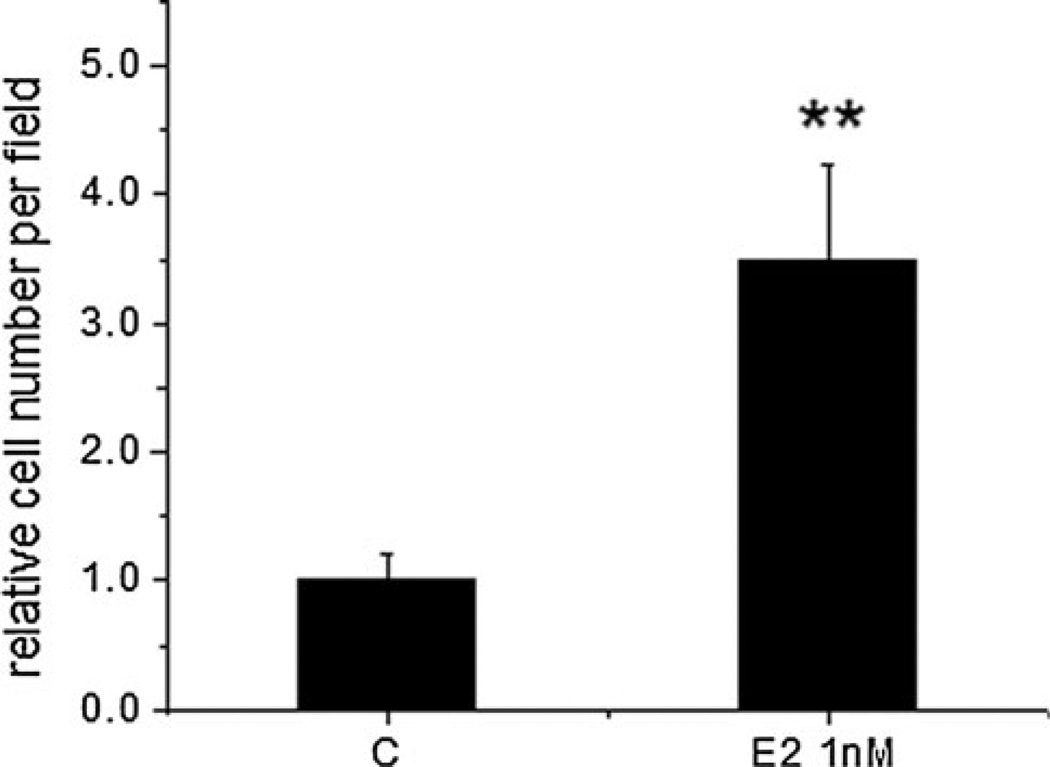
To examine the effect of estradiol on the invasiveness of 4T1 cells, we utilized an invasion chamber assay. We showed that 1 nM estradiol increased the motility of 4T1 cells. In addition, this dose of estradiol is a physiological dosage and is similar to the levels reported in plasma for peak estradiol in women [49]. Therefore, we selected this concentration to conduct the invasion assay. Figure 2 shows that estradiol at 1 nM increased the number of cells that invaded the membrane to 3.5-fold over the untreated control (P < 0.01), indicating that estradiol stimulated the ability of 4T1 cells to invade the surrounding biocoated membrane. To examine the effect of estradiol on the viability of 4T1 cells, we utilized a MTT assay. Estradiol (1–100 nM) did not affect cell viability in phenol red-free media (data not shown).
Fig. 2.
Estradiol (E2) increased the invasion of 4T1 cells. Murine 4T1 cells were incubated in biocoated invasion chambers for 24 h and invasiveness was evaluated by counting cells that travelled through the biocoated PET membrane per field. Data (mean ± SEM) are normalized to the untreated control cells (C) and are representative of two independent experiments. ** Indicates significant difference at the level of 0.01 when compared to the untreated cells
Optimization of an experimental BC metastasis model of 4T1 cells
In the present study, we utilized an experimental metastasis model with the injection of 4T1 cells into mice via the lateral TV. The model was optimized by determining the optimal amount of cells to be injected into mice. The study duration for the TV1 (5 × 103 4T1 cells), TV2 (5 × 104) and TV3 (2 × 10 ) groups was 21, 18 and 14 days, respectively (Table 1). The significant correlation between the inoculated cell number and the duration of the study (Pearson’s r = 0.999, P < 0.05) indicated that study duration was negatively correlated with inoculated cell number. Furthermore, the metastasis occurrence rate for the TV1 group (56 %) was significantly different from the TV2 (100 %) (P < 0.01) and TV3 groups (100 %) (P < 0.01), indicating that inoculated cell number was positively associated with metastasis occurrence rate (Table 1). In the in vitro study, we observed that estradiol increased the motility and invasion in cultured 4T1 cells. This increase in motility and invasion could potentially stimulate BC metastasis in whole animals. Therefore, we conducted a study in whole animals in which 5 × 103 cells were injected into the lateral TV of mice to evaluate the potential ability of estradiol to increase BC metastasis into lung.
Table 1.
Optimization of the experimental BC metastasis model of 4T1 cells
| Group | Cell number |
Study duration (day) |
Metastasis occurrence rate: number of mice with metastasis/total number of mice |
|---|---|---|---|
| TV1 | 5 × 103 | 21 | 9/16 (56 %)a |
| TV2 | 5 × 104 | 18 | 15/15 (100 %)b |
| TV3 | 2 × 105 | 14 | 13/13 (100 %)b |
Murine 4T1 cells were inoculated into mice via the lateral TV with the indicated cell numbers. Animals in individual groups were sacrificed at the same time when more than three mice in this group showed physiological abnormalities. Study duration was then recorded and mice with detectable bioluminescent signals were counted at the end of the experiment. Metastasis occurrence rate was calculated as the number of mice with detectable bioluminescent signals divided by the total number of mice
Different letters indicate significant difference at the level of 0.05 between groups
BLI monitoring of BC metastasis into lung in mice receiving 5 × 103 4T1 cells via the lateral TV
To examine the effect of estradiol on BC metastasis in whole animals, we subcutaneously implanted an estradiol pellet (1 mg estradiol: 19 mg cholesterol) into each mouse in the estradiol group, and 5 × 103 4T1 cells were injected into mice via the lateral TV 4 days after pellet implantation to allow stabilization of blood levels of estradiol. The result of BLI monitoring showed that no bioluminescent signals were detected by BLI on either day 4 (D4) or D7 post cell injection. Distinct bioluminescent signals were detectable on D11 in the lung area after a latent period of 10 days, suggesting the growth of metastatic 4T1 tumors on the lungs. After initial detection by BLI, animals with detectable bioluminescent signals exhibited a rapid increase in photon emission until the end of the study. Histological staining (H&E) of collected organs confirmed BLI results, showing that the lung was the common site for BC metastasis induced by injecting 4T1 cells via the TV. No metastatic tumors were detected in organs other than the lungs in the present study (data not shown).
Estradiol accelerated lung metastasis in mice receiving 5 × 103 4T1 cells via the lateral TV
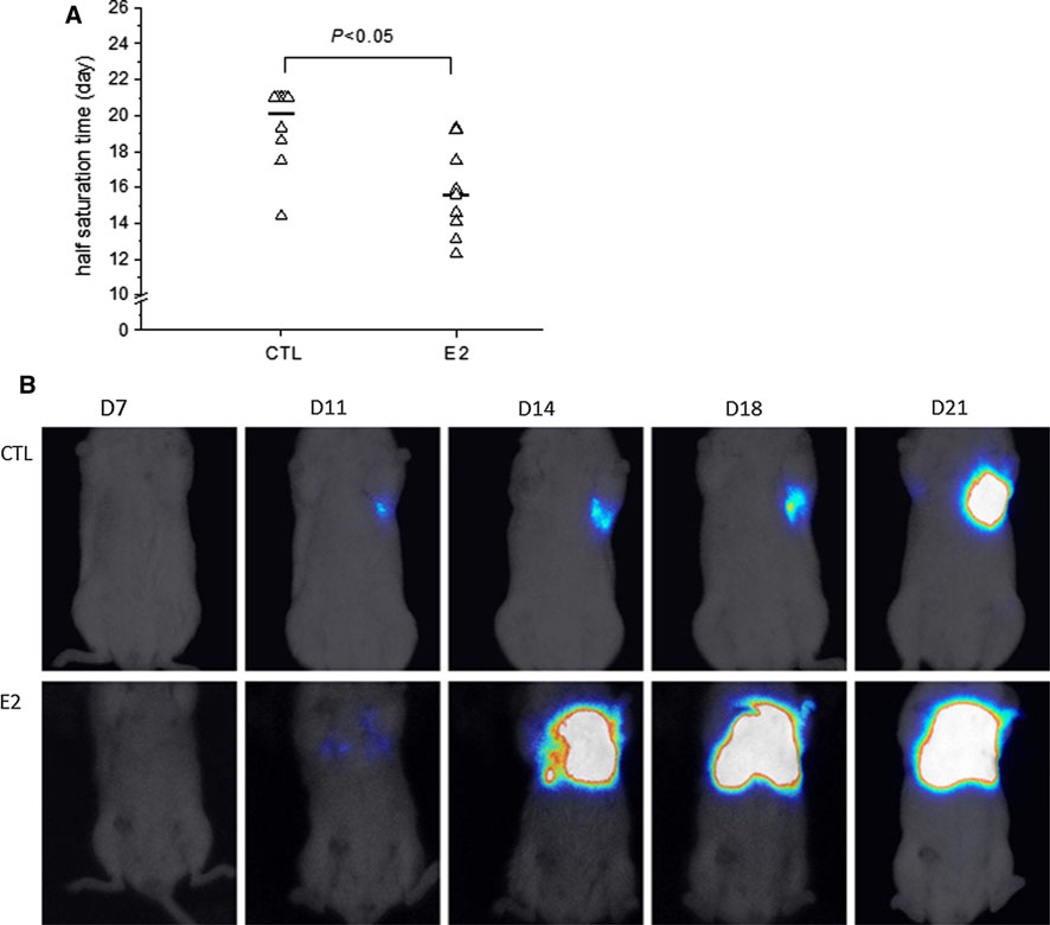
By the end of the study, survival analysis showed that there was a trend of decrease in survival time in the estradiol-treated mice when compared with the control mice (P = 0.07, data not shown), suggesting that estradiol potentially affected the progression of ER-negative BC metastasis induced by 4T1 cells. For humane reasons, all animals were sacrificed on D21, thus, the survival test could not be carried out past D21. To accurately examine the progression of metastasis, we calculated the half saturation time (ST1/2) of bioluminescent signals for each mouse. The median ST1/2 was 20.1 days for the control group, while treatment with estradiol significantly decreased the median ST1/2 to 15.6 days (P < 0.05, Fig. 3a), suggesting that estradiol-treated mice developed lung metastasis faster than the control mice. Figure 3b displays a series of bioluminescent images of a mouse from the control group (top panel) and a series of bioluminescent images of a mouse from the estradiol group (bottom panel) recorded on D7, D11, D14, D18 and D21, showing that estradiol accelerated bioluminescent signals emitted from metastatic tumors on lungs to reach saturation.
Fig. 3.
Estradiol accelerated lung metastasis progression in mice receiving 5 × 103 4T1 cells via the lateral TV. a Estradiol decreased the half time for bioluminescent signals emitted from metastatic tumors on the lungs to reach saturation (P < 0.05). Half time for bioluminescent signals to reach saturation (ST1/2) was recorded for each mouse. When bioluminescent signal from an individual mouse did not reach saturation by the end of the study, ST1/2 for this mouse was set as 21 (the duration of the study). Mice without detected bioluminescent signals were excluded. Triangle: individual ST1/2, dash median ST1/2 for experimental groups, b Top panel a series of bioluminescent images of a mouse from the control group (CTL) recorded on D7, D11, D14, D18 and D21. Bottom panel a series of bioluminescent images of a mouse from the estradiol group (E2) recorded on D7, Dll, D14, D18 and D21. Bioluminescent signals from the estradiol mouse reached saturation faster than from the control mouse
Estradiol increased metastatic tumor burden in lungs in mice receiving 5 × 103 4T1 cells from the lateral TV by histological analysis
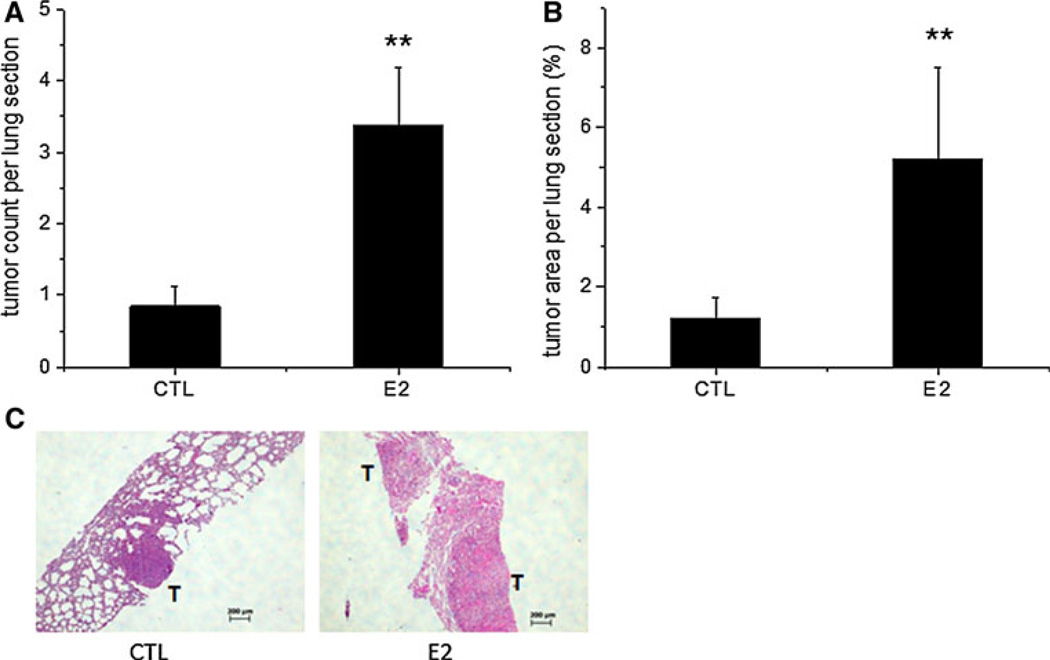
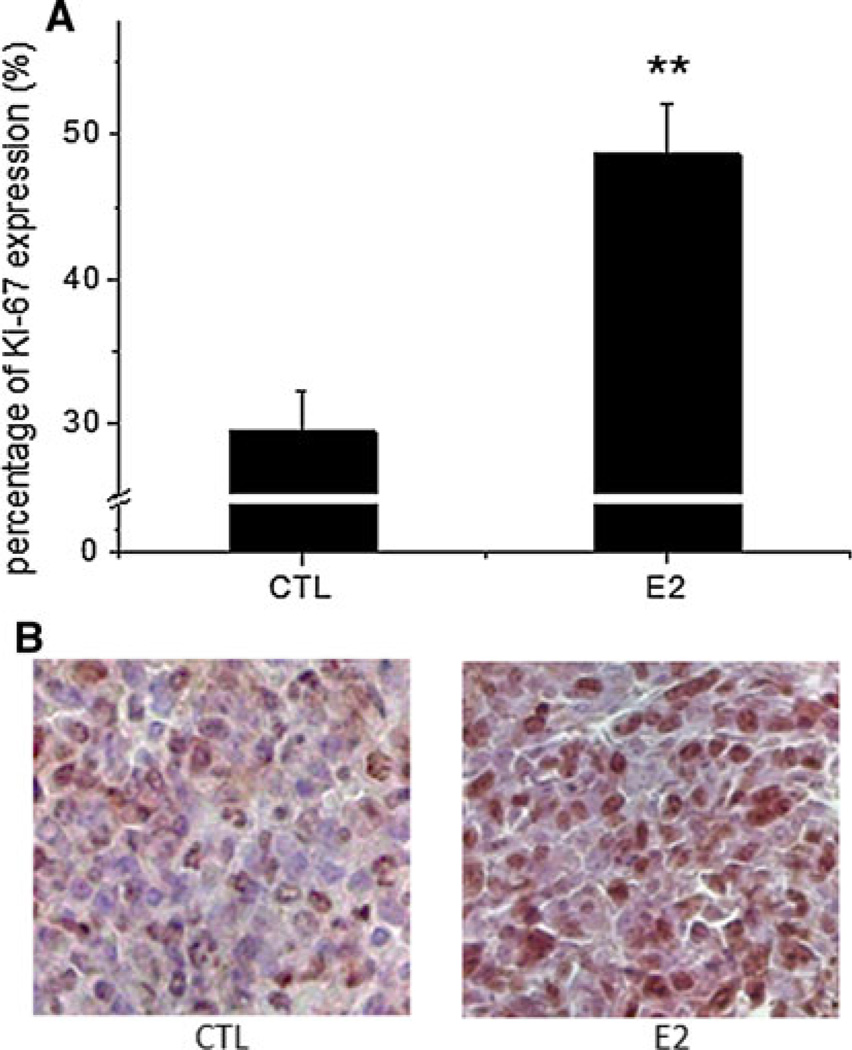
Since the lung was the most common site for BC metastasis in the 4T1 TV model as detected by BLI, we examined the effect of estradiol on microscopic metastasis in the lungs. Estradiol significantly increased tumor colony formation to three tumor colonies per lung section when compared with one tumor colony per lung section in the control group (P < 0.01, Fig. 4a). Estradiol also significantly increased tumor area to 5.2 %, when compared with 1.2 % in the control group (P < 0.01, Fig. 4b). Figure 4c shows two representative images of lung tissues stained with H&E from the control and estradiol groups. Estradiol (right panel) increased metastatic tumor colony formation with enlarged tumor size when compared with the mouse from the control group (left panel). To investigate the potential molecular mechanism by which estradiol increased lung metastasis, Ki-67 immunohistochemical staining, which is used to measure proliferative cells, was performed in metastatic tumors in the sectioned lung tissues. Estradiol significantly increased the percentage of tumor cells that expressed Ki-67 protein to 48.6 % when compared with 29.4 % in the control group (P < 0.01, Fig. 5a), indicating that estradiol increased metastatic lung tumor formation, at least in part, by increasing proliferative tumor cells in the lungs. Figure 5b shows two representative images of lung tissues from the control and estradiol groups. Estradiol (right panel) increased the percentage of tumor cells that expressed Ki-67 protein (brown) when compared with the mouse from the control group (left panel).
Fig. 4.
Estradiol (E2) increased metastatic tumor burden in the lungs in mice receiving 5 × 103 4T1 cells via the lateral TV. Lungs were embedded in paraffin, sectioned and stained with H&E. Metastatic tumor colonies were counted and tumor areas (percentage of the whole lung tissue) were measured in each lung section. Estradiol increased metastatic tumor colony formation (a) and metastatic tumor area (b) in the lung tissue, c Left panel a representative image of stained lung tissue in the control group with 1 tumor colony and smaller tumor area; Right panel a representative image of stained lung tissue in the estradiol group with two tumor colonies and larger tumor size. Data are presented as mean ± SEM. ** Indicates significant difference at the level of 0.01 when compared to the control group (CTL). T tumor colony; Scale bar 200 µm
Fig. 5.
Estradiol (E2) increased the proliferation of the metastatic tumor cells in the lungs in mice receiving 5 × 103 4T1 cells via the lateral TV. a E2 increased Ki-67 expression in metastatic tumor cells in the lungs. Lungs were embedded in paraffin, sectioned and processed to examine Ki-67 expression. Positive (brown) and negative (blue) cells in the metastatic tumors were counted and the results are presented as the percentage of proliferating cells in a given area of metastatic tumors. Data are presented as mean ± SEM. ** Indicates significant difference at the level of 0.01 when compared to the control group (CTL). b Left panel, a representative image of stained metastatic tumors in the lungs from the control group (CTL) with less proliferative cells (brown); Right panel, a representative image of stained metastatic tumors in the lungs from the estradiol group (E2) with more proliferative cells. Magnification: 400×. (Color figure online)
Discussion
The present study investigated the effect of estradiol on BC metastasis using ER-negative murine 4T1 cells. We showed that estradiol increased the motility and invasion of 4T1 cells in vitro. We also demonstrated that estradiol accelerated metastatic progression in an experimental BC metastasis model in whole animals. In addition to accelerating metastatic progression, estradiol increased the number and size of metastatic 4T1 tumors in the lungs. This stimulatory effect on ER-negative BC metastasis likely resulted from estradiol increasing cell proliferation in metastatic tumors in the lungs and directly stimulating motility and invasion of 4T1 cells. The pure ER antagonist, ICI 182,780, did not inhibit the stimulatory effect of estradiol on the motility of cultured 4T1 cells, indicating a possible involvement of non-ER-mediated pathways.
We used an experimental model for ER-negative BC metastasis by injecting murine mammary caner 4T1 cells into the lateral TV of mice. We used varying numbers of 4T1 cells injected into the TV and found that inoculated cell number was negatively related to study duration and positively related to metastasis occurrence rate in lung. Estradiol has been shown to increase ER-negative BC metastasis [34], so, it became critical to choose a metastasis model that would result in sufficient study duration time without prolonging animal suffering and would also lead to moderate metastasis, allowing us to observe the potential increase in BC metastasis by estradiol. In the present study, injection of 5 × 103 4T1 cells via the lateral TV resulted in the study duration of 21 days and a metastasis occurrence rate of 56 %, and was, therefore, selected as the optimal cell number to use in this model to study the effect of estradiol on BC metastasis. The use of estradiol pellet implantation (1–2 mg estradiol) has been reported to produce circulating estradiol levels of 2–3 nM in mice [44, 50], which was similar to the estradiol levels observed in premenopausal women [51, 52]. Our previous studies revealed that these levels of plasma estradiol rapidly stimulated the growth of human breast MCF-7 tumors in mice [44]. Therefore, in the present study, we subcutaneously implanted estradiol pellets containing 1 mg estradiol into mice and injected 4T1 cells into the TV to examine the effect of estradiol on 4T1 tumor metastasis.
We used engineered 4T1 cells that express luciferase and bioluminescence imaging, a non-invasive technique, to follow the progression of metastasis of these engineered cells. Bioluminescent signals that indicated the growth of metastatic tumors were detected on the lungs after a latent period of 10 days post the injection of 5 × 103 4T1 cells in ‘real time’. Cancer metastasis is an inefficient process, due to the destruction of cancer cells in the blood stream by shear stress and the immune system and a slow rate of extravasation and proliferation at a secondary site [53]. Therefore, without continuous dissemination of cancer cells from primary tumors into circulation, a large number of 4T1 cells would not survive in the blood stream or proliferate at the secondary site after TV injection. The small percentage of cells that do survive might require a longer time to proliferate in the lungs and be detected by BLI, resulting in the latent period of 10 days which we observed in the present study. Furthermore, with the injection of 2 × 105 4T1 cells, bioluminescent signals were detectable on D5 post cell injection (data not shown), suggesting that the latent period decreased as the number of cancer cells increased. Our observations are consistent with previous reports demonstrating that injection of cancer cells via the lateral TV into mice resulted in a latent period of metastasis onset [54] and a short study duration due to severe pulmonary metastasis [55].
Here we demonstrated that estradiol decreased the half time for bioluminescent signals to reach saturation (ST1/2) from 20.1 days in the control group to 15.6 days in the estradiol group, indicating that estradiol accelerated BC metastasis induced by 4T1 cells in mice. Combined with histological analysis on excised lung tissues, the stimulation of estradiol on BC metastasis likely resulted from estradiol stimulating the survival and proliferation of cancer cells at the secondary site. The underlying mechanisms by which estradiol enhances BC metastasis are complex. Cell motility and invasiveness are important properties needed by malignant cells to escape from a primary site to secondary organs, and the stimulatory effect of estradiol on cell motility and invasion has been reported in ER-positive cells. For example, estradiol increased proliferation and migration of human umbilical vein endothelial cells through the RhoA/Rock pathway [56]. Estradiol also enhanced the migration of human endometrial cancer cells, Hec 1A and Hec 1B, by regulating the FAK/c-Src pathway [57]. Additionally, estradiol-induced the migration and invasion of human BC T-47D cells via the FAK and N-WASP pathways [58]. The primary mechanism for cell migration is the remodeling of the actin cytoskeleton [59]. Estrogen has been shown to rapidly provoke actin cytoskeleton reorganization, by activating ER and then interacting with actin-binding proteins including moesin [60], ezrin [61] and focal adhesion kinase [58], leading to the formation of membrane specialized structures that facilitate ER-positive BC cell migration and invasion. While the mechanism by which estradiol alters cell motility and migration via ER-mediated pathways has been explored, the role estradiol plays in ER-negative BC remains unclear. ER-negative BC accounts for 30 % of BC cases at diagnosis and presents with a more aggressive progression [12]. Thus, it is important to understand the role that estradiol may have in ER-negative BC.
We utilized ER-negative 4T1 cells to examine the effect of estradiol on ER-negative BC metastasis and demonstrated that estradiol increased the number of metastatic tumors in lungs. The size of these metastatic tumors in the estradiol group was also increased as indicated by H&E staining. Our observations were consistent with recent reports showing that estradiol enhanced ER-negative BC and metastasis [33, 34]. Banka et al. [34] observed that estradiol increased lung tumor burden of ER-negative D121 cells and wet lung weight, while the growth of primary tumors was unaffected [34]. Gupta et al. [33] observed that estradiol enhanced tumor formation of weakly tumorigenic, ER-negative breast epithelial HMLE-RAS cells, which were injected into the mammary fat pad. This effect was caused by the systemic induction of angiogenesis and stromal cell recruitment by estradiol, not by the direct action of estradiol on tumor cells, since these cells lack ER expression [33]. These reports suggest that estradiol could affect BC progression and metastasis induced by ER-negative cancer cells through systemic effects on the host rather than by the direct binding with ER and eliciting downstream changes in cancer cells themselves.
Despite these reports, the possibility that estradiol exhibited a direct effect on ER-negative cancer cells was generally overlooked, likely because estradiol did not affect the proliferation of some ER-negative cancer cells in vitro and the growth of primary tumors in vivo [34, 38]. In agreement to these reports, we also observed that estradiol did not stimulate the growth of 4T1 cells in vitro. However, when examining the metastatic 4T1 tumors on the lung tissues, we demonstrated that the percentage of proliferative cells was increased by estradiol, suggesting that estradiol affected cell proliferation differently when in cell culture than when in metastatic tumors in vivo. The mechanism by which estradiol enhanced metastasis of 4T1 tumors in mice and increased Ki-67 expression of 4T1 tumor cells metastasized to lungs remained unclear. Since estradiol stimulated the motility and invasion of 4T1 cells in vitro, this process may involve matrix metalloproteinases (MMPs). MMPs are a family of secreted or transmembrane proteins that are capable of digesting extracellular matrix and basement membrane components under physiologic conditions [62]. During metastasis, in addition to these physiological functions, recent studies have shown that MMPs are key regulators of tumor growth, at both primary and metastatic sites [62]. Nilsson et al. [63] first showed that estradiol decreased intracellular and secreted protein levels of MMP-2/MMP-9 in ER-positive MCF-7 cells in vitro [63]. They also showed that tamoxifen, an ER antagonist, increased MMPs protein levels and negated the effect of estradiol on MMPs protein levels [63], which indicated a possible involvement of ER pathways. However, how estradiol affects MMPs in ER-negative BC still remains unclear and needs further investigation.
Non-genomic ER transduction pathways are independent of classic ER genomic transcriptional pathways. In these non-genomic pathways, E2 induces rapid cellular effects by protein–protein interactions, which involves several signal transduction pathways, such as PLC/PKC, p38/MAPK, JAK/STAT [29]. For example, in MCF-7 cells, E2 induces ER association with both Src and Shc, leading to Shc/Src/ Ras/ERK activation and cycle progression [64, 65]. In addition, different growth factor receptors, such as EGFR and IGF1-R, have been reported to modulate subcellular localization of and interaction with ER in MCF-7 cells [66, 67]. Regarding ER expression in 4T1 cells, Banka et al. [34] have shown that 4T1 cells are negative in ER protein expression [34]. On the other hand, 4T1.2 cells, another clone of 4T1 cells, have been reported to be positive for gene expression of ER [68]. Thus, we conducted a western blot analysis and confirmed that 4T1 cells we used in the study are negative of ER expression. Therefore, it is more likely that E2 exhibits its effects on 4T1 cells through non-ER pathways. This is an important potential mechanism - currently under investigation in our laboratory.
In conclusion, the present study shows that estradiol accelerates ER-negative BC metastasis and increases metastatic tumor colony formation and tumor size in the lungs in mice. This effect potentially results from the ability of estradiol to increase the proliferation of metastatic 4T1 tumor cells on the lungs. Estradiol also enhances the motility and invasion of 4T1 cells, which appears to be regulated by non-ER-mediated mechanisms. As the need for effective therapies against ER-negative BC remains, a better understanding of the underlying mechanisms may help to design and evaluate new therapies for ER-negative BC.
Acknowledgement
This study was supported by the National Cancer Institute [CA77355 to W.G.H.]; the National Institute on Aging, the National Institute for Complementary and Alternative Medicine and the Office of Dietary Supplements, the Women’s Health Initiative [P01 AG024387 to W.G.H. and Y.H.J.]; and the National Institute for Complementary and Alternative Medicine, the Office of Dietary Supplements and the National Cancer Institute [P50AT006268 to W.G.H.]. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NICAM, ODS, NCI or the National Institutes of Health.
Footnotes
Conflict of interests None declared.
Contributor Information
Xujuan Yang, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Aashvini Belosay, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Mengyuan Du, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
Timothy M. Fan, Department of Veterinary Clinical Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA
Russell T. Turner, Skeletal Biology Laboratory, School of Biological and Population Health Sciences, Oregon State University, Corvallis, OR 97331, USA
Urszula T. Iwaniec, Skeletal Biology Laboratory, School of Biological and Population Health Sciences, Oregon State University, Corvallis, OR 97331, USA
William G. Helferich, Email: helferic@illinois.edu, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA.
References
- 1.Jemal A, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagemeister FB, Jr, et al. Causes of death in breast cancer: a clinicopathologic study. Cancer. 1980;46(1):162–167. doi: 10.1002/1097-0142(19800701)46:1<162::aid-cncr2820460127>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53(1):5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29(3):465–482. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen PR, et al. A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol. 1989;7(3):355–366. doi: 10.1200/JCO.1989.7.3.355. [DOI] [PubMed] [Google Scholar]
- 6.Lee YT. Patterns of metastasis and natural courses of breast carcinoma. Cancer Metastasis Rev. 1985;4(2):153–172. doi: 10.1007/BF00050693. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz KB. The year in basic science: update of estrogen plus progestin therapy for menopausal hormone replacement implicating stem cells in the increased breast cancer risk. Mol Endocrinol. 2008;22(12):2743–2750. doi: 10.1210/me.2008-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma H, et al. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 10.Kaaks R, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 11.Diaz LK, Sneige N. Estrogen receptor analysis for breast cancer: current issues and keys to increasing testing accuracy. Adv Anat Pathol. 2005;12(1):10–19. doi: 10.1097/00125480-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Skliris GP, et al. Estrogen receptor alpha negative breast cancer patients: estrogen receptor beta as a therapeutic target. J Steroid Biochem Mol Biol. 2008;109(1–2):1–10. doi: 10.1016/j.jsbmb.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Fox EM, Davis RJ, Shupnik MA. ERbeta in breast cancer-onlooker, passive player, or active protector? Steroids. 2008;73(11):1039–1051. doi: 10.1016/j.steroids.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omoto Y, et al. Estrogen receptor (ER) beta1 and ERbe-tacx/beta2 inhibit ERalpha function differently in breast cancer cell line MCF7. Oncogene. 2003;22(32):5011–5020. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- 15.Buteau-Lozano H, et al. Transcriptional regulation of vascular endothelial growth factor by estradiol and tamoxifen in breast cancer cells: a complex interplay between estrogen receptors alpha and beta. Cancer Res. 2002;62(17):4977–4984. [PubMed] [Google Scholar]
- 16.Chang EC, et al. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147(10):4831–41842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 17.Matthews J, et al. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol. 2006;20(3):534–543. doi: 10.1210/me.2005-0140. [DOI] [PubMed] [Google Scholar]
- 18.Roger P, et al. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61(6):2537–2541. [PubMed] [Google Scholar]
- 19.Leygue E, et al. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58(15):3197–3201. [PubMed] [Google Scholar]
- 20.Jarvinen TA, et al. Estrogen receptor beta is coexpressed with ERalpha and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am J Pathol. 2000;156(1):29–35. doi: 10.1016/s0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuqua SA, et al. Estrogen receptor beta protein in human breast cancer: correlation with clinical tumor parameters. Cancer Res. 2003;63(10):2434–2439. [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neill PA, et al. Wild-type oestrogen receptor beta (ERbetal) mRNA and protein expression in Tamoxifen-treated post-menopausal breast cancers. Br J Cancer. 2004;91(9):1694–1702. doi: 10.1038/sj.bjc.6602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platet N, et al. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51(1):55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Neven P, et al. Endocrine treatment and prevention of breast and gynaecological cancers. Eur J Cancer. 2002;38(Suppl 6):S1–S11. doi: 10.1016/s0959-8049(02)00267-8. [DOI] [PubMed] [Google Scholar]
- 25.Vergote I, et al. The oestrogen receptor and its selective modulators in gynaecological and breast cancer. Eur J Cancer. 2000;36(Suppl 4):S1–S9. doi: 10.1016/s0959-8049(00)00203-3. [DOI] [PubMed] [Google Scholar]
- 26.Hartman J, Strom A, Gustafsson JA. Estrogen receptor beta in breast cancer-diagnostic and therapeutic implications. Steroids. 2009;74(8):635–641. doi: 10.1016/j.steroids.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Warner M, Gustafsson JA. The role of estrogen receptor beta (ERbeta) in malignant diseases-a new potential target for antiproliferative drugs in prevention and treatment of cancer. Biochem Biophys Res Commun. 2010;396(1):63–66. doi: 10.1016/j.bbrc.2010.02.144. [DOI] [PubMed] [Google Scholar]
- 28.Yue W, et al. Effects of estrogen on breast cancer development: role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127(8):1748–1757. doi: 10.1002/ijc.25207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006;238(1):1–14. doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 31.Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339(8784):1–15. [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339(8785):71–85. [PubMed] [Google Scholar]
- 33.Gupta PB, et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67(5):2062–2071. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- 34.Banka CL, et al. Estrogen induces lung metastasis through a host compartment-specific response. Cancer Res. 2006;66(7):3667–3672. doi: 10.1158/0008-5472.CAN-05-4416. [DOI] [PubMed] [Google Scholar]
- 35.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 36.Miller FR. Tumor subpopulation interactions in metastasis. Invasion Metastasis. 1983;3(4):234–242. [PubMed] [Google Scholar]
- 37.Miller FR, Miller BE, Heppner GH. Characterization of metastatic heterogeneity among subpopulations of a single mouse mammary tumor: heterogeneity in phenotypic stability. Invasion Metastasis. 1983;3(1):22–31. [PubMed] [Google Scholar]
- 38.Hong X, et al. EBAG9 inducing hyporesponsiveness of T cells promotes tumor growth and metastasis in 4T1 murine mammary carcinoma. Cancer Sci. 2009;100(5):961–969. doi: 10.1111/j.1349-7006.2009.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–1405. [PubMed] [Google Scholar]
- 40.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58(7):1486–1493. [PubMed] [Google Scholar]
- 41.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im2002s39. Chapter 20: p. Unit-Uni2. [DOI] [PubMed] [Google Scholar]
- 42.Adams LS, et al. Blueberry phytochemicals inhibit growth and metastatic potential of MDA-MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2010;70(9):3594–3605. doi: 10.1158/0008-5472.CAN-09-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valster A, et al. Cell migration and invasion assays. Methods. 2005;37(2):208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Ju YH, et al. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131(11):2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 45.Ju YH, et al. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis. 2006;27(6):1292–1299. doi: 10.1093/carcin/bgi370. [DOI] [PubMed] [Google Scholar]
- 46.Hiraga T, et al. Zoledronic acid inhibits visceral metastases in the 4Tl/luc mouse breast cancer model. Clin Cancer Res. 2004;10(13):4559–4567. doi: 10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- 47.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 48.Ju YH, et al. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27(4):856–863. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- 49.Pike MC, et al. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 50.Gottardis MM, Robinson SP, Jordan VC. Estradiol-stimulated growth of MCF-7 tumors implanted in athymic mice: a model to study the tumoristatic action of tamoxifen. J Steroid Biochem. 1988;30(1–6):311–314. doi: 10.1016/0022-4731(88)90113-6. [DOI] [PubMed] [Google Scholar]
- 51.Alliende ME. Mean versus individual hormonal profiles in the menstrual cycle. Fertil Steril. 2002;78(1):90–95. doi: 10.1016/s0015-0282(02)03167-9. [DOI] [PubMed] [Google Scholar]
- 52.Kaaks R, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97(10):755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 53.Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8(5):444–448. doi: 10.1016/S1470-2045(07)70140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nogawa M, et al. Monitoring luciferase-labeled cancer cell growth and metastasis in different in vivo models. Cancer Lett. 2005;217(2):243–253. doi: 10.1016/j.canlet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Uneda S, et al. Anti-endoglin monoclonal antibodies are effective for suppressing metastasis and the primary tumors by targeting tumor vasculature. Int J Cancer. 2009;125(6):1446–1453. doi: 10.1002/ijc.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oviedo PJ, et al. Estradiol induces endothelial cell migration and proliferation through estrogen receptor-enhanced RhoA/ ROCK pathway. Mol Cell Endocrinol. 2011;335(2):96–103. doi: 10.1016/j.mce.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 57.Acconcia F, Barnes CJ, Kumar R. Estrogen and tamoxifen induce cytoskeletal remodeling and migration in endometrial cancer cells. Endocrinology. 2006;147(3):1203–1212. doi: 10.1210/en.2005-1293. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez AM, et al. Estrogen receptor-alpha promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP . Mol Endocrinol. 2010;24(11):2114–2125. doi: 10.1210/me.2010-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kedrin D, et al. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12(2–3):143–152. doi: 10.1007/s10911-007-9046-4. [DOI] [PubMed] [Google Scholar]
- 60.Giretti MS, et al. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS ONE. 2008;3(5):e2238. doi: 10.1371/journal.pone.0002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng S, et al. 17beta-Estradiol enhances breast cancer cell motility and invasion via extra-nuclear activation of actin-binding protein ezrin. PLoS ONE. 2011;6(7):e22439. doi: 10.1371/journal.pone.0022439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89(17):1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 63.Nilsson UW, Garvin S, Dabrosin C. MMP-2 and MMP-9 activity is regulated by estradiol and tamoxifen in cultured human breast cancer cells. Breast Cancer Res Treat. 2007;102(3):253–261. doi: 10.1007/s10549-006-9335-4. [DOI] [PubMed] [Google Scholar]
- 64.Migliaccio A, et al. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15(6):1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z, et al. The role of adapter protein Shc in estrogen non-genomic action. Steroids. 2004;69(8–9):523–529. doi: 10.1016/j.steroids.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 66.Song RX, et al. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101(7):2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Z, Barnes CJ, Kumar R. Human epidermal growth factor receptor 2 status modulates subcellular localization of and interaction with estrogen receptor alpha in breast cancer cells. Clin Cancer Res. 2004;10(11):3621–3628. doi: 10.1158/1078-0432.CCR-0740-3. [DOI] [PubMed] [Google Scholar]
- 68.Restall C, et al. A novel histone deacetylase inhibitor augments tamoxifen-mediated attenuation of breast carcinoma growth. Int J Cancer. 2009;125(2):483–487. doi: 10.1002/ijc.24350. [DOI] [PubMed] [Google Scholar]