Abstract
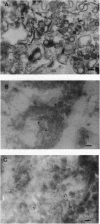
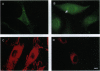
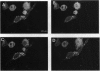
Vesicles containing endothelin 1 (ET-1) were isolated from bovine aortic endothelial cells (BAECs) by fractionation of homogenates on sucrose density gradients by ultracentrifugation. The vesicles were localized at the 1.0/1.2 M sucrose interface using a specific anti-ET-1-(16-21) RIA. Identification of ET-1 and big ET-1 in this fraction was confirmed by HPLC analysis combined with RIA. Morphological examination of the ET-1-enriched fraction by electron microscopy identified clusters of vesicles approximately 100 nm in diameter. Immunostaining of ultrathin cryosections prepared from the vesicle fraction for ET-1 or big ET-1 showed clusters of 15-nm gold particles attached to or within vesicles. Immunofluorescence staining of whole BAECs using a specific ET-1-(16-21) IgG purified by affinity chromatography revealed punctate granulation of the cell cytoplasm viewed under light microscopy. This distinct pattern of staining was shown by confocal light microscopy to be intracellular. Immunofluorescence staining of whole cells with a polyclonal antiserum for big ET-1-(22-39) showed a defined perinuclear localization of precursor molecule. Hence, several different approaches have demonstrated that ET-1 and big ET-1 are localized within intracellular vesicles in BAECs, suggesting that these subcellular compartments are an important site for processing of big ET-1 by endothelin-converting enzyme.
Full text
PDF
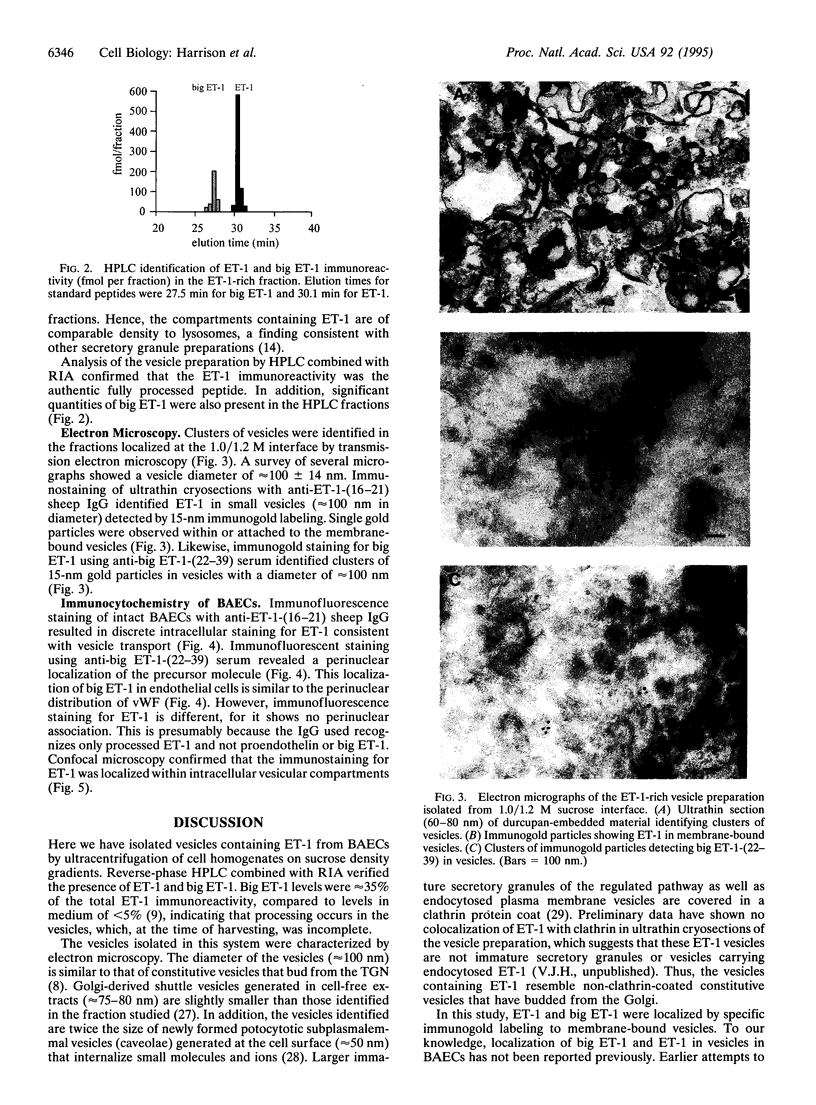
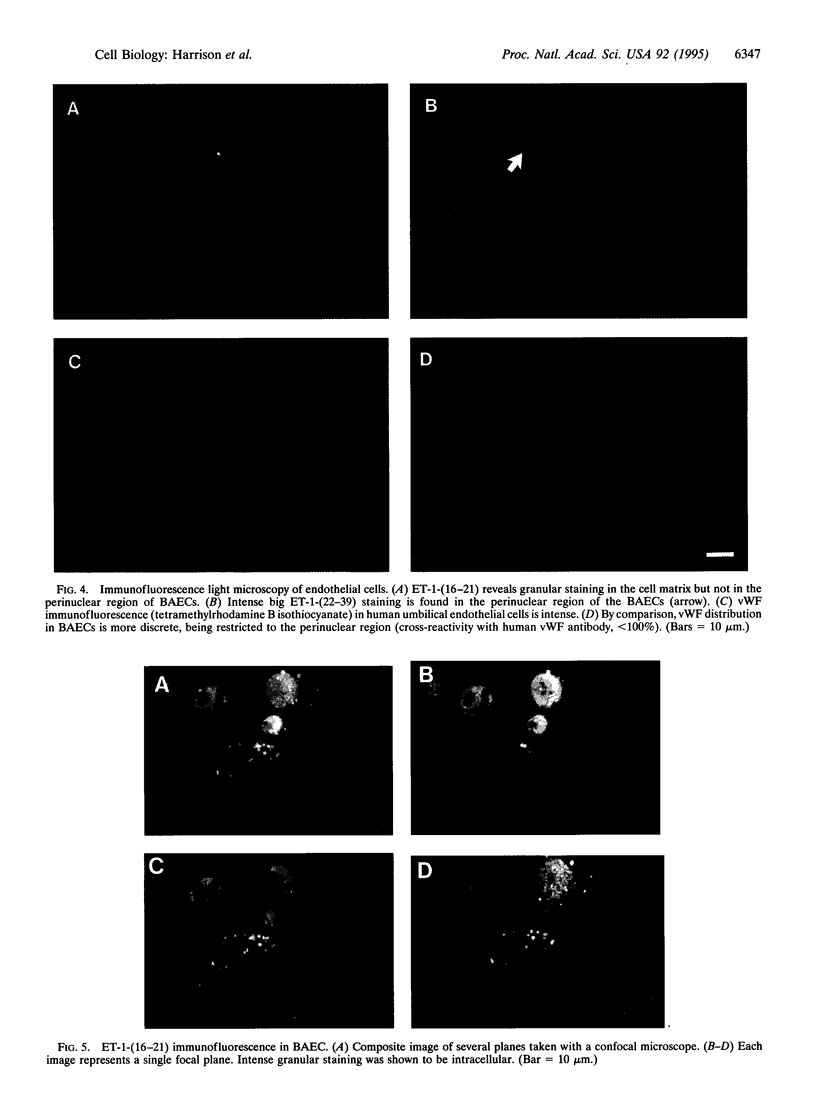

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Potocytosis of small molecules and ions by caveolae. Trends Cell Biol. 1993 Mar;3(3):69–72. doi: 10.1016/0962-8924(93)90065-9. [DOI] [PubMed] [Google Scholar]
- Barnes K., Kenny A. J., Turner A. J. Localization of aminopeptidase N and dipeptidyl peptidase IV in pig striatum and in neuronal and glial cell cultures. Eur J Neurosci. 1994 Apr 1;6(4):531–537. doi: 10.1111/j.1460-9568.1994.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Barnes K., Turner A. J., Kenny A. J. An immunoelectron microscopic study of pig substantia nigra shows co-localization of endopeptidase-24.11 with substance P. Neuroscience. 1993 Apr;53(4):1073–1082. doi: 10.1016/0306-4522(93)90490-7. [DOI] [PubMed] [Google Scholar]
- Beckers J. M., Rothman J. E. Transport between Golgi cisternae. Methods Enzymol. 1992;219:5–12. doi: 10.1016/0076-6879(92)19004-p. [DOI] [PubMed] [Google Scholar]
- Brew K., Shaper J. H., Olsen K. W., Trayer I. P., Hill R. L. Cross-linking of the components of lactose synthetase with dimethylpimelimidate. J Biol Chem. 1975 Feb 25;250(4):1434–1444. [PubMed] [Google Scholar]
- Corder R., Harrison V. J., Khan N., Anggård E. E., Vane J. R. Effects of phosphoramidon in endothelial cell cultures on the endogenous synthesis of endothelin-1 and on conversion of exogenous big endothelin-1 to endothelin-1. J Cardiovasc Pharmacol. 1993;22 (Suppl 8):S73–S76. doi: 10.1097/00005344-199322008-00021. [DOI] [PubMed] [Google Scholar]
- Corder R., Khan N., Harrison V. J. A simple method for isolating human endothelin converting enzyme free from contamination by neutral endopeptidase 24.11. Biochem Biophys Res Commun. 1995 Feb 6;207(1):355–362. doi: 10.1006/bbrc.1995.1195. [DOI] [PubMed] [Google Scholar]
- Ewenstein B. M., Warhol M. J., Handin R. I., Pober J. S. Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. J Cell Biol. 1987 May;104(5):1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Snyder S. H. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3886–3890. doi: 10.1073/pnas.79.12.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban P. A., Irminger J. C. Sorting and processing of secretory proteins. Biochem J. 1994 Apr 1;299(Pt 1):1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison V. J., Corder R., Anggård E. E., Vane J. R. Evidence for vesicles that transport endothelin-1 in bovine aortic endothelial cells. J Cardiovasc Pharmacol. 1993;22 (Suppl 8):S57–S60. doi: 10.1097/00005344-199322008-00017. [DOI] [PubMed] [Google Scholar]
- Herbert E., Uhler M. Biosynthesis of polyprotein precursors to regulatory peptides. Cell. 1982 Aug;30(1):1–2. doi: 10.1016/0092-8674(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Ikura T., Sawamura T., Shiraki T., Hosokawa H., Kido T., Hoshikawa H., Shimada K., Tanzawa K., Kobayashi S., Miwa S. cDNA cloning and expression of bovine endothelin converting enzyme. Biochem Biophys Res Commun. 1994 Sep 30;203(3):1417–1422. doi: 10.1006/bbrc.1994.2343. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Loesch A., Bodin P., Burnstock G. Colocalization of endothelin, vasopressin and serotonin in cultured endothelial cells of rabbit aorta. Peptides. 1991 Sep-Oct;12(5):1095–1103. doi: 10.1016/0196-9781(91)90065-w. [DOI] [PubMed] [Google Scholar]
- Macarthur H., Warner T. D., Wood E. G., Corder R., Vane J. R. Endothelin-1 release from endothelial cells in culture is elevated both acutely and chronically by short periods of mechanical stretch. Biochem Biophys Res Commun. 1994 Apr 15;200(1):395–400. doi: 10.1006/bbrc.1994.1462. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Opgenorth T. J., Wu-Wong J. R., Shiosaki K. Endothelin-converting enzymes. FASEB J. 1992 Jun;6(9):2653–2659. doi: 10.1096/fasebj.6.9.1612289. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Madsen O., Vassalli J. D., Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985 Sep;42(2):671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Perrelet A., Powell S. K., Quinn D. L., Moore H. P. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell. 1987 Dec 24;51(6):1039–1051. doi: 10.1016/0092-8674(87)90590-3. [DOI] [PubMed] [Google Scholar]
- Orcl L., Palmer D. J., Amherdt M., Rothman J. E. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993 Aug 19;364(6439):732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Docherty K., Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J Cell Biochem. 1984;24(2):121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Takahashi M., Matsushita Y., Iijima Y., Tanzawa K. Purification and characterization of endothelin-converting enzyme from rat lung. J Biol Chem. 1993 Oct 5;268(28):21394–21398. [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Xu D., Emoto N., Giaid A., Slaughter C., Kaw S., deWit D., Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994 Aug 12;78(3):473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]