Abstract
Background
Dementia is associated with disruptions in sleep and sleep quality for patients and their family caregivers. Little is known about the impact of frontotemporal dementia (FTD) on sleep.
Objective
The purpose of this study was to characterize sleep in patients with frontotemporal dementia and their family caregivers.
Methods
Twenty-two patient-caregiver dyads were enrolled: Thirteen behavioral variant FTD (bvFTD) and nine semantic dementia (SD). Sleep and sleep quality data were collected for two weeks using diaries and Actiwatches.
Results
Patients with bvFTD and SD spent more time in bed at night compared to their caregivers. Nighttime behaviors were reported more frequently by caregivers for the bvFTD patients and strongly correlated with caregiver distress. Actigraphy data demonstrated normal sleep efficiency and timing of the nighttime sleep period for both patients and their caregivers. Caregivers of patients with bvFTD reported poorer sleep quality compared to the SD caregivers. A greater number of bvFTD caregivers compared to SD reported negative aspects of sleep quality for themselves and used sleep medications more frequently.
Conclusion
The clinical manifestations of bvFTD appear to be associated with different and more distressing impacts on the caregiver sleep quality than SD.
Keywords: frontotemporal dementia, semantic dementia, caregiving, actigraphy, sleep, sleep quality
The neurological deterioration associated with dementia contributes to disturbances in nighttime behavior and sleep. Disrupted nighttime sleep occurs in many types of dementia. In Alzheimer’s disease (AD), such disruptions include insomnia, frequent nighttime awakenings, decreased total nighttime sleep, increased daytime sleep, and evening agitation (Dowling et al., 2005). Nightime sleep disruption is even more common in dementia with Lewy bodies (DLB) compared to AD. Patients with DLB suffer more movement disorders during sleep and more daytime sleepiness (Bliwise et al., 2011). Patients with vascular dementia experience disruption in sleep wake cycles and decreased sleep efficiency (Aharon-Peretz et al., 1991). Nighttime sleep disruption is difficult to treat and pharmacological management is associated with negative side effects (McCurry & Ancoli-Israel, 2003).
Much less is known about sleep in frontotemporal dementia (FTD). FTD refers to a range of neurodegenerative disorders characterized by focal atrophy of the frontal and/or anterior temporal lobes of the brain, resulting in profound behavioral, cognitive, and emotional symptoms (Brun, 1987; Neary et al., 1998; Rosen et al., 2005). Two subtypes of FTD include the behavorial variant (bvFTD) and semantic dementia (SD). Sleep disruption, characterized by increased nocturnal activity, decreased morning activity, and excessive daytime sleepiness, have been reported, but not well characterized, in FTD (Anderson, Hatfield, Kipps, Hastings, & Hodges, 2009; Harper et al., 2001; Merrilees, Hubbard, Mastick, Miller, & Dowling, 2009).
Sleep is an important issue for the family members who care for patients with dementia. Approximately two thirds of adult family caregivers complain of disrupted sleep (McCurry et al., 1999; Wilcox & King, 1999). Dementia family caregivers sleep less and have poorer ratings of sleep quality compared to noncaregivers (McKibbin et al., 2005; von Känel et al., 2012). Nighttime behaviors of patients with dementia are often associated with sleep problems in family caregivers (McCurry, Logsdon, Teri, & Vitiello, 2007), and sleep disruption is a major reason why family members institutionalize their care recipients (Hope, Keene, Gedling, Fairburn, & Jacoby, 1998; Yaffe et al., 2002). Poor sleep quality has been shown to contribute to depression and elevated biomarkers of increased atherosclerotic risk among family caregivers of persons with AD (Rowe, McCrae, Campbell, Benito, & Cheng, 2008; Simpson & Carter, 2013; von Känel et al., 2010), and more research describing the nature of sleep disruptions and their impact on sleep quality in patients with FTD and their caregivers are needed. Caregivers of patients with FTD have not been the focus of sleep research, although a case of a spouse caregiver of a patient with bvFTD whose ratings of emotional distress for the patient’s nighttime behavior increased during a three-year period of caregiving was reported (Merrilees et al., 2009). The purposes of this study were to characterize sleep (using actigraphy and subjective assessments) in patients with mild to moderate bvFTD and SD and their primary family caregivers, and to compare patient and caregiver data.
Methods
Participants
Subjects were recruited from an ongoing National Institutes of Healthfunded Program Project Grant examining FTD at the University of California, San Francisco Memory and Aging Center. To be included, patients needed to have a diagnosis of FTD, be living at home, and have a family caregiver willing to participate. Clinical diagnoses were established by consensus agreement of a panel of experts consisting of a neurologist, neuropsychologist, and a clinical nurse specialist, applying Neary criteria (Neary et al., 1998). Consent for participation in this study was obtained according to approved Institutional Review Board guidelines (including special protection for patients with cognitive impairment who rely on oversight by their surrogate). Twenty-two patient-caregiver dyads were enrolled: Thirteen bvFTD and nine SD. All patients resided at home with their spouse caregivers.
Apparatus
Sleep and activity data were collected using MiniMitter Actiwatch monitors (AW-64). Developed in the early 1970s, actigraphy has become an accepted method for studying sleep patterns in patients with dementia (Ancoli-Israel et al., 2003; Littner et al., 2003). Actigraphy is movement-based monitoring used widely in sleep and circadian rhythm research based on the premise that activity is more prominent during wake periods and less prominent during sleep (Ancoli-Israel et al., 2003; Morgenthaler et al., 2007). Actiwatches are wristwatch size devices that use accelerometers to monitor the occurrence, degree, and speed of motion. A signal reflecting magnitude and duration of motion is generated, amplified and digitized by an onboard circuit. This information is stored in memory as activity counts. The Actiwatches were programmed to collect data at one-minute epochs continuously over the two-week data collection period. Data were analyzed for bedtime (from “lights off” to “lights on”), and the sleep interval that lies within the bedtime interval (the period between sleep start and sleep end). Percentage sleep, the ratio of time asleep to amount of time in bed, was used as a measure of sleep efficiency, and is a ratio of time asleep to amount of time in bed. Normal sleep efficiency in adults aged 55–60 is 80.6% (SD = 11.7) and 79.2% (SD = 10.1) in adults 61 years and older (Bliwise, 2005). Other sleep-related outcome variables included: (a) length of time in bed [in hours]; (b) sleep interval duration [minutes]; (c) percent sleep [percent of time asleep from sleep onset to final wake time]; (d) total minutes awake within the sleep interval; (e) number of wake bouts within the sleep interval; and (f) wake bout duration [mean duration of wake bouts within the sleep interval in minutes].
Other Measures
Data collected on patients included demographics, dementia severity, cognitive performance, and behavioral symptoms. Caregiver data included demographics, sleep quality, and emotional distress related to the presence of nighttime disruption in the patient. Caregivers also maintained a “sleep diary” or record of sleep/wake times for both the patient and themselves that was used to aid in scoring the actigraphy data.
Dementia severity
The Clinical Dementia Rating (CDR) was used to stage the severity of dementia (Morris, 1993). Scores range from 0 (no dementia) to 3 (severe dementia). The CDR has good reliability and validity (Morris et al., 1997). The Mini-Mental Status Examination (MMSE) is a brief, 30-point scale with established reliability and validity as a measure of cognitive function (Folstein, Folstein, & McHugh, 1975).
Neurobehavior
The Neuropsychiatric Inventory (NPI), a structured interview with established reliability and validity, was used to assess 12 neurobehavioral domains in patients and the associated severity of caregiver’s distress (Cummings et al., 1994). The behavioral domains were: delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, nighttime behavior, and eating/appetite. There was a “yes” or “no” screening question for each domain and if the respondent answered affirmatively, additional questions were asked. For example, to assess nighttime behavior the following screening questions were asked: “Does the patient have difficulty sleeping (do not count as present if the patient simply gets up once or twice per night only to go to the bathroom and falls back asleep immediately)?” “Is he/she up at night?” “Does he/she wander at night, get dressed, or disturb your sleep?” If the caregiver answered yes, then additional questions were asked regarding the presence of excessive daytime sleeping, early morning awakenings, and any other nighttime behaviors. The severity and frequency of each symptom was rated with higher scores indicating greater behavioral symptomatology. For the purposes of this study, only the domain for nighttime behavior was included. The nighttime behavior score was derived by multiplying the frequency and severity scores. Additionally, the score reflecting the emotional distress experienced by the caregiver related to nighttime behavior (ranging from 0 [no distress] to 5 [very severely distressful] with higher scores indicative of greater emotional distress was included (Cummings, 1997).
Caregiver sleep quality
Characteristics of caregivers’ sleep during the last month were assessed using the Pittsburgh Sleep Quality Index (PSQI), a standardized quantitative measure of sleep quality developed to identify “good” and “poor” sleepers. A global PSQI score of greater than 5 indicates poor sleep quality and correlates well with clinical and/or laboratory measures (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Daytime dysfunction was measured using Question 8: “During the past month, how much of a problem has it been for you to keep up enough enthusiasm to get things done?” Rating response options were 0 = no problem at all), 1 = very slight problem, 2 = somewhat of a problem, and 3 = a very big problem. Sleeping medication use was measured using Question 6: “During the past month, how often have you taken medicine to help you sleep (prescribed or over the counter”)? Responses options were 0 = not during the past month, 1 = less than once a week, 2 = once or twice a week, and 3 = three or more times a week.
Procedure
After consenting to participate in the study, patients and their primary family caregivers were fitted with an Actiwatch and received verbal and written instructions. The Actiwatches were programmed to begin monitoring activity on the Monday afternoon following the research visit. Subjects were instructed to affix the Actiwatches to their nondominant wrist and to wear them at all times for the ensuing two weeks. In addition to written instructions, caregivers also received sleep diaries and the PSQI to complete at home. At the end of the two-week data collection period, the watches, diaries and questionnaires were returned to study staff in a self-addressed, prepaid mailer, and the data were downloaded and scored.
Statistical Analysis
Actigraphy records were analyzed for both patient and caregiver dyads on the medium sensitivity setting. Areas of validated “watch off” time were validated by reviewing diary reports and confirming these times in the actigraphs. Additionally, all actigraphs were reviewed for any periods greater than two hours when there was no recorded activity, indicating the watch was most likely off the wrist. These “watch off” times were deleted from the analysis. Records for both members of each dyad were “matched” by deleting identical periods on both records to ensure accuracy in comparison. For example if the patient had removed the watch one night, the data for both patient and caregiver were excluded for that night. Seven of the 22 participants had data removed in this process, and the overall mean hours of data removed was 31.5 (SD 61.6 hours). To facilitate visual comparison, the actogram activity scale was calibrated to be the same for both data sets. Raw actigraphy data were subjected to a scoring algorithm in the Actiware software. Bed and rise times were interpreted by the analyst based on diary entries and the raw data. Lights out/attempt to sleep was scored as the first epoch with at least a 200 count drop in activity over three minutes and sustained, and lights on/last wake in the morning was scored as the first epoch with at least a 200 count increase in activity over three minutes and sustained. Sleep was calculated by the software algorithm based on immobility where 10 continuous minutes of inactivity was categorized as sleep. Epochs were then scored as sleep, starting with the first minute of inactivity. The rules to determine sleep and wake parameters were based on the experience reviewing and scoring hundreds of actigraphy records for patients with neurodegenerative disorders (e.g. Parkinson’s disease, dementia). The rules took into account differences in mobility in these populations compared to the general population, for which the default scoring algorithms are calculated.
Statistical Package for the Social Sciences (SPSS) software was used for additional data analyses. Nonparametric independent samples analyses were employed to compare patients with bvFTD to SD and bvFTD caregivers to SD caregivers. Paired samples were used to compare the bvFTD and SD patient groups with their respective caregiver groups.
Results
Subject Characteristics
Patient and caregiver characteristics are summarized in Table 1. Of the 22 caregivers, 14 were female: Nine in the bvFTD group and five in the SD group. The mean MMSE scores for bvFTD patients was 24.5 (SD = 3.8) compared to 16.4 (SD = 9.1) for patients with SD (p < .02). CDR scores were higher (indicating greater dementia severity) in patients with bvFTD compared to those with SD (M = 1.6, SD = 0.6; M = 1.0, SD = 0.6, respectively; p < .02).
Table 1.
Participant Characteristics
| Behavioral Variant FTD | Semantic Dementia | |||||
|---|---|---|---|---|---|---|
| Characteristic | Statistic | Patients (n = 13) |
Caregivers (n = 13) |
Patients (n = 9) |
Caregivers (n = 9) |
p |
| Gender (Male) | n | 9 | 4 | 5 | 4 | |
| % | 69.2 | 30.8 | 55.6 | 44.4 | ||
| Age | Mean | 61.5 | 59.9 | 66.2 | 63.0 | |
| SD | 5.9 | 9.1 | 8.9 | 10.8 | ||
| MMSE | Mean | 24.5 | 16.4 | .009a | ||
| SD | 3.8 | 9.1 | ||||
| CDR | Mean | 1.6 | 1.0 | .03a | ||
| SD | 0.6 | 0.6 | ||||
| PSQI (All)b | Mean | 7.8 | 4.9 | |||
| SD | 4.2 | 2.5 | ||||
| PSQI (Male)b | Mean | 4.0 | 3.67 | |||
| SD | 0.0 | 2.1 | ||||
| PSQI (Female)b | Mean | 9.67 | 5.75 | |||
| SD | 3.89 | 2.75 | ||||
Note. FTD = frontotemporal dementia. MMSE = Mini Mental Status Exam; score range is 0 – 30. CDR = Clinical Dementia Rating Scale; range is 0 – 3. PSQI = Pittsburgh Sleep Quality Index; range is 0 – 21 with scores > 5 indicating poor sleep quality.
Independent samples t-test.
Only caregivers responded to the PSQI. High scores indicate pooer sleep quality.
Sleep
The NPI nighttime sleep disruption rating results are presented in Table 2. Nighttime disruption was reported more often in patients with bvFTD than SD (85% versus 33%). Descriptively, nighttime disruption was more often rated as occurring frequently or very frequently in patients with bvFTD compared those with SD. There was a strong positive correlation between nighttime behaviors in patients with bvFTD and caregiver distress related to sleep disruption (rho = .67, p < .05), but not in the SD group.
Table 2.
Caregiver Nighttime Disturbance Ratings from the Neuropsychiatric Inventory
| bvFTD (n = 11) |
Semantic Dementia (n = 3) |
|||
|---|---|---|---|---|
| Nighttime Disturbance | Mean | SD | Mean | SD |
| Frequency | 5.5 | 2.8 | 4.7 | 3.1 |
| Severity | 1.4 | 0.6 | 1.3 | 0.5 |
| Frequency × Severitya | 5.4 | 2.8 | 4.6 | 3.0 |
| Emotional Distress | 2.1 | 1.2 | 0.7 | 1.2 |
Note. FTD = frontotemporal dementia. Information is for caregivers who reported nighttime disturbance in the patient.
Frequency × Severity is the behavioral domain.
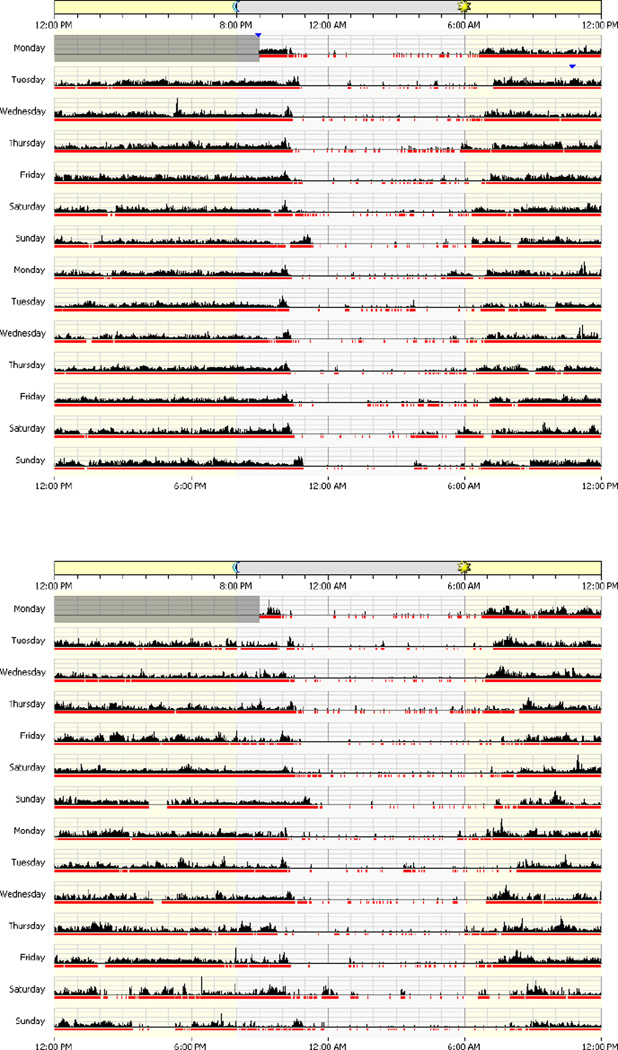
Actigraphy and diary data are presented in Table 3. Wilcoxon sign-rank tests of time spent in bed for the patients and their caregivers revealed significant differences for the bvFTD dyads (Z = 2.06, p = .04) and the SD dyads (Z = 2.67, p = .004). Both bvFTD and SD patient groups spent significantly more time in bed than their caregivers. Although both patient groups showed a slightly higher percentage of sleep compared to their caregivers, all four groups had sleep efficiencies greater than 80%. There were no significant differences in duration of the sleep interval, number of wake bouts or wake bout duration between the groups. An example of actigraphs for one patient/caregiver dyad is displayed in Figure 1.
Table 3.
Sleep and Sleep Behaviors
| Behavioral Variant FTD | Semantic Dementia | ||||
|---|---|---|---|---|---|
| Characteristic | Statistic | Patients (n = 13) |
Caregivers (n = 13) |
Patients (n = 9) |
Caregivers (n = 9) |
| Bedtime (hours:minutes) | Mean | 9:13 pm | 10:37 pm | 9:50 pm | 10:50 pm |
| SD | 1:21 | 0:57 | 1:19 | 0:39 | |
| Rise time | Mean | 7:23 am | 7:14 am | 7:35 am | 6:45 am |
| SD | 1:13 | 1:00 | 0:41 | 0:20 | |
| Time in bed (hours) | Mean | 9.78a | 8.65a | 10.14b | 8.28b |
| SD | 1.59 | 0.98 | 1.36 | 0.66 | |
| Sleep Duration (hours) | Mean | 9.21 | 8.06 | 9.63 | 7.70 |
| SD | 1.68 | 0.82 | 1.28 | 0.73 | |
| Percentage Sleep | Mean | 88.57 | 86.81 | 89.76 | 86.19 |
| SD | 6.73 | 4.79 | 4.55 | 5.02 | |
| ATSI (Minutes) | Mean | 64.29 | 63.99 | 60.26 | 65.20 |
| SD | 43.14 | 23.01 | 29.55 | 25.34 | |
| Wake Bouts (Number) | Mean | 26.32 | 29.66 | 26.61 | 27.59 |
| SD | 8.69 | 10.30 | 9.73 | 6.75 | |
| Wake Bouts (Duration) | Mean | 2.27 | 2.18 | 2.16 | 27.59 |
| SD | 0.69 | 0.46 | 0.43 | 0.57 | |
Note. FTD = frontotemporal dementia. ATSI = awake time in sleep intervals. Bedtime and rise time were obtained from diary data. Other variables were derived from actigraph recordings.
Within-dyad difference is significant (z = 2.67, p = .04).
Within-dyad difference is significant (z = 2.06, p = .004).
Figure 1.
Exemplar patient (top) and caregiver (bottom) actigraph readings.
Caregiver Sleep Quality
Caregiver sleep quality data are presented in Table 1. The average PSQI global score was 7.8 (SD = 4.2) for the bvFTD caregivers and 4.9 (SD = 2.5) for the SD caregivers, indicating poor sleep quality for the bvFTD caregivers and adequate quality for the SD caregivers, on average, based on the published cutpoint. Overall, female caregivers reported poorer sleep quality (higher PSQI scores) than male caregivers. In addition, 92% of bvFTD caregivers reported that they experienced daytime dysfunction during the past month by indicating that it had been a problem for them to keep up enough enthusiasm to get things done. Only 37% of SD caregivers reported this same problem. Female, but not male, caregivers reported using sleep medications. Fifty-four percent of the female bvFTD caregivers reported taking medicine for sleep, with 41% of these using sleep medication > 3 times a week. Only one SD caregiver reported using sleep medication and this was less than one time per week.
Discussion
Caregiver reports show that patient nighttime disruption is an important feature of FTD. In this cohort of patients with CDR scores indicating mild disease severity, nighttime behaviors were reported in 85% of patients with bvFTD and correlated strongly with caregiver distress. The number of caregivers of patients with SD who reported any nighttime behaviors in the patient was too small to comment on the association between nightime behaviors and caregiver distress. The bvFTD caregivers were more likely to rate themselves as having poor sleep quality. The bvFTD caregivers were taking sleep medications three times a week or more. In addition, a higher percentage of bvFTD caregivers compared to SD, reported trouble with daytime enthusiasm. Yet, despite these subjective ratings of poor sleep quality, actigraphy data demonstrate sleep efficiencies considered to be within the normal range.
These data suggest that caregiver complaints about sleep could be influenced by factors other than sleep disruption in the patient. Subjective reports of sleep disruption do not always correlate with objective measures of sleep (Hoekert, der Lek, Swaab, Kaufer, & Van Someren, 2006; McCurry, Vitiello, Gibbons, Logsdon, & Teri, 2006). For example, in a study comparing subjective ratings with objective measures of sleep efficiency, the presence of depression, lower levels of social support, being overcommitted at work, and being less happy were factors associated with poorer self-ratings of sleep efficiency despite objective measures indicating normal sleep efficiency (Jackowska, Dockray, Hendrickx, & Steptoe, 2011). In another study comparing female caregivers to noncaregivers, caregivers rated their sleep quality as poorer despite similar sleep patterns in both groups (Castro et al., 2009). In the author’s experience, caregivers report that worry and anxiety about the patient (e.g., vigilience about safety) and other issues (e.g., financial, legal, and health care-related decisions) prevent them from sleeping soundly. This study did not explore these factors.
Results regarding poor sleep quality are important in considering the potential negative consequences that caregiving has on health. Disrupted sleep has been associated with medical and psychiatric illnesses such as depression, anxiety, cognitive dysfunction, and reduction in quality of life (Neikrug & Ancoli-Israel, 2010). Poor sleep is also associated with negative health outcomes in reaction times, functional performance (Koslowsky & Babkoff, 1992), and excessive daytime sleepiness (Neikrug & Ancoli-Israel, 2010). People experiencing poor sleep often use sleep medicines, alcohol, and daytime caffeine as strategies for managing sleep disruption. Nurses play a critical role as advocates for the health and well-being of dementia family caregivers. Caregivers typically ignore their own health as a result of the time and burden of providing care to the person with dementia, thus, compounding the risks to their own physical and emotional well-being. Results from this study reinforce the need to attend to subjective complaints about sleep and sleep quality. Nurses are in a position to assess for sleep disruption among caregivers and to promote strategies that promote good sleep.
Results from this study underscore the necessity of using both quantitative/objective measures of sleep and behavior and subjective ratings. While caregiver reports are valuable, such ratings may be biased and influenced by caregiver fatigue and burden (McCurry, Gibbons, Logsdon, & Teri, 2004). Community-based monitoring is valuable because it facilitates research in patients with earlier, milder stages of disease and allows maximum freedom for the subjects to engage in their typical activity. Monitoring in laboratory or institutional settings can create problems with data being confounded by institutional variables (e.g., lack of outdoor light, imposed bedtime) (Ancoli-Israel, Clopton, Klauber, Fell, & Mason, 1997; Ancoli-Israel et al., 1997).
Limitations
There are limitations to this study. The inclusion of actigraphy data from age-matched normal controls would enhance knowledge of the potential differences between patients, caregivers, and noncaregivers. While nighttime disruption was endorsed, it is not clear whether the disruption was due to nighttime awakenings, trouble falling or staying asleep, or other types of disturbances. It may be that certain disruptions are more stressful than others, but this was not assessed in this study. Also, a potential limitation of the PSQI is that subjects are asked to rate sleep items over the past month which likely does not capture day-to-day variability, nor does it match the two-week actigraphy data collection. The presence of primary sleep disorders (e.g., sleep apnea, rapid eye movement sleep-behavior disorder, periodic limb movements of sleep) were not assessed in either the patients or caregivers, caregiver depression, or other factors that could influence sleep and sleep quality, or patient’s use of medicines that could affect sleep. Data on whether patients and caregivers shared the same bedroom—a factor that could influence aspects relating to sleep and sleep quality—were also not collected.
Conclusions
Although nighttime disturbances are reported by caregivers of patients with bvFTD and SD and associated with caregiver distress, objective measures of sleep efficiency and the timing of the sleep period appeared relatively normal. The caregivers of patients with bvFTD reported worse sleep quality compared to SD caregivers. The clinical manifestations of bvFTD produce different and more distressing impacts on the caregiver. These cohort differences suggest separate analysis of FTD subtypes is warranted and could provide data to inform interventions to minimize negative health outcomes for family caregivers and improve dementia care. Objective measurement of features of sleep in patients and their family caregivers holds promise as a method for accurately assessing sleep and sleep quality.
Acknowledgments
The authors acknowledge support for this article was provided by The Integra Foundation Neuroscience Nursing Foundation Research Grant Program, NIH 5 P01 AG019724, and The John A. Hartford Center of Geriatric Excellence.
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Jennifer Merrilees, Memory and Aging Center, University of California, San Francisco.
Erin Hubbard, Department of Physiological Nursing, University of California, San Francisco.
Judy Mastick, Department of Physiological Nursing, University of California, San Francisco.
Bruce L. Miller, Memory and Aging Center, University of California, San Francisco.
Glenna A. Dowling, Department of Physiological Nursing, University of California, San Francisco.
References
- Aharon-Peretz J, Masiah A, Pillar T, Epstein R, Tzischinksy O, Lavie P. Sleep-wake cycles in multi-infarct dementia and dementia of the Alzheimer type. Neurology. 1991;41:1616–1619. doi: 10.1212/wnl.41.10.1616. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997a;20:24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, Fell R. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 1997b;20:18–23. [PubMed] [Google Scholar]
- Anderson KN, Hatfield C, Kipps C, Hastings M, Hodges JR. Disrupted sleep and circadian patterns in frontotemporal dementia. European Journal of Neurology. 2009;16:317–323. doi: 10.1111/j.1468-1331.2008.02414.x. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement W, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Saunders; 2005. pp. 24–38. [Google Scholar]
- Bliwise DL, Mercaldo ND, Avidan AY, Boeve BF, Greer SA, Kukull WA. Sleep disturbance in dementia with Lewy bodies and Alzheimer's disease: A multicenter analysis. Dementia and Geriatric Cognitive Disorders. 2011;31:239–246. doi: 10.1159/000326238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A. Frontal lobe degeneration of non-Alzheimer type. I. neuropathology. Archives of Gerontology and Geriatrics. 1987;6:193–208. doi: 10.1016/0167-4943(87)90021-5. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, III, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Castro CM, Lee KA, Bliwise DL, Urizar GG, Woodward SH, King AC. Sleep patterns and sleep-related factors between caregiving and non-caregiving women. Behavioral Sleep Medicine. 2009;7:164–179. doi: 10.1080/15402000902976713. [DOI] [PubMed] [Google Scholar]
- Cummings JL. The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48:10S–16S. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJW. Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer's disease. International Psychogeriatrics. 2005;17:221–236. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, McKee AC, Satlin A, Harlan PC, Goldstein R, Volicer L. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Archives of General Psychiatry. 2001;58:353–360. doi: 10.1001/archpsyc.58.4.353. [DOI] [PubMed] [Google Scholar]
- Hoekert M, der Lek RF, Swaab DF, Kaufer D, Van Someren EJ. Comparison between informant-observed and actigraphic assessments of sleep-wake rhythm disturbances in demented residents of homes for the elderly. American Journal of Geriatric Psychiatry. 2006;14:104–111. doi: 10.1097/01.JGP.0000192481.27931.c5. [DOI] [PubMed] [Google Scholar]
- Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. International Journal of Geriatric Psychiatry. 1998;13:682–690. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Jackowska M, Dockray S, Hendrickx H, Steptoe A. Psychosocial factors and sleep efficiency: Discrepancies between subjective and objective evaluations of sleep. Psychosomatic Medicine. 2011;73:810–816. doi: 10.1097/PSY.0b013e3182359e77. [DOI] [PubMed] [Google Scholar]
- Koslowsky M, Babkoff H. Meta-analysis of the relationship between total sleep deprivation and performance. Chronobiology International. 1992;9:132–136. doi: 10.3109/07420529209064524. [DOI] [PubMed] [Google Scholar]
- Littner M, Kushida CA, McDowell Anderson W, Bailey D, Berry RB, Davila DG, Johnson SF. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: An update for 2002. Sleep. 2003;26:337–341. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Ancoli-Israel S. Sleep dysfunction in Alzheimer's disease and other dementias. Current Treatment Options in Neurology. 2003;5:261–272. doi: 10.1007/s11940-003-0017-9. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Gibbons LE, Logsdon RG, Teri L. Anxiety and nighttime behavioral disturbances. Awakenings in patients with Alzheimer's disease. Journal of Gerontological Nursing. 2004;30:12–20. doi: 10.3928/0098-9134-20040101-05. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Teri L, Gibbons LE, Kukull WA, Bowen JD, Larson EB. Characteristics of sleep disturbance in community-dwelling Alzheimer's disease patients. Journal of Geriatric Psychiatry and Neurology. 1999;12:53–59. doi: 10.1177/089198879901200203. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: Contributing factors and treatment implications. Sleep Medicine Review. 2007;11:143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry SM, Vitiello MV, Gibbons LE, Logsdon RG, Teri L. Factors associated with caregiver reports of sleep disturbances in persons with dementia. American Journal of Geriatric Psychiatry. 2006;14:112–120. doi: 10.1097/01.JGP.0000192499.25940.da. [DOI] [PubMed] [Google Scholar]
- McKibbin CL, Ancoli-Israel S, Dimsdale J, Archuleta C, von Känel R, Mills P, Grant I. Sleep in spousal caregivers of people with Alzheimer's disease. Sleep. 2005;28:1245–1250. doi: 10.1093/sleep/28.10.1245. [DOI] [PubMed] [Google Scholar]
- Merrilees J, Hubbard E, Mastick J, Miller BL, Dowling GA. Rest-activity and behavioral disruption in a patient with frontotemporal dementia. Neurocase: The Neural Basis of Cognition. 2009;15:515–526. doi: 10.1080/13554790903061371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Swick TJ. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Woodbury P. Clinical Dementia Rating training and reliability in multicenter studies: The Alzheimer's Disease Cooperative Study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult—A mini-review. Gerontology. 2010;56:181–189. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MA, McCrae CS, Campbell JM, Benito PA, Cheng J. Sleep pattern differences between older adult dementia caregivers and older adult noncaregivers using objective and subjective measures. Journal of Clinical Sleep Medicine. 2008;4:362–369. [PMC free article] [PubMed] [Google Scholar]
- Simpson C, Carter P. Short-term changes in sleep, mastery & stress: Impacts on depression and health in dementia caregivers. Geriatric Nursing. 2013 doi: 10.1016/j.gerinurse.2013.07.002. [DOI] [PubMed] [Google Scholar]
- von Känel R, Ancoli-Israel S, Dimsdale JE, Mills PJ, Mausbach BT, Ziegler MG, Grant I. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56:41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox S, King AC. Sleep complaints in older women who are family caregivers. The Journals of Gerontology: Series B: Psychological Sciences. 1999;54:P189–P198. doi: 10.1093/geronb/54b.3.p189. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, Covinsky KE. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]