Abstract
BACKGROUND:
The magnitude and implication of variation in end-of-life decision-making among ICUs in the United States is unknown.
METHODS:
We reviewed data on decisions to forgo life-sustaining therapy (DFLSTs) in 269,002 patients admitted to 153 ICUs in the United States between 2001 and 2009. We used fixed-effects logistic regression to create a multivariable model for DFLST and then calculated adjusted rates of DFLST for each ICU.
RESULTS:
Patient factors associated with increased odds of DFLST included advanced age, female sex, white race, and poor baseline functional status (all P < .001). However, associations with several of these factors varied among ICUs (eg, black race had an OR for DFLST from 0.18 to 2.55 across ICUs). The ICU staffing model was also found to be associated with DFLST, with an open ICU staffing model associated with an increased odds of a DFLST (OR = 1.19). The predicted probability of DFLST varied approximately sixfold among ICUs after adjustment for the fixed patient and ICU effects and was directly correlated with the standardized mortality ratios of ICUs (r = 0.53, 0.41–0.68).
CONCLUSION:
Although patient factors explain much of the variability in DFLST practices, significant effects of ICU culture and practice influence end-of-life decision-making. The observation that an ICU’s risk-adjusted propensity to withdraw life support is directly associated with its standardized mortality ratio suggests problems with using the latter as a quality measure.
One in five Americans die during or shortly after an ICU stay; therefore, admission to an ICU is a time when many decisions to forgo life-sustaining therapy (DFLSTs) are made.1 These decisions include do-not-resuscitate orders, decisions to withhold or withdraw mechanical ventilation (MV) or other specific interventions, and more general decisions to redirect the overriding goals of care from curative to palliative. Prior studies have shown that most patients dying in an ICU do so following a DFLST.2,3
Proponents of shared decision-making4 suggest that DFLSTs should be influenced by patients’ values and by providers’ assessments of prognosis. For the majority of patients in the ICU who cannot express their wishes at the time decisions need to be made,5,6 it is advocated that such preferences be elicited from advance directives or surrogate decision-makers. Evidence that a variety of patient characteristics influences the odds of DFLST7‐11 is consistent with the view that patients’ preferences do guide such decisions.
If a compendium of patient characteristics can be identified that reliably predicts who will and who will not have a DFLST, then after adjustment for such characteristics among large samples of patients in the ICU, rates of DFLSTs should be reasonably consistent among ICUs. Whether patient characteristics such as age and race are causally associated with different preferences for the aggressiveness of end-of-life care, or are mere markers of such preferences, adjustment for such factors should substantially reduce the among-ICU variability in DFLST rates if these critical decisions are being made in ways that promote patients’ values. We, therefore, evaluated the extent to which patient characteristics are associated with DFLSTs among a large, contemporary sample of patients admitted to ICUs across the United States. We evaluated the operating characteristics of a predictive model for DFLSTs overall and then determined the extent of among-ICU variability in overall DFLST rates after adjusting for the characteristics included in this model. We used similar approaches to determine whether certain patient factors, such as age and race, are consistently or variably associated with DFLSTs among ICUs. Finally, we explored the relationship between an ICU’s probability of initiating a DFLST for a given patient and that ICU’s standardized mortality ratio (SMR) to assess the potential consequences of differing DFLST rates for quality reporting.
Materials and Methods
Data Source
Using the Project IMPACT database (Cerner Corp), we performed a retrospective cohort study on patients admitted to ICUs between 2001 and 2009. Project IMPACT is a voluntary, fee-based ICU clinical information system that is commonly used in critical care outcomes research.12‐15 Each enrolled ICU employs a staff member who is trained to use a standardized web-based instrument to collect data on individual patients, processes of care, and ICU characteristics.
Patients and Outcome Variable
To preserve independence between observations, we declared patients as eligible only during their index ICU admission; additional ICU admissions during a single hospitalization or during subsequent hospitalizations were excluded. We restricted our dataset to patients who were “full code” at the time of ICU admission, thereby enabling assessments of new DFLSTs. Additionally, we excluded patients who were (1) ineligible for severity adjustment using the Mortality Prediction Model III (MPM0-III) (typically, because of burns or cardiac surgery),16 (2) < 18 years old, (3) being managed in the ICU only for purposes of organ recovery, or (4) admitted to an ICU that collected data in Project IMPACT for < 1 year or contributed < 20 patients during the 3-month period in which the patient was admitted (e-Fig 1 (442.4KB, pdf) ).
The primary outcome was a DFLST, defined as any change in “code status” from no limitations on care at the time of ICU admission to any limitation(s) on care prior to ICU discharge. Limitations on care included (1) a do-not-resuscitate order; (2) an order to withhold CPR plus one or more of the following: MV, cardioversion, dialysis, and/or other potentially life-prolonging therapies; or (3) an order for comfort measures only or hospice care. For ICU survivors, a DFLST was coded if any of the forgoing limitations on care were coded at the time of ICU discharge. For patients who died in the ICU, code status at discharge was not recorded uniformly. We, therefore, considered a DFLST to have occurred if there was no event code for CPR on either the day of death or the preceding day. We confirmed the validity of this approach by finding that all the 3,298 patients who died in the ICU without a DFLST by our coding scheme had a CPR event code.
Statistical Analysis
We assessed the relationships between patient characteristics and the outcome of DFLST using logistic regression models. After computing unadjusted relationships, we created multivariable models by including covariates based on prior studies and/or physiologic or mechanistic hypotheses or if associations with the outcome in unadjusted analyses had an OR of > 1.2 or < 0.8 and an associated P value of < .001 (because of the large sample size). To adjust for severity of illness, we used the log of the MPM0-III score, which was validated in this dataset for predicting the probability of in-hospital mortality.16 We entered a fixed effect for year to avoid confounding by temporal changes in DFLST practices. Additionally, each ICU was modeled as a fixed effect to account for correlation of outcomes within center and to enable measurement of center effects. We used backward selection based on the likelihood ratio test, as well as tests for interaction, to develop the final model.
Best-fit regression models were first run among all eligible patients. We then performed two analyses restricted to patients less likely to make substantial contributions to real-time decision-making: (1) patients who had undergone prolonged acute MV (PAMV), defined as MV for at least 96 consecutive hours during their ICU stay,17 and (2) patients admitted with a Glasgow Coma Score (GCS) of 3 to 5 at the time of ICU admission. Many such patients are unable to participate in medical decision-making and may, therefore, be more vulnerable to variable end-of-life practices among providers, ICUs, or health systems. We also ran the same models among stratified samples of patients who did vs did not survive their initial ICU stay. In each case, we tested model discrimination using the area under the receiver operating characteristic curve.18 We then created a second model in which we included several measured ICU factors, including ICU staffing model, nocturnal intensivist coverage, and the presence of a critical care fellowship, to examine the influence of these measurable ICU effects on DFLST.
We ran the fully adjusted model in each ICU individually to evaluate among-ICU variability in the strengths of relationships between the prespecified patient factors of age (< 65 years vs ≥ 65 years) and race (white vs black) on DFLSTs. Finally, we calculated each ICU’s SMR and used Pearson correlations to investigate the relationship between SMR and adjusted DFLST rates. The SMR is defined as the ratio between observed and expected death rates, with the expected number of deaths based on the sum of the MPM0-III score for patients in the ICU.
We handled missing data in our primary analyses by excluding the two ICUs that accounted for > 50% of missing data on DFLST and the additional 1,420 patients in other ICUs (0.5%) for whom coding of limitations on care at the time of ICU discharge was missing. To assess the impact of missing data, we conducted sensitivity analyses in which we first coded all patients missing data on DFLST as having had a DFLST and then as having not had a DFLST. By using these two extreme assumptions and comparing the point estimates of predictor variables with those obtained in the primary model, we gauged the maximal potential influence of missing data.
All analyses were performed using STATA 12.1 (StataCorp LP). The institutional review board of the University of Pennsylvania approved this study (protocol number 816393; IRB board No. 7).
Results
The full dataset contained 400,128 patients admitted to 196 ICUs between April 1, 2001 and February 29, 2009. Exclusions are shown in e-Figure 1 (442.4KB, pdf) . The final analytic dataset included 270,442 patients admitted to 153 ICUs in 105 hospitals in the United States with no limitations on care in place at the time of ICU admission.
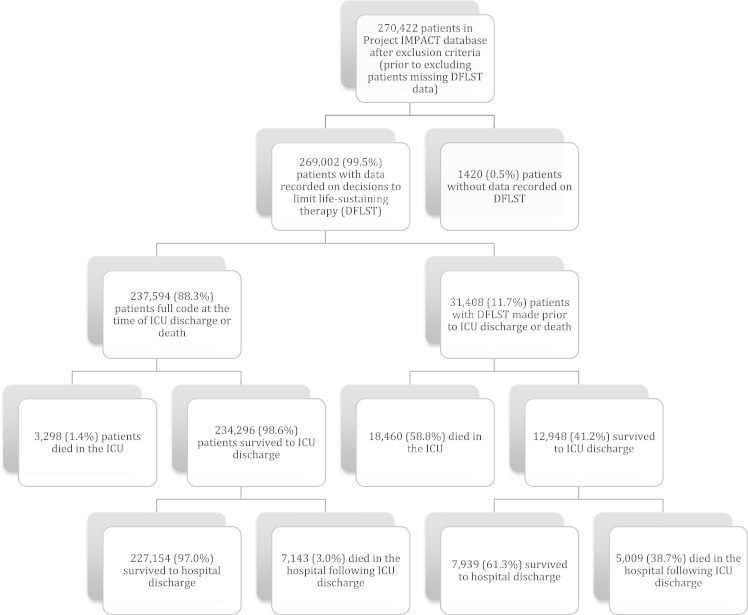
Among the 269,002 patients (99.5%) with information on DFLST at discharge or death, 31,408 (11.7%) had a DFLST made in the ICU (Fig 1). The sample had considerable diversity regarding age, sex, race, insurance status, and functional limitations on ICU admission, each of which was associated with DFLST in unadjusted analyses (e-Table 1 (442.4KB, pdf) ). In models adjusted for the predicted probability of death, year of admission, admission diagnosis, need for MV or vasopressors, and several comorbid conditions (e-Table 2 (442.4KB, pdf) ), patient characteristics independently associated with the odds of a new DFLST included older age, increased functional limitations prior to ICU admission, and being a medical rather than surgical patient; black race and other nonwhite races were associated with decreased odds of DFLST (Table 1). Sensitivity analyses examining the impact of missing outcome data revealed that no point estimate of any covariate changed by > 10% when all missing patients were coded as either having a DFLST or not having a DFLST (e-Table 3 (442.4KB, pdf) ).
Figure 1 .
– Patient flowchart showing the rate and outcome of DFLST among patients in the Project IMPACT database.
TABLE 1 .
] Adjusted Patient Characteristics Associated With DFLST
| Patient Characteristics at ICU Admission | Multivariable Models | |||||
| All Patients (N = 269,002) | Patients Requiring PAMV (n = 27,030) | Patients With GCS 3 to 5 on Admission (n = 14,923) | ||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | ||||||
| < 65 y | Reference | … | … | … | … | … |
| 65-74 y | 1.50 (1.43–1.58) | a | 1.62 (1.46–1.79) | a | 1.30 (1.14–1.49) | a |
| 75-84 y | 2.18 (2.07–2.30) | a | 2.50 (2.24–2.79) | a | 1.69 (1.45–1.95) | a |
| >84 y | 3.44 (3.23–3.67) | a | 3.60 (3.06–4.23) | a | 2.17 (1.76–2.68) | a |
| Race/ethnicity | ||||||
| White | Reference | … | … | … | … | … |
| Black | 0.73 (0.70–0.77) | a | 0.69 (0.62–0.77) | a | 0.65 (0.57–0.73) | a |
| Other | 0.79 (0.74–0.85) | a | 0.74 (0.64–0.86) | a | 0.84 (0.72–0.99) | .04 |
| Sex | ||||||
| Male | Reference | … | … | … | … | … |
| Female | 1.09 (1.05–1.12) | a | 1.09 (1.02–1.16) | b | 1.14 (1.05–1.24) | b |
| Functional status on admission | ||||||
| Independent | Reference | … | … | … | … | … |
| Partially dependent | 1.78 (1.71–1.85) | a | 1.43 (1.31–1.57) | a | 1.13 (0.99–1.29) | .06 |
| Fully dependent | 2.29 (2.16–2.42) | a | 1.28 (1.14–1.44) | a | 1.48 (1.29–1.71) | a |
| Insurance | ||||||
| Private | Reference | … | … | … | … | … |
| Medicare | 1.12 (1.07–1.17) | a | 1.13 (1.02–1.25) | .02 | 1.23 (1.08–1.40) | b |
| Medicaid | 1.03 (0.96–1.10) | .42 | 1.02 (0.90–1.16) | .70 | 0.95 (0.82–1.11) | .51 |
| Self-pay | 1.15 (1.08–1.22) | a | 1.06 (0.93–1.19) | .45 | 1.15 (1.01–1.31) | .04 |
| Other | 1.04 (0.94–1.15) | .40 | 1.13 (0.94–1.37) | .18 | 1.22 (0.97–1.53) | .08 |
| Source of ICU admission | ||||||
| ED | Reference | … | … | … | … | … |
| Direct admission | 1.06 (1.00–1.13) | .06 | 1.05 (0.92–1.19) | .49 | 1.10 (0.94–1.28) | .24 |
| General care floor | 1.56 (1.49–1.64) | a | 1.29 (1.17–1.42) | a | 0.87 (0.75–1.00) | .05 |
| Step-down unit | 1.45 (1.34–1.56) | a | 1.20 (1.04–1.39) | .01 | 0.70 (0.55–0.90) | b |
| Procedure | 0.76 (0.70–0.81) | a | 1.00 (0.86–1.16) | .99 | 0.71 (0.59–0.86) | a |
| SNF, rehab, LTACH | 0.77 (0.65–0.91) | b | 0.78 (0.56–1.08) | .13 | 0.49 (0.31–0.76) | b |
| Another ICU | 1.12 (1.01–1.24) | .03 | 1.01 (0.86–1.19) | .87 | 0.88 (0.65–1.17) | .38 |
| Other | 0.77 (0.68–0.87) | a | 0.77 (0.58–1.03) | .08 | 0.99 (0.68–1.44) | .97 |
| Patient type | ||||||
| Elective surgery | Reference | … | … | … | … | … |
| Emergency surgery | 1.58 (1.47–1.70) | a | 0.95 (0.82–1.11) | .52 | 1.05 (0.82–1.34) | .71 |
| Medical | 2.09 (1.93–2.27) | a | 1.30 (1.11–1.52) | a | 1.33 (1.04–1.71) | .02 |
DFLST = decision to forgo life-sustaining therapy; GCS = Glasgow Coma Score; LTACH = long-term acute care hospital; PAMV = prolonged acute mechanical ventilation; SNF = skilled nursing facility.
P < .001.
P < .01.
The final patient-level model performed best among patients who did not require PAMV or had a low GCS (pseudo-R2, 0.35; area under the receiver operating characteristic curve, 0.90) (Table 2). Discrimination of this model was significantly better than that among patients requiring PAMV (P < .001) and among those with a GCS of 3 to 5 (P < .001) (Table 2). The associations of patient factors with DFLST were similar among patients dying in the ICU and those surviving to ICU discharge, and the fit and discrimination of the patient-characteristic model were significantly better among survivors than among patients who died in the ICU (e-Table 4 (442.4KB, pdf) ).
TABLE 2 .
] Model Fit Comparison
| Patient Population | No. Patients (n) | Pseudo-R2 | Area Under ROC Curve | P Value |
| All patients | 269,002 | 0.34 | 0.89 | NA |
| Patients without PAMV and with GCS > 5 | 230,987 | 0.35 | 0.90 | … |
| Patients requiring PAMV | 27,030 | 0.24 | 0.82 | < .001a |
| Patients with GCS 3 to 5 on admission | 14,923 | 0.25 | 0.82 | < .001a |
NA = not applicable; ROC = receiver operating characteristic. See Table 1 for expansion of other abbreviations.
P value is for comparison with model for patients without PAMV and with GCS > 5.
When we added the fixed ICU effects of staffing intensity, nocturnal coverage model, and presence of critical care fellows, we found that the lower-intensity ICU staffing model, defined as ICUs that were open with or without optional critical care consult, was associated with an increased odds of a DFLST (OR, 1.19 [1.15–1.23]) (Table 3). In multivariate analysis, there was no difference among a variety of nocturnal staffing models or the presence of critical care fellows on the odds of a DFLST.
TABLE 3 .
] Fixed ICU Effects
| ICU Characteristic | % Patients | OR (95% CI) | P Value |
| ICU staffing model | |||
| Closed or open with mandatory consult | 34.3 | Reference | … |
| Open with optional consult | 65.7 | 1.15 (1.11–1.19) | < .0001 |
| Nocturnal staffing model | |||
| Critical care physician | 30.5 | Reference | … |
| Other physician | 59.9 | 0.99 (0.95–1.02) | .416 |
| No physician coverage | 9.6 | 0.95 (0.89–1.00) | .060 |
| Critical care fellowship program | |||
| No | 61.6 | Reference | … |
| Yes | 38.4 | 1.02 (0.99–1.06) | .205 |
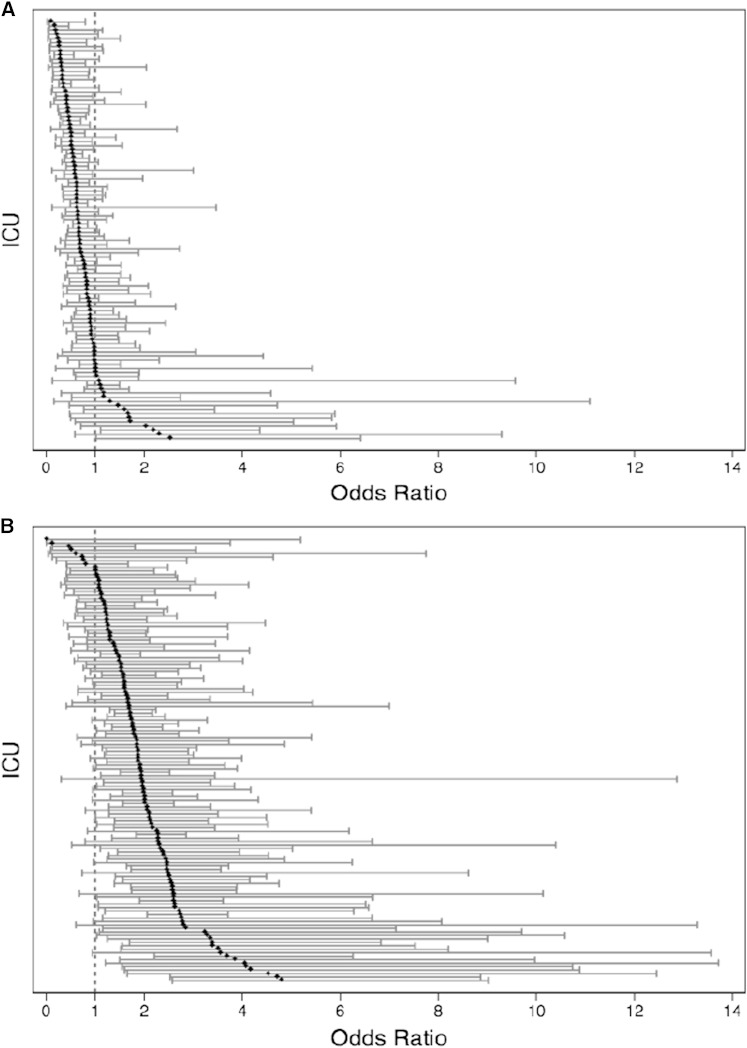
Among the 102 ICUs in which > 5% of admitted patients were black, the OR relating race (black vs white) with DFLST varied considerably (range of ICU-specific ORs, 0.18–2.55) (Fig 2A). The percentage of black patients within each ICU ranged from 5.4% to 90.7% (mean, 24.5%), but this was unrelated to the strength of the effect of race on DFLST (OR, 1.12 [0.89–1.34]; P = .26). An analysis of whether the association between age and DFLST varied among ICUs revealed similarly large variability in the magnitude of the effect, although in nearly all ICUs, patients ≥ 65 years of age were more likely to have DFLSTs than were younger patients (Fig 2B). The effect of female sex on the odds of DFLST ranged from 0.60 to 2.86, but the majority of the point estimates were equal to or slightly greater than one, suggesting that the relationship between sex and DFLST is relatively conserved across ICUs.
Figure 2 .
– A, Adjusted odds of DFLST for black patients. Each point represents the adjusted odds of a DFLST for black patients in each ICU accompanied by 95% CI. Results stem from fully adjusted models. The reference is white patients. Total number of ICUs is 105 because ICUs with < 5% black patients were excluded from this analysis. B, Adjusted odds of DFLST for patients ≥ 65 years old. Each point represents the adjusted odds of a DFLST for patients ≥ 65 years old in each ICU accompanied by 95% CI. Results stem from fully adjusted models. The reference is age < 65 years. See Figure 1 legend for expansion of abbreviation.
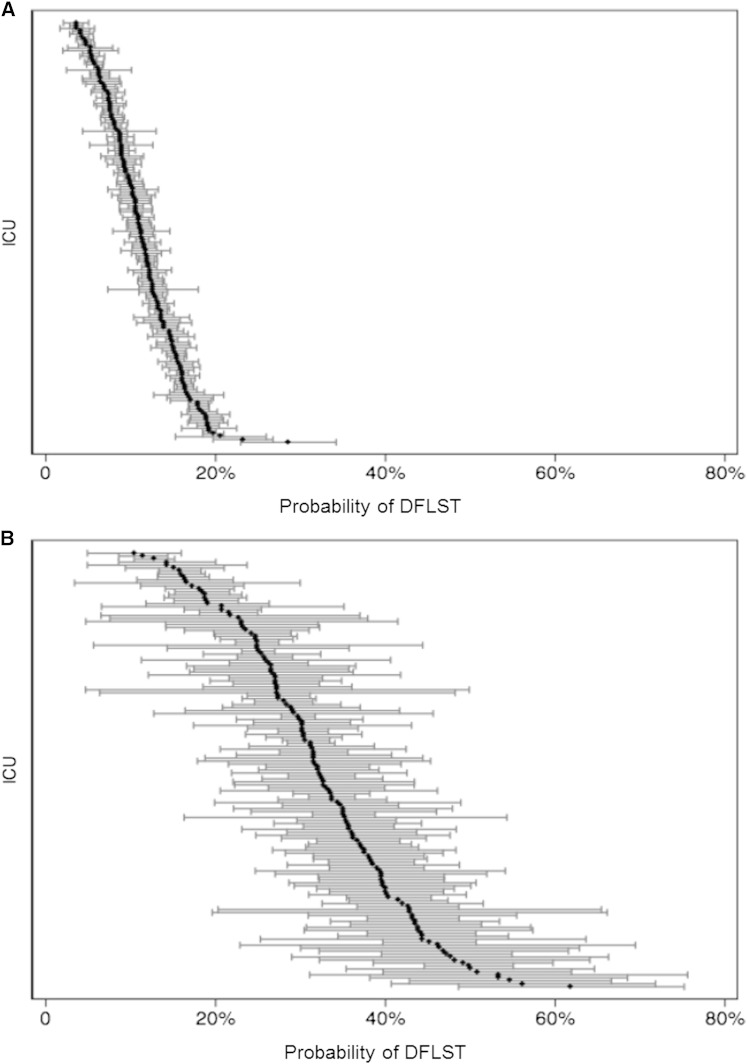
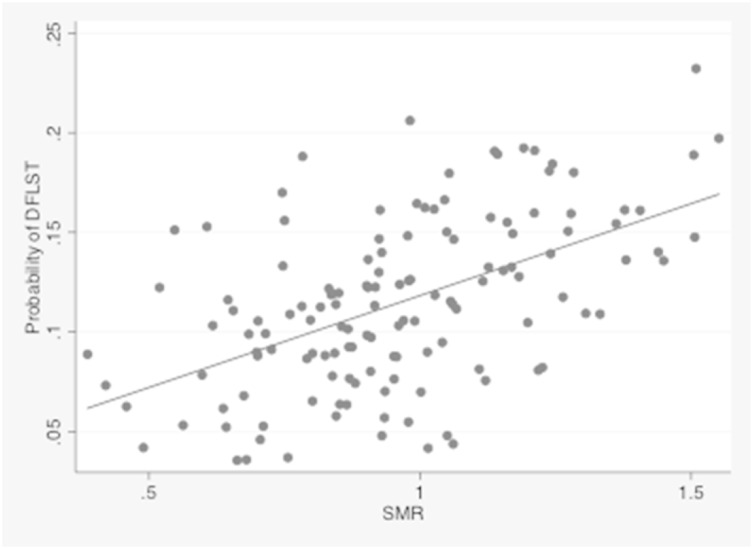
After adjustment for all measured patient factors, the predicted probability of DFLST among ICUs varied nearly sixfold (median, 11.5%; range, 3.5%–20.6%) (Fig 3A). Among patients undergoing PAMV, DFLST rates were expectedly higher, and among-ICU variability was similarly large (median, 32.3%; range, 12.3%–61.7%) (Fig 3B). Among ICUs, the predicted DFLST rate was strongly associated with its SMR, such that ICUs that were more likely to limit life support had worse risk-adjusted mortality rates (Pearson’s r, 0.53 [0.41–0.68]; P < .001) (Fig 4).
Figure 3 .
– A, Adjusted predicted probability of DFLST by ICU. The adjusted predicted probability of a DFLST was calculated from the fully adjusted model. The median was 11.5%, with a range from 3.5% to 20.6%. B, Adjusted predicted probability of DFLST by ICU for patients requiring prolonged acute mechanical ventilation (PAMV). The median predicted probability of DFLST among patients requiring PAMV was 32.3%, with a range from 12.3% to 61.7%. See Figure 1 for expansion of other abbreviation.
Figure 4 .
– Adjusted probability of DFLST by ICU and SMR. The adjusted DFLST rate was directly correlated to the corresponding ICU’s SMR (Pearson’s r, 0.53 [0.41–0.68]; P < .001). SMR = standardized mortality ratio. See Figure 1 for expansion of other abbreviation.
Discussion
Making decisions to limit life-sustaining therapies in an ICU is a complex process that may be influenced by the characteristics of the patients,7‐11 family members,19 providers,20 and institutions in which the decisions are made.21 Prior studies have made clear that making such decisions is strongly associated with patients’ clinical and demographic characteristics.3,7,9‐11,22‐29 These relationships are unlikely to be directly causal. Rather, older age and greater functional limitations, for example, are likely to be markers of preferences for less aggressive care at the end of life. Thus, the consistent associations between patient factors and DFLST observed in this and prior studies, and the outstanding discrimination of the patient-characteristics model found in the current study, support the view that patient characteristics and preferences strongly influence end-of-life decision-making in the ICU.
The current study reveals several novel insights into how end-of-life decisions are made in the ICU. First, patient characteristics explain more variation in the odds of a DFLST and better discriminate between patients who would or would not have a DFLST among patients likely to have participated in these decisions than among patients who may be less able to participate. This finding indicates that when patients are unable to participate in decision-making, the values and preferences of surrogate decision-makers, or providers, or the characteristics of ICUs and hospitals may hold greater sway over the decisions that are made. Further support for the great role non-patient-centered influences play in DFLSTs stems from the considerable variability identified in adjusted rates of DFLST among ICUs. Although the possibility of incomplete adjustment for differences in case mix limits the ability to quantify this variability with precision, the finding of nearly sixfold variability among ICUs suggests that the ICU to which a given patient is admitted influences his or her odds of having a DFLST.
A second important finding of our study concerns the influence of several patient factors on DFLSTs. We found that women were more likely to have DFLSTs than were men, whether or not they survived their ICU stay. Because this relationship was relatively conserved among ICUs, this observation likely indicates that critically ill women have less aggressive treatment preferences than men or are more likely to have surrogates who make DFLSTs than do men. By contrast, we found that race, and to some extent age, have variable effects on DFLST across ICUs. Overall, as in prior studies,9,10,30‐33 black and other nonwhite patients were less likely than white patients to have DFLSTs. However, a novel finding of this study is that this effect is not conserved among all ICUs and, in fact, in a minority of ICUs, black patients were more likely to have DFLSTs. This suggests that observed race-based variations in DFLST may be explained, at least in part, by how providers within ICUs communicate with patients of different races. Because the size of the race effect was not associated with the percentage of black patients cared for in a given ICU, these ICU effects are unlikely to be attributable to certain providers being less experienced in caring for black patients. We were unable to adjust for education, religion, and income, all of which may confound the relationship between race and DFLST. Future investigations are needed to better understand how and why ICUs vary in their approaches to end-of-life decision-making among minorities.
The increased odds of a DFLST in ICUs that are staffed with an open staffing model, defined as ICUs that are open with or without optional critical care consult, is also worthy of discussion. It is possible that patients in open ICUs are cared for primarily by physicians who have known them over time and are, therefore, better positioned to have discussions and make recommendations regarding the use of life support. Alternatively, it may be that intensivists have a bias toward technologically aggressive care and are less likely to limit or withdraw care than are internists or subspecialists. It is also possible that open ICUs are more likely to admit patients who are unlikely to benefit from ICU care and that such patients are more likely to have limits placed on life-sustaining therapies. Finally, ICU staffing may be associated with some other process of care or ICU structure that was not measured in this study. This novel association warrants further investigation.
In contrast to a study in Italian ICUs,34 our study found that ICUs with a higher adjusted probability of DFLST also had higher SMRs. There are at least two possible explanations for our results. First, it is possible that in the United States, ICUs with higher rates of DFLST are truly of poorer quality. Alternatively, and perhaps more likely, the SMR may be a poor quality indicator by virtue of defining quality as survival, and, therefore, being insensitive to other measures of quality, such as quality of death and dying and appropriate resource use near the end of life. Given the implications of this potential unintended consequence of measuring, let alone publicly reporting, SMRs, further research is needed to confirm this finding.
This study is limited by the fact that unmeasured patient factors may influence the probability of a DFLST being made. However, the models we generated exhibited good fit and excellent discrimination, suggesting that the addition of data on socioeconomic status, education, religion, or other factors would not alter the results appreciably. For similar reasons, unmeasured patient characteristics are unlikely to explain the variance in predicted probabilities of DFLST among ICUs. An additional limitation of this study is that, as is the case for all analyses of registry data, coding inaccuracies and missing data could bias the results. However, the concordance of our results regarding patient factors associated with DFLST with those from prior smaller studies, and the insensitivity of our results to extreme assumptions about the values of missing data points, suggest that any attributable bias is minimal. Finally, without measures of true patient-centered outcomes, such as families’ perceptions of the quality of dying,35 it is uncertain whether DFLST rates are too high in some ICUs, too low in others, or both.
Conclusions
By suggesting that substantial ICU-level variability in DFLST rates persist after accounting for patient characteristics, this study highlights opportunities for improving the patient-centeredness of end-of-life decision-making across the United States. Specifically, understanding differences in how physicians reach and convey prognostic judgments, and how ICU organizational factors influence DFLSTs, may enable targeted interventions to improve the quality of end-of-life care in all ICUs. Finally, the novel finding that the adjusted rate of DFLST of an ICU is directly correlated with its SMR has potential policy implications in an era of increasing physician and hospital accountability and quality reporting.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: C. M. Q. takes responsibility for the integrity of the work as a whole. C. M. Q. contributed to the concept and design of the study, data acquisition, analysis, and interpretation, and drafting and revision of the manuscript; S. J. R. and M. O. H. contributed to the design of the study, data analysis, and interpretation and revision of the manuscript; and S. D. H. contributed to the concept and design of the study, data acquisition, analysis, and interpretation, and revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- DFLST
decision to forgo life-sustaining therapy
- GCS
Glasgow Coma Score
- MPM0-III
Mortality Prediction Model III
- MV
mechanical ventilation
- PAMV
prolonged acute mechanical ventilation
- SMR
standardized mortality ratio
Footnotes
FOR EDITORIAL COMMENT SEE PAGE 532
FUNDING/SUPPORT: Dr Quill was supported by National Institutes of Health T32HL098054 Training in Critical Care Health Policy Research.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Angus DC, Barnato AE, Linde-Zwirble WT, et al. ; Robert Wood Johnson Foundation ICU End-Of-Life Peer Group. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638-643. [DOI] [PubMed] [Google Scholar]
- 2.Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163-1167. [DOI] [PubMed] [Google Scholar]
- 3.Pochard F, Azoulay E, Chevret S, et al. ; French PROTOCETIC Group. French intensivists do not apply American recommendations regarding decisions to forgo life-sustaining therapy. Crit Care Med. 2001;29(10):1887-1892. [DOI] [PubMed] [Google Scholar]
- 4.Davidson JE, Powers K, Hedayat KM, et al. ; American College of Critical Care Medicine Task Force 2004-2005, Society of Critical Care Medicine. Clinical practice guidelines for support of the family in the patient-centered intensive care unit: American College of Critical Care Medicine Task Force 2004-2005. Crit Care Med. 2007;35(2):605-622. [DOI] [PubMed] [Google Scholar]
- 5.Silveira MJ, Kim SY, Langa KM. Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care. 2008;12(suppl 3):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips RS, Wenger NS, Teno J, et al. ; SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Choices of seriously ill patients about cardiopulmonary resuscitation: correlates and outcomes. Am J Med. 1996;100(2):128-137. [DOI] [PubMed] [Google Scholar]
- 8.The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598. [PubMed] [Google Scholar]
- 9.Nathens AB, Rivara FP, Wang J, Mackenzie EJ, Jurkovich GJ. Variation in the rates of do not resuscitate orders after major trauma and the impact of intensive care unit environment. J Trauma. 2008;64(1):81-88. [DOI] [PubMed] [Google Scholar]
- 10.Jayes RL, Zimmerman JE, Wagner DP, Knaus WA. Variations in the use of do-not-resuscitate orders in ICUS. Findings from a national study. Chest. 1996;110(5):1332-1339. [DOI] [PubMed] [Google Scholar]
- 11.Azoulay E, Metnitz B, Sprung CL, et al. ; SAPS 3 investigators. End-of-life practices in 282 intensive care units: data from the SAPS 3 database. Intensive Care Med. 2009;35(4):623-630. [DOI] [PubMed] [Google Scholar]
- 12.Brown SE, Ratcliffe SJ, Kahn JM, Halpern SD. The epidemiology of intensive care unit readmissions in the United States. Am J Respir Crit Care Med. 2012;185(9):955-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Company overview of Cerner Project IMPACT, Inc. Bloomberg Businessweek website. http://investing.businessweek.com/research/stocks/private/snapshot.asp?privcapId = 24623915. Accessed February 12, 2014.
- 14.Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP; DELAY-ED Study Group. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007;35(6):1477-1483. [DOI] [PubMed] [Google Scholar]
- 15.Nasraway SA, Jr, Albert M, Donnelly AM, Ruthazer R, Shikora SA, Saltzman E. Morbid obesity is an independent determinant of death among surgical critically ill patients. Crit Care Med. 2006;34(4):964-970. [DOI] [PubMed] [Google Scholar]
- 16.Higgins TL, Teres D, Copes WS, Nathanson BH, Stark M, Kramer AA. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III). Crit Care Med. 2007;35(3):827-835. [DOI] [PubMed] [Google Scholar]
- 17.Zilberberg MD, de Wit M, Pirone JR, Shorr AF. Growth in adult prolonged acute mechanical ventilation: implications for healthcare delivery. Crit Care Med. 2008;36(5):1451-1455. [DOI] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemenshow S. Applied Logistic Regression. 2nd ed Hoboken, NJ: John Wiley and Sons; 2000. [Google Scholar]
- 19.Schenker Y, Crowley-Matoka M, Dohan D, Tiver GA, Arnold RM, White DB. I don’t want to be the one saying ‘we should just let him die’: intrapersonal tensions experienced by surrogate decision makers in the ICU. J Gen Intern Med. 2012;27(12):1657-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garland A, Connors AF. Physicians’ influence over decisions to forego life support. J Palliat Med. 2007;10(6):1298-1305. [DOI] [PubMed] [Google Scholar]
- 21.Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClish DK, Powell SH, Montenegro H, Nochomovitz M. The impact of age on utilization of intensive care resources. J Am Geriatr Soc. 1987;35(11):983-988. [DOI] [PubMed] [Google Scholar]
- 23.Goodlin SJ, Zhong Z, Lynn J, et al. Factors associated with use of cardiopulmonary resuscitation in seriously ill hospitalized adults. JAMA. 1999;282(24):2333-2339. [DOI] [PubMed] [Google Scholar]
- 24.Stolman CJ, Gregory JJ, Dunn D, Ripley B. Evaluation of the do not resuscitate orders at a community hospital. Arch Intern Med. 1989;149(8):1851-1856. [PubMed] [Google Scholar]
- 25.O’Donnell H, Phillips RS, Wenger N, Teno J, Davis RB, Hamel MB. Preferences for cardiopulmonary resuscitation among patients 80 years or older: the views of patients and their physicians. J Am Med Dir Assoc. 2003;4(3):139-144. [DOI] [PubMed] [Google Scholar]
- 26.Barnato AE, Angus DC. Value and role of intensive care unit outcome prediction models in end-of-life decision making. Crit Care Clin. 2004;20(3):345-362. [DOI] [PubMed] [Google Scholar]
- 27.Wenger NS, Pearson ML, Desmond KA, et al. Epidemiology of do-not-resuscitate orders. Disparity by age, diagnosis, gender, race, and functional impairment. Arch Intern Med. 1995;155(19):2056-2062. [PubMed] [Google Scholar]
- 28.Fowler RA, Sabur N, Li P, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ. 2007;177(12):1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapoport J, Teres D, Lemeshow S. Resource use implications of do not resuscitate orders for intensive care unit patients. Am J Respir Crit Care Med. 1996;153(1):185-190. [DOI] [PubMed] [Google Scholar]
- 30.Marrie TJ, Fine MJ, Kapoor WN, Coley CM, Singer DE, Obrosky DS. Community-acquired pneumonia and do not resuscitate orders. J Am Geriatr Soc. 2002;50(2):290-299. [DOI] [PubMed] [Google Scholar]
- 31.Smith AK, Earle CC, McCarthy EP. Racial and ethnic differences in end-of-life care in fee-for-service Medicare beneficiaries with advanced cancer. J Am Geriatr Soc. 2009;57(1):153-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnato AE, Chang CC, Saynina O, Garber AM. Influence of race on inpatient treatment intensity at the end of life. J Gen Intern Med. 2007;22(3):338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnato AE, Berhane Z, Weissfeld LA, Chang CC, Linde-Zwirble WT, Angus DC; Robert Wood Johnson Foundation ICU End-of-Life Peer Group. Racial variation in end-of-life intensive care use: a race or hospital effect? Health Serv Res. 2006;41(6):2219-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertolini G, Boffelli S, Malacarne P, et al. End-of-life decision-making and quality of ICU performance: an observational study in 84 Italian units. Intensive Care Med. 2010;36(9):1495-1504. [DOI] [PubMed] [Google Scholar]
- 35.Patrick DL, Curtis JR, Engelberg RA, Nielsen E, McCown E. Measuring and improving the quality of dying and death. Ann Intern Med. 2003;139(5 pt 2):410-415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement