Abstract
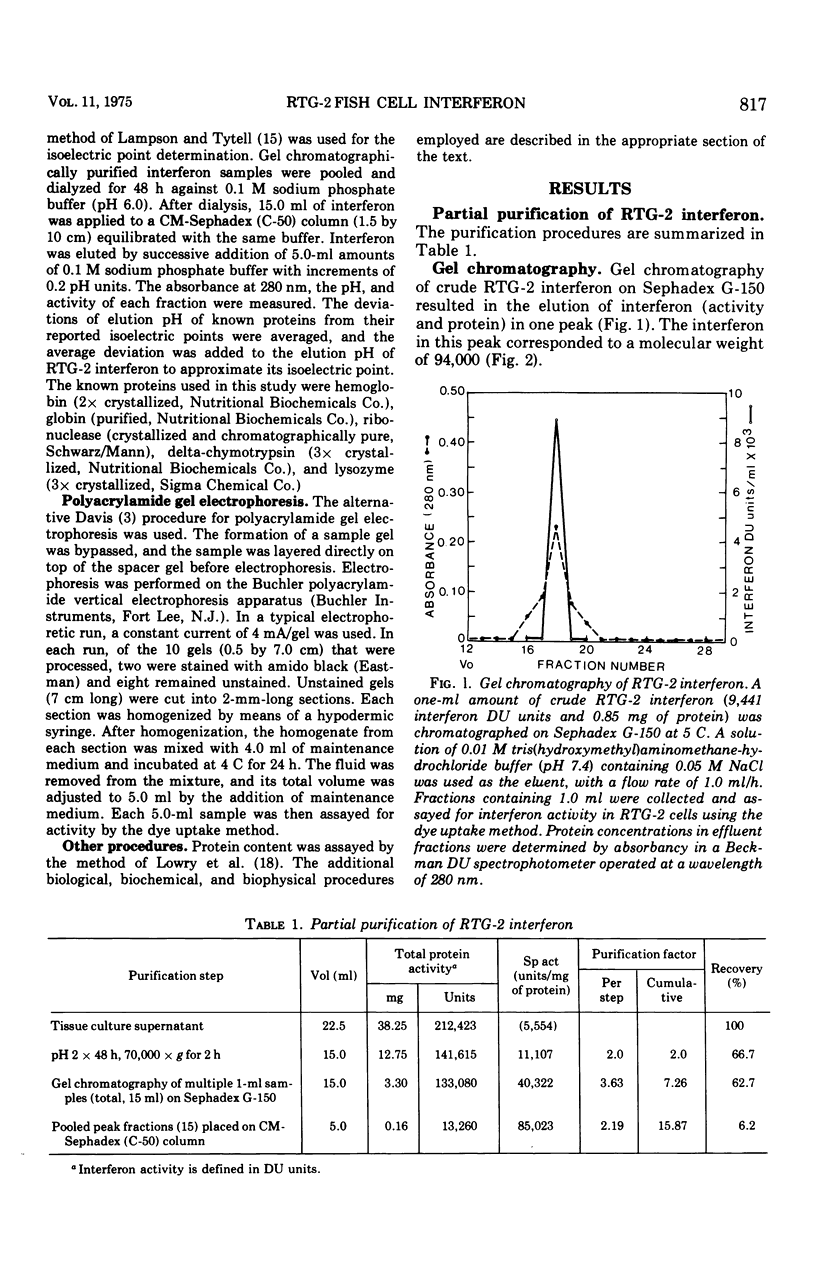
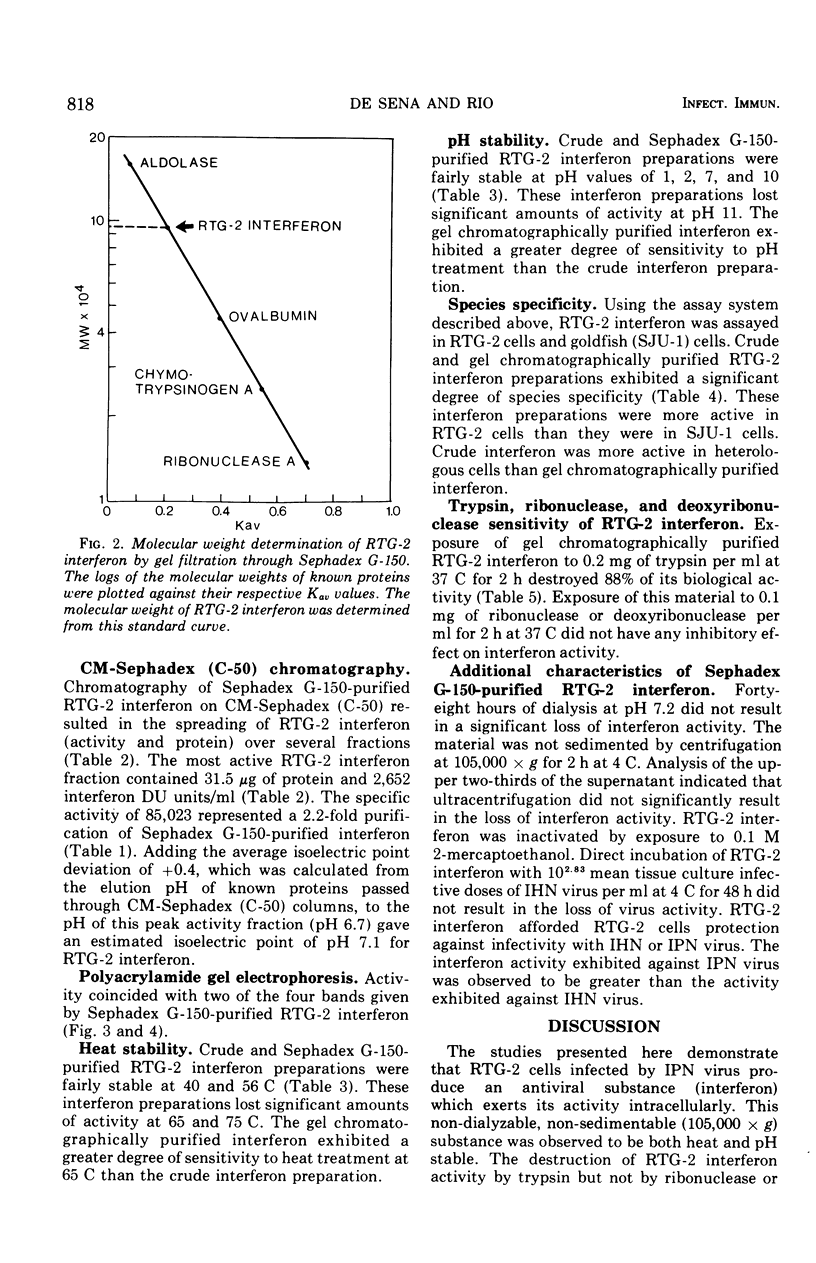
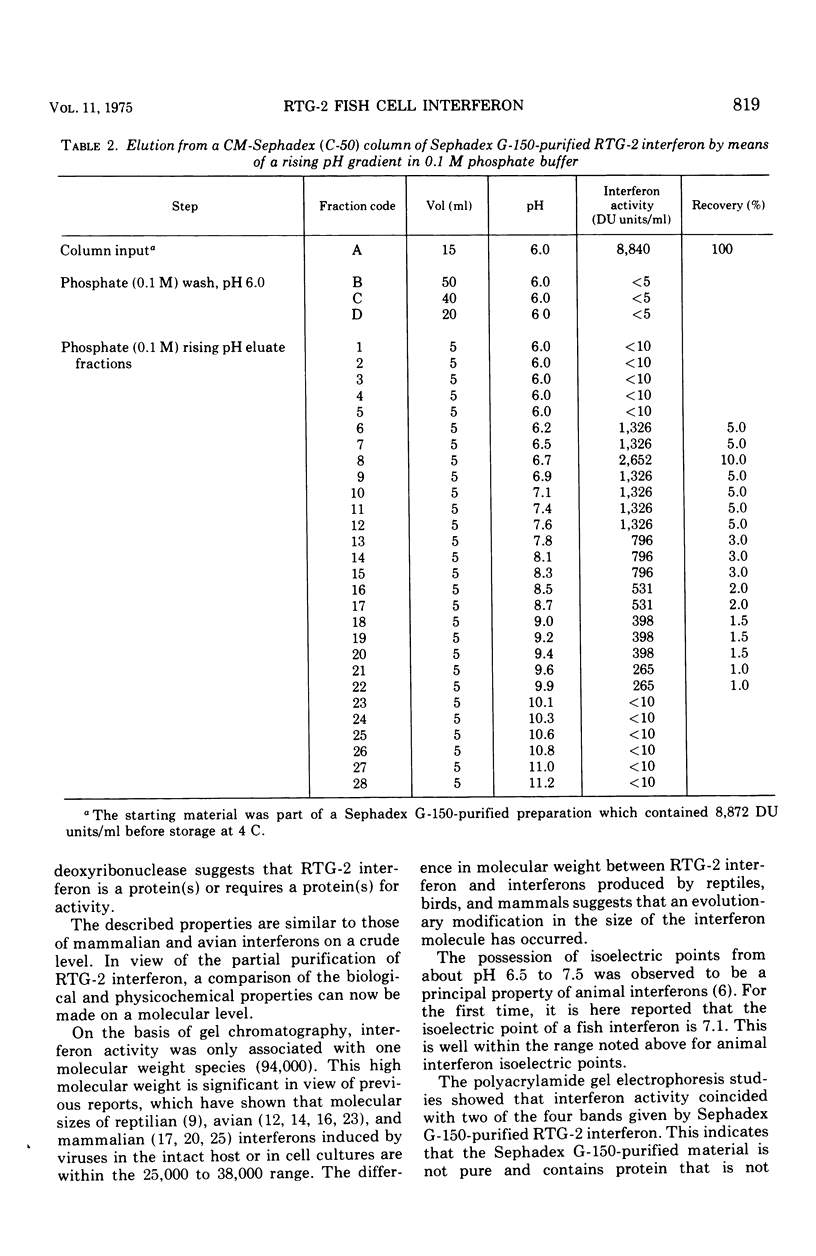
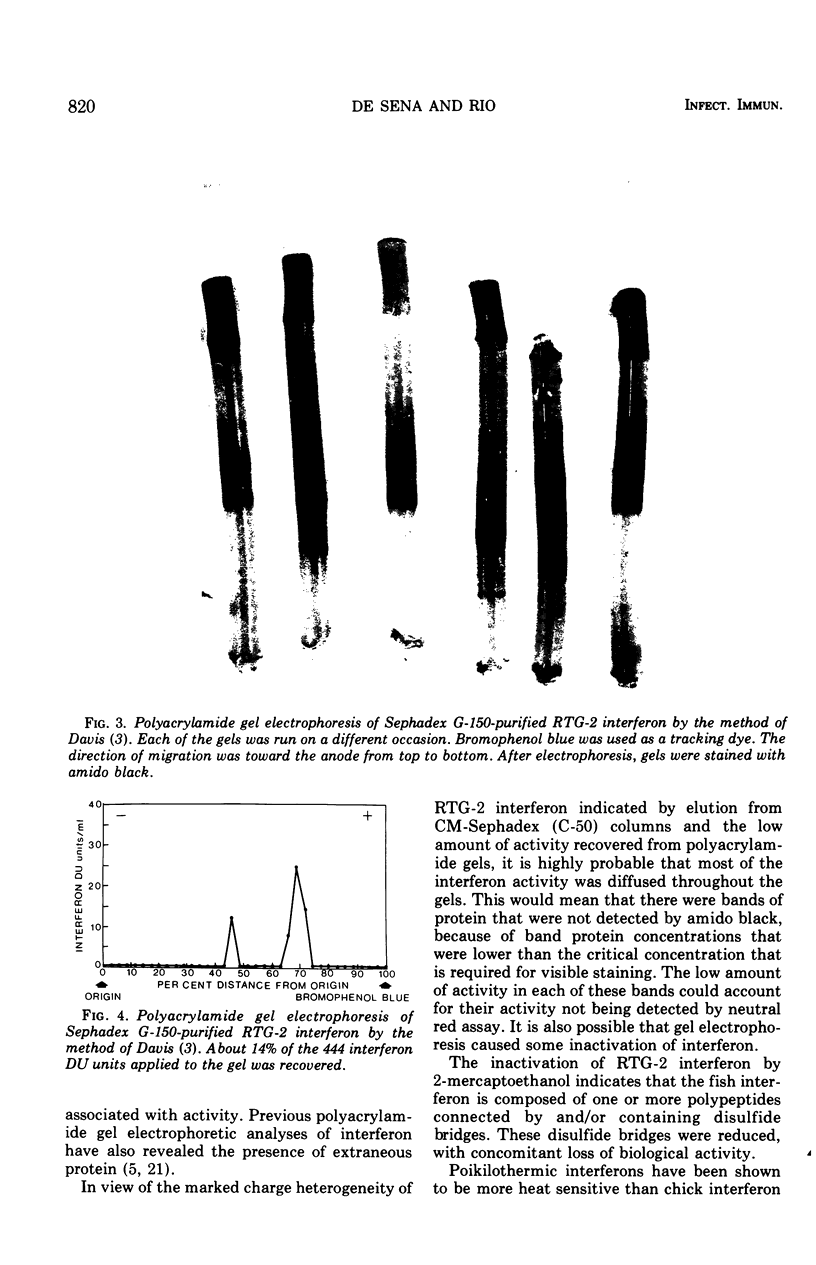
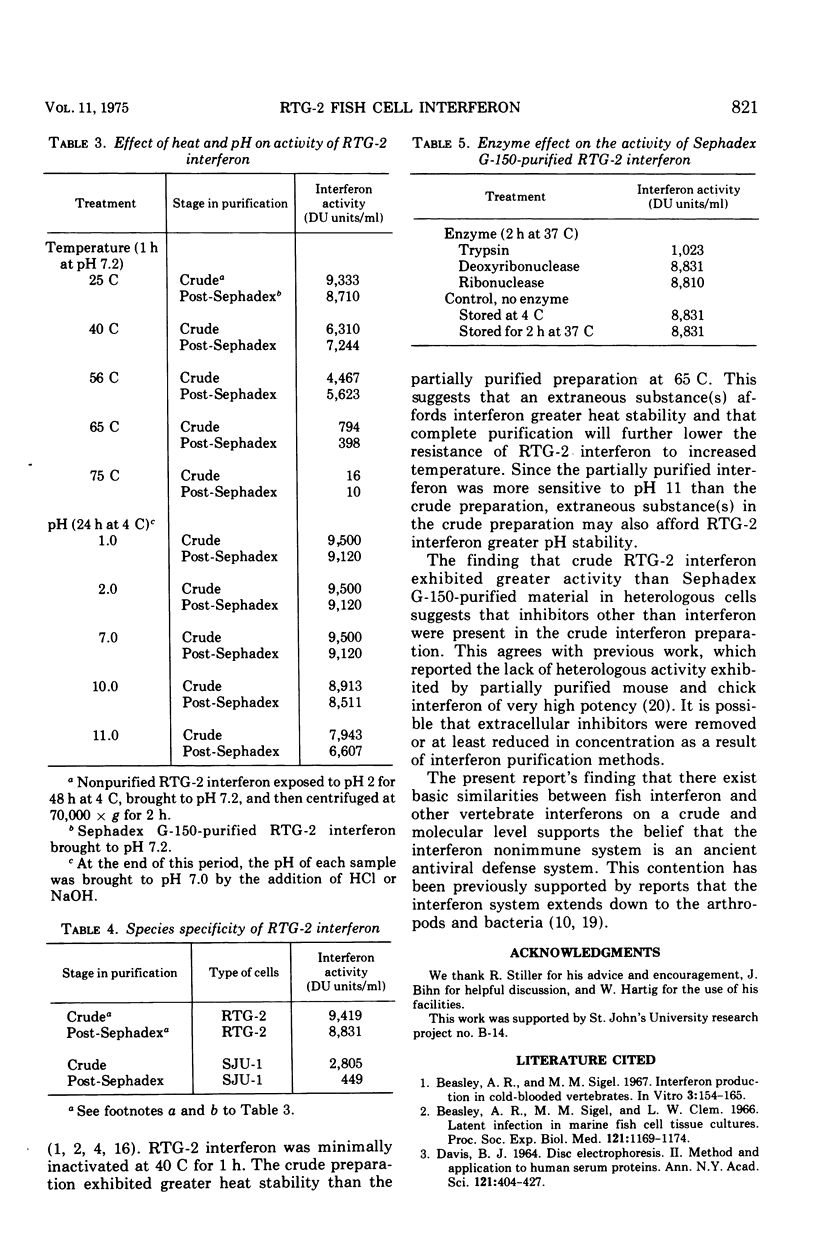
Interferon produced by rainbow trout gonadal cells (RTG-2) was partially purified. The physical, chemical, and biological properties of this in vitro produced fish cell interferon were studied. Purification was achieved by ultracentrifugation, molecular sieve gel chromatography, ion exchange chromatography, and polyacrylamide gel electrophoresis. The isoelectric point of RTG-2 interferon, as determined by CM-Sephadex (C-50) chromatography, was 7.1. Filtration through Sephadex G-150 showed that RTG-2 interferon had a molecular weight of 94,000. The partially purified material was not sedimented at 105,000 times g for 2 h at 4 C. The fish cell interferon was non-dialyzable and exhibited heat and pH stability. The partially purified material was inactivated by treatment with trypsin or 2-mercaptoethanol, but was resistant to treatment with deoxyribonuclease or ribonuclease. RTG-2 interferon which was induced by infectious pancreatic necrosis virus exhibited antiviral activity against challenge with infectious hematopoietic necrosis virus or infectious pancreatic necrosis virus. Partially purified RTG-2 interferon exhibited greater species specificity than the crude material.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley A. R., Sigel M. M., Clem L. W. Latent infection in marine fish cell tissue cultures. Proc Soc Exp Biol Med. 1966 Apr;121(4):1169–1174. doi: 10.3181/00379727-121-30997. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FALCOFF E., FAUCONNIER B. IN VITRO PRODUCTION OF AN INTERFERON-LIKE INHIBITOR OF VIRAL MULTIPLICATION BY A POIKILOTHERMIC ANIMAL CELL, THE TORTOISE (TESTUDO GRECA). Proc Soc Exp Biol Med. 1965 Mar;118:609–612. doi: 10.3181/00379727-118-29918. [DOI] [PubMed] [Google Scholar]
- Galabov A., Petrunova S., Savov Z. Molecular weight of virus-induced tortoise interferon in cell cultures. Experientia. 1973;29(7):900–901. doi: 10.1007/BF01946357. [DOI] [PubMed] [Google Scholar]
- Garzon S., Kurstak E. Interférence sélective au niveau de tissus entre le virus de la polyédrie nucléaire (VPN) et le virus de la densonucléose (VDN) et présence d'une substance de type interféron chez un arthropode. Rev Can Biol. 1969 Jun;28(2):89–94. [PubMed] [Google Scholar]
- Gravell M., Malsberger R. G. A permanent cell line from the fathead minnow (Pimephales promelas). Ann N Y Acad Sci. 1965 Aug 10;126(1):555–565. doi: 10.1111/j.1749-6632.1965.tb14302.x. [DOI] [PubMed] [Google Scholar]
- JUNGWIRTH C., BODO G. BESTIMMUNG DES MOLEKULARGEWICHTES VON INTERFERON DURCH GELFILTRATION. Biochem Z. 1964 Mar 12;339:382–389. [PubMed] [Google Scholar]
- KREUZ L. E., LEVY A. H. PHYSICAL PROPERTIES OF CHICK INTERFERON. J Bacteriol. 1965 Feb;89:462–469. doi: 10.1128/jb.89.2.462-469.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. K., Loh P. C. Some properties of an established fish cell line from Xiphophorus helleri (red swordtail). In Vitro. 1973 Sep-Oct;9(2):73–80. doi: 10.1007/BF02616003. [DOI] [PubMed] [Google Scholar]
- LAMPSON G. P., TYTELL A. A., NEMES M. M., HILLEMAN M. R. CHARACTERIZATION OF CHICK EMBRYO INTERFERON INDUCED BY A DNA VIRUS. Proc Soc Exp Biol Med. 1965 Feb;118:441–448. doi: 10.3181/00379727-118-29870. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A. A simple method for estimating isoelectric points. Anal Biochem. 1965 May;11(2):374–377. doi: 10.1016/0003-2697(65)90026-6. [DOI] [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Nemes M. M., Hilleman M. R. Multiple molecular species of interferons of mouse and of rabbit origin. Proc Soc Exp Biol Med. 1966 Feb;121(2):377–384. doi: 10.3181/00379727-121-30783. [DOI] [PubMed] [Google Scholar]
- MERCER C. K., MILLS R. F. Interference with multiplication of Pc phage by a factor produced by exposure of Pseudomonas aeruginosa to inactivated Pc phage. J Gen Microbiol. 1960 Oct;23:253–256. doi: 10.1099/00221287-23-2-253. [DOI] [PubMed] [Google Scholar]
- MERIGAN T. C. PURIFIED INTERFERONS: PHYSICAL PROPERTIES AND SPECIES SPECIFICITY. Science. 1964 Aug 21;145(3634):811–813. doi: 10.1126/science.145.3634.811-a. [DOI] [PubMed] [Google Scholar]
- Oie H. K., Loh P. C. Reovirus type 2: induction of viral resistance and interferon production in fathead minnow cells. Proc Soc Exp Biol Med. 1971 Feb;136(2):369–373. doi: 10.3181/00379727-136-35266. [DOI] [PubMed] [Google Scholar]
- PHILLIPS A. W., WOOD R. D. SOME PHYSICAL PROPERTIES OF CHICK INTERFERON. Nature. 1964 Feb 22;201:819–820. doi: 10.1038/201819b0. [DOI] [PubMed] [Google Scholar]
- ROTEM Z., CHARLWOOD P. A. Molecular weights of interferons from different animal species. Nature. 1963 Jun 15;198:1066–1068. doi: 10.1038/1981066a0. [DOI] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C. Established eurythermic line of fish cells in vitro. Science. 1962 Mar 23;135(3508):1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]



