Abstract
The response to injury is one of wound healing and fibrogenesis, which ultimately leads to fibrosis. The fibrogenic response to injury is a generalized one across virtually all organ systems. In the liver, the injury response, typically occuring over a prolonged period of time, leads to cirrhosis (although it should be pointed out that not all patients with liver injury develop cirrhosis). The fact that many different diseases result in cirrhosis suggests a common pathogenesis. The study of hepatic fibrogenesis over the past 2 decades has been remarkably active, leading to a considerable understanding of this process. It has been clearly demonstrated that the hepatic stellate cell is a central component in the fibrogenic process. It has also been recognized that other “effector” cells are important in the fibrogenic process, including resident fibroblasts, bone marrow derived cells, fibrocytes, and even perhaps cells derived from epithelial cells (i.e., through epithelial to mesenchymal transition or EMT). A key aspect of the biology of fibrogenesis is that the fibrogenic process is dynamic; thus, even advanced fibrosis (or cirrhosis) is reversible. Together, an understanding of the cellular basis for liver fibrogenesis, along with multiple aspects of the basic pathogenesis of fibrosis, have highlighted many exciting potential therapeutic opportunities. Thus, while the most effective “anti-fibrotic” therapy is treatment of the underlying disease, in situations in which this not possible, specific anti-fibrotic therapy is likely to not only become feasible, but will soon become a reality. The goal of this review is to highlight the mechanisms underlying fibrogenesis that may be translated into future anti-fibrotic therapies and to review the current state of clinical development.
Introduction
The response to chronic injury is a generalized one, with features common among multiple organ systems. This feature suggests thematically related pathogenic events across organs. In the liver, many different kinds of injury, including viral hepatitis, alcohol, fatty liver, biliary tract disease, iron or copper overload, cystic fibrosis, and others cause fibrogenesis, and subsequently cirrhosis.
Over the past 2 decades, much has been learned about the biology and pathophysiology of fibrosis. Understanding the mechanisms underlying fibrosis has pointed out several potential therapeutic approaches. Preclinical studies have been particularly informative, and have highlighted many possible therapies. Although therapies that are directed at the underlying disease process, including anti-viral therapies for patients with hepatitis B and hepatitis C virus infection, have proven to be effective at reducing and/or reversing fibrosis, specific and effective anti-fibrotic therapy remains elusive. The objective of this review will be to emphasize fundamental concepts underlying hepatic fibrogenesis, and to review translational therapeutics.
Fibrogenesis – Pathophysiology
The fibrogenic process
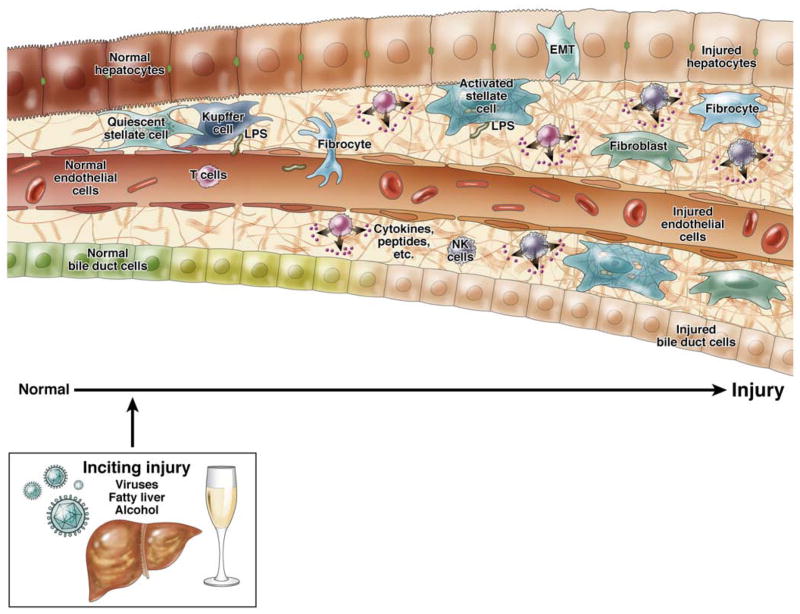
A critical aspect of the fibrogenic response is that injury, typically to hepatocytes stimulates the injury response (Figure 1). Multiple forms of injury, including hepatitis, metabolic disease (i.e, in particular the metabolic syndrome) biliary injury, toxins (including alcohol), heavy metals, cause a variety of complicated and often integrated effects in the liver. For example, viral hepatitis causes activation of T cells, with recruitment of other inflammatory cells, as well as inflammatory mediators, and this leads to the fibrogenic wounding response (Figure 1). Alcohol mediated hepatocyte injury causes a classic inflammatory lesion, including TNF, which leads to hepatitis, and a fibrogenic wounding response. It should be emphasized multiple different cell types play a role in the injury mileu. For example, injury to endothelial cells, either directly or indirectly causes them to produce abnormal extracellular matrix, which in turn stimulates fibrogenesis by stellate cells 1.
Figure 1. Liver injury and fibrogenesis.
In the liver, many different types of injury (i.e., chronic hepatitis, ethanol, metabolic disease, biliary tract disease, iron, copper, etc…) lead to hepatocyte injury, and then typically an inflammatory response. This injury process is complicated, but in aggregate, it stimulates a wound healing response, which involves a number of different systems. Paramount in this process is often including recruitment of inflammatory cells. Among other properties, inflammatory cells produce a variety of mediators, cytokines, and other factors, which in turn are responsible for stimulation and/or recruitment of other cells. Key among these other cells include effector cells, highlighted in the figure and including stellate cells, fibrocytes, fibroblasts, and even fibroblasts derived though epithelial to mesenchymal transition (EMT). These effectors produce extracellular matrix proteins (see text), and importantly interact with other cells in the wounding mileu. Additionally, it is important to emphasize that many forms of injury lead to activation and transformation of other cells in the liver, such as endothelial and bile duct epithelial cells. Injury to these cells in turn leads to a variety of downstream effects. Each injured endothelial bile duct epithelial cells are capable of stimulatulation of effector cells to produce extracellular matrix consitutents.
A central event in the hepatic wounding response is enhanced extracellular matrix production, or fibrogenesis (Figure 1). Irrespective of the specific cause of liver injury (in both experimental models and human cirrhosis), the wound process leads to increased synthesis of extracellular matrix. The fibrogenic process is characterized by increases in a multiple matrix components, including the interstitial collagens, basement membrane collagens, proteoglycans and matrix glycoproteins such as laminin and fibronectin 2; specific changes in matrix composition are highly similar in all forms of liver injury and hepatic fibrogenesis. Among the most prominent extracellular matrix proteins are the collagens (type I>III>IV), but increases in other matrix proteins are also prominent. It is important to emphasize that the wounding process is a dynamic one that includes aspects of matrix synthesis and deposition as well as degradation 3. This point is exemplified by a robust body of literature data indicating that experimental 4–6 and clinical fibrosis 7–9 and even clinical cirrhosis is reversible 10–17. In one study in patients with chronic hepatitis B infection and cirrhosis 14, 436 of 651 patients were assigned to receive lamivudine and 215 to receive placebo; 7.8 percent of patients receiving lamivudine and 17.7 percent of those receiving placebo developed hepatocellular carcinoma, spontaneous bacterial peritonitis, bleeding gastroesophageal varices, or had death related to liver disease (P=0.001). Additionally, the Child-Pugh score increased in 3.4 percent of the patients receiving lamivudine and 8.8 percent of those receiving placebo (P=0.02). Thus, not only is advanced fibrosis reversible, but resolution of fibrosis is also associated with improved clinical outcomes.
Hepatic stellate cells and their activation in fibrogenesis
A key concept in the wounding response is that during the fibrogenic response, there is activation of effector cells. Evidence now supports the presence of a number of effector cells including stellate cells 18, peri-portal and peri-central fibroblasts 19, fibrocytes 20, myofibroblasts, and perhaps fibrogenic cells derived from hepatocytes through epithelial to mesenchymal transition (or EMT) 21, 22.
Stellate cells (also known previously as lipocytes, Ito, perisinusoidal cells), perisinusoidal, pericyte-like cells of mesenchymal origin, have garnered great attention as effectors of the fibrogenic response. In the normal liver, these cells function as a major retinoid reservoir for the body, storing much of the body’s vitamin A 23, 24. Given their pericyte-like appearance, they may also function as regulators of blood flow 23. Notwithstanding, one of their most notable features occurs after liver injury. In this situation, stellate cells transform from “quiescent” (normal) to an “activated” (injured liver) state is a central component to the liver wounding process (Figure 1). The activation process is remarkably complex, with multiple and dynamic features. Phenotypically, it consists of many important cellular changes; characteristic features include loss of vitamin A, acquisition of stress bundles, and development of prominent rough endoplasmic reticulum (Figure 2). Perhaps the most prominent feature of activation is the striking increase in production and secretion of extracellular matrix proteins, including types I, III and IV collagens, fibronectin, laminin and proteoglycans, and others 2, 25. An additional important feature of activation is de novo expression of smooth muscle specific proteins, such as smooth muscle α actin 26. This feature further identifies stellate cells as liver specific myofibroblasts, a cell type typical of fibrogenesis in all organs 27, 28.
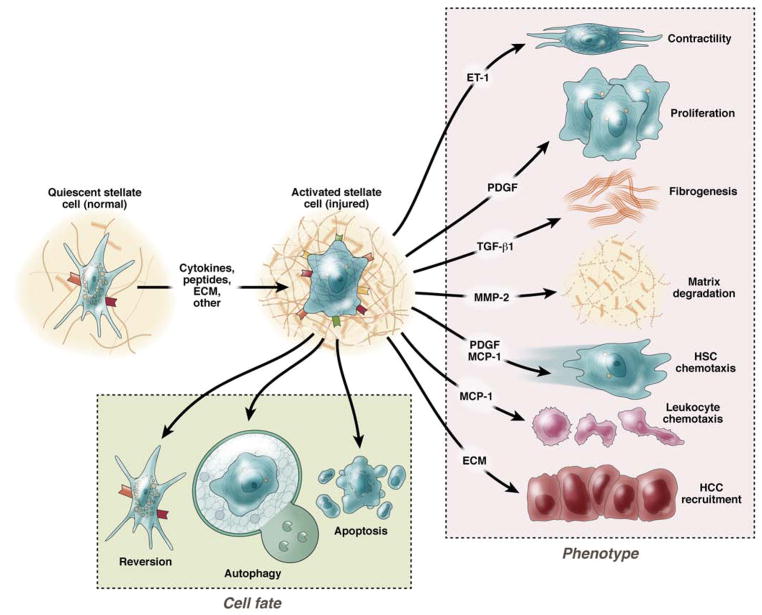
Figure 2. Stellate cell activation.
A key pathogenic feature underlying liver fibrosis and cirrhosis is activation of hepatic stellate cells (note that activation of other effector cells is likely to parallel that of stellate cells). The activation process is complex, both in terms of the events that induce activation and the effects of activation. Multiple and varied stimuli participate in the induction and maintenance of activation, including, but not limited to cytokines, peptides, and the extracellular matrix itself. Recently, signaling through TLR4 on stellate cells has been identified as important in activation. Key phenotypic features of activation include production of extracellular matrix, loss of retinoids, proliferation, of upregulation of smooth muscle proteins, secretion of peptides and cytokines (which have autocrine effects on stellate cells and paracrine effects on other cells such as leukocytes and malignant cells), and upregulation of various cytokine and peptide receptors. Additionally, evidence indicates that stellate cells exhibit several cell fates, highlighted at the bottom of the figure, and each of these appear to play a role in the biology of fibrogenesis.
Although the most prominent features of activation include enhanced extracellular matrix production, and the expression of smooth muscle α actin, activation is also associated with other important cellular phenotypes including enhanced proliferation, release of proinflammatory cytokines 29, release of matrix degrading enzymes and their inhibitors, and recruitment and activation of other cell types such as hepatocellular cancer and cholangiocarcinoma cells 30, 31 and inflammatory cells 32. When deisgning therapeutics focused on liver fibrogenesis, is important to emphasize that each of these features of activation (and fibrogenesis) represent a potential target for therapy. Important elements of the activation process are highlighted below.
Stellate cell fibrogenesis
Multiple factors play key pathogenic roles in stellate cell fibrogenesis. Prominent among these factors are cytokines, small peptides, and the extracellular matrix itself. Transforming growth factor beta-1 (TGF-β1) appears to be the most profibrogenic cytokine in the liver 33–35. TGFβ1 is produced by Kupffer cells, sinusoidal endothelial cells, bile duct epithelial cells, hepatocytes and by stellate cells and has prominent paracrine/autocrine effects on stellate cells 36, 37. When TGFβ1 is overexpressed in the liver, it leads to promiinent fibrosis 33 and when inhibited during experimental liver injury, fibrosis is reduced 38. TGFβ-1 signaling in stellate cells is remarkably complex 39, acting via direct (and to a lesser extent, indirect) pathways to stimulate of extracellular matrix production in stellate cells. Although none appears to be as potent as TGFβ1, a variety of other cytokines and peptides have profibrogenic effects on stellate cells (Table 1), including connective tissue growth factor (CTGF) 40, 41, endothelin-1 6, leptin 42, angiotensin II 43, and others.
Table 1.
Cytokines and growth factors important in stellate cell fibrogenesis
| Profibrogenic | Antifibrogenic |
|---|---|
| Transforming growth factor-β | Interferon γ |
| Transforming growth factor-α | Interferon α |
| Connective tissue growth factor | Adiponectin |
| * Insulin-like growth factor (I, II) | ** Endostatin |
| * Platelet derived growth factor | Hepatocyte growth factor |
| * Monocyte chemotactic factor | |
| * Fibroblast growth factor | |
| ** Interleukin-1 | |
| ** Interleukin-4 | |
| * Interleukin-6 | |
| * Thrombin | |
| Endothelin-1 | |
| Norepinephrine | |
| Angiotensin II | |
| Thrombospondin (1,2) | |
| Leptin | |
| ** Lipopolysaccaride |
Agents whose effect is largely via stimulation of proliferation
Indirect effects on stellate cells
Including fragments
It should also be emphasized that cytokines and growth factors that drive stellate cell proliferation are important in the fibrogenic response because they help expand the total number of fibrogenic (stellate) cells. In essentially all forms of fibrosing liver injury, the number of activated effector cells is increased. Although the major mitogen driving cellular proliferation appears to be PDGF, a variety of other factors appear to be important in stimulation of stellate cell proliferation and include epidermal growth factor, fibroblast growth factor, insulin-like growth factor, thrombin, PAR agonists, monocyte chemotactic factor (MCP-1), insulin like growth factors (IGF-1 and 2), interleukin-6, CTGF, endothelin-1, angiotensin II, and others. While many of these compounds have isolated proliferative effects (i.e. PDGF), others (i.e. endothelin-1, angiotensin II, CTGF) stimulate both proliferation and fibrogenesis.
The vasoactive peptides endothelin-1 and angiotensin II, each of which have pleotrophic cell biologic and molecular effects, are notable not only because they have been emphasized in the pathogenesis of hepatic fibrogenesis 6, 43–45, but also because these compounds have vasoactive properties, and as such, may be important in the pathogenesis of portal hypertension. This raises the possibility that therapy directed at them could affect both fibrogenesis and portal hypertension. Other biologically active peptides (including unidentified compounds) may also be important in fibrogenesis. For example, dopamine beta-hydroxylase deficient mice, which cannot make norepinephrine, are resistant to fibrogenesis 46. Thus, antagonism of these systems is attractive.
A number of cytokines and peptides appear to have anti-activation or anti-fibrogenic properties towards stellate cells. Although the number of the agents is considerably less than the number reported to be pro-fibrogenic and/or stimulate proliferation, included in this group are interferon γ 47, interferon α 48, adiponectin 49, hepatocyte growth factor 50, and possibly STAP 51.
Evolving evidence indicates that the extracellular matrix and the local environment plays an important role in modulating stellate cell activation. For example, culture of stellate cells on a basement membrane mimicking the normal basement membrane inhibits stellate cell activation and matrix synthesis 52, while culture of stellate cells on abnormal substrates such as the EDA isoform of fibronectin leads to increased activation of stellate cells 1. Further, data suggest that stellate cells sense their surrounding environment 53. For example, it was demonstrated that stellate cells became activated preferentially while exposed to a stiff substrate (compared to a softer substrate), and that this stiffness-dependent activation required adhesion to matrix proteins and the generation of mechanical tension 54. It has also been shown that integrins, which link the extracellular matrix to stellate (and other cells) play an important role in transmitting fibrogenic and contractile signals 55. Recently, integrin linked kinase (ILK), an integrin-intracellular signaling molecule, has been shown to transmit fibrogenic signals in stellate cells 56, 57.
It should also be pointed out that fibrogenesis is a dynamic process with elements of extracellular matrix synthesis as well as degradation. During fibosis progression, there is not only increased expression of extracellular matrix protiens as highlighted above, but also metalloproteinases (MMPs) and in particular their tissue inhibitors (TIMPs). Evolving evidence suggests that early in the injury process, increases in expression of MMP-2 and membrane type 1-MMP lead to degradation of normal basement membrane matrix, which appears to facilitate stellate cell activation 58–60. Additionally, overexpression of the TIMPs (TIMP-1 and TIMP-2) contributes to the profibrogenic phenotype 58. This dynamic interplay of matrix synthesis and degradation is complex, but an attractive therapeutic target. As proof of concept, overexpression of MMP8 has been shown to lead to partial reversal of fibrosis 61.
Stellate cell contractility
Activation of stellate cells is accompanied by an increase in expression of proteins characteristic of contractile cells (i.e., such as smooth muscle α actin and smooth muscle myosins 26, 62). Stellate cell contraction has been reported to be mediated by Ca++ dependent and independent mechanisms 63–66. Stellate cell contraction has a multitude of effects in the injured liver including in perisinuoidal constriction and portal hypertension, and may also lead to the collapse and shrunken state of cirrhotic livers 45. Stellate cell contractility is likely tied to multiple different systems, including the endothelin, angiotensin, adrenergic, and perhaps other systems 44, 45, 66–71.
Other stellate cell activation phenotypes
Beyond the phenotypes highlighted above, during liver injury and activation, stellate cells exhibit a number of important features (Figure 2). For example apoptosis (i.e., programmed cell death) is prominent in stellate cells and appears to be an important mechanism for fibrosis regression 5. The data suggest that a balance between cell proliferation and apoptosis is important in determining the dynamics of the total overall stellate cell population in the liver. Based on these data, stimulation of stellate cell apoptosis could be an attractive therapeutic approach 72. However, it has also been shown that stellate cell apoptosis may stimulate stellate cell activation, and thus may not be desirable 73. Additionally, stellate cells may undergo senescence 74 or revert to a normal phenotype 75. Recently, autophagy, a catabolic mechanism involviing cell degradation of unnecessary or dysfunctional cellular components through the lysosomal pathway, appears to play a role in stellate cell activation 76–78. In mice with stellate cell specific deletion of autophagy-related protein 7 (Atg7), a protein important in mammalian autophagy, led to reduced activation following liver injury, leading to reduced fibrosis in vivo 78.
Approach to therapy for fibrosis
It is important to emphasize that the most effective anti-fibrotic therapies are those that target the primary stimulus to fibrogenesis (Table 2). For example, eradication or inhibition of hepatitis B virus (HBV) 7, 9 or hepatitis C virus (HCV) 8 leads to reversion of fibrosis, and is associated with improved clinical outcomes 11, 12, 14. Fibrosis (and cirrhosis) in patients with autoimmune hepatitis who respond to medical treatment (prednisone or equivalent) is reversible 13, 17. Fibrosis may improve in patients with alcoholic liver disease who respond to anti-inflammatory therapy such as corticosteroids 79, 80. Fibrosis reverts in patients with hemochromatosis during iron depletion 81, 82 and after relief of bile duct obstruction 15. Additionally, in patients with non-alcoholic steatohepatitis (NASH) treated with the peroxisomal proliferator active receptor (PPAR) gamma agonist, rosiglitazone reduced both steatosis and fibrosis 83.
Table 2.
Liver Diseases in which treatment of the underlying process may reverse fibrosis
| Disease | Comments |
|---|---|
| Hepatitis B | Antiviral treatment improves outcomes |
| Hepatitis C | Viral eradiction improves outcomes |
| Autoimmune hepatitis | Corticosteroids may improve outcomes |
| Alcoholic hepatitis | Corticosteroids may improve outcomes |
| Bile duct obstruction | Biliary decompression improves histology |
| Hemochromatosis | Iron depletion may improve outcomes |
| Primary biliary cirrhosis | UDCA, MTX have weak effects |
| Non-alcoholic steatohepatitis | PAR ligands have weak effects |
See text for discussion and references.
Abbreviations: MTX = methotrexate; PPAR = peroxisomal proliferator activated receptor
Experimental studies have demonstrated that many different interventions are capable of inhibiting (usually preventing) fibrogenesis. Such therapies have been targeted at inhibition of collagen synthesis, matrix deposition, modulation of stellate cell activation, stimulation of matrix degradation or stimulation of stellate cell death. A number of these preclinical approaches have been transitioned to clinical trials in humans (Table 3). The summary presented below indicates that as of the current writing, a specific anti-fibrotic that fits the profile of an ideal agent - one that is potent, safe, orally bioavailable, and inexpensive - is not yet available.
Table 3.
Potential anti-fibrotic therapies tested in humans
| Agent | Disease | Comments | Status |
|---|---|---|---|
| Compounds with anti-inflammatory, anti-oxidant or general effects | |||
| Interleukin-10 | HCV | Increased viral load | Not suitable for therapy |
| PPC | ETOH | Minimal if any effect | Not recommended |
| SAM | ETOH | Minimal if any effect | Not recommended |
| Silymarin | HCV/ETOH | Further studies pending | |
| Anti-TNFα | ETOH | Increased mortality | Likely dangerous |
| UDCA | Multiple | Modestly effective, safe | May be acceptable (PBC) |
| Vitamin E | HCV/NASH | Modestly effective, safe | May be acceptable |
| Pentoxifylline | ETOH | Minimally effective, safe | May be acceptable |
| Compounds with specific anti-fibrotic effects | |||
| Colchicine | Misc | Minimal if any effect | Not recommended |
| Interferon gamma | HCV | Minimal if any effect | Not suitable for therapy |
| Farglitizar | NASH | No clear effect | Not suitable for therapy |
| ARBs | Misc | Minimal if any effect | May be acceptable |
See the text for specific discussion of mechanism and for references.
Abbreviations: PPC = Polyenylphosphatidylcholine; SAM = s-adenosylmethionine, TNF = tumor necrosis factor; PPAR = peroxisomal proliferator activated receptor, ETOH = alcohol, HCV = hepatitis C virus, NASH = non alcoholic steatohepatitis, misc = miscellaneous, UDCA = ursodeoxycholic acid; PBC = primary biliary cirrhosis; ARB = angiotensin receptor blocker.
Specific anti-fibrotic targets and therapies
Colchicine is a plant alkaloid that inhibits polymerization of microtubules, and has anti-fibrotic properties in experimental animal models 84. Although it has been studied in a number of clinical trials 85–88, including in primary biliary cirrhosis, alcoholic cirrhosis, as well as in miscellaneous other liver diseases 86, evidence supporting its effectiveness remains lacking.
Interleukin-10, an anti-inflammatory and immunomodulatory cytokine can down regulate production of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin-1, and interleukin-2 from T cells. When administered to patients with HCV, interleukin-10 reduced hepatic inflammation and fibrosis scores (mean change from 5.0 ± 0.2 to 4.5 ± 0.3, p <0.05). However, serum HCV RNA levels increased during therapy and thus has not been pursued.
Several studies have shown that interferon γ has potent inhibitory effects on stellate cells, inhibiting multiple aspects of stellate cell activation including fibrogenesis 47, 89. A preliminary recent report in patients with chronic hepatitis C infection and fibrosis indicated that a subgroup of patients had an anti-fibrotic response 90. However, a larger randomized study found that interferon γ failed to have an antifibrotic effect in patients with HCV and advanced fibrosis, presumably because it enrolled patients with advanced cirrhosis and treated them for too short a time period 91.
The peroxisomal proliferator activated receptor (PPAR) system has gained considerable attention in the hepatic fibrogenesis field 92–94. PPAR γ in particular is reduced during stellate cell activation, and PPAR γ ligands inhibit activation and synthesis of extracellular matrix 92–94. Further, the adipocytokine, adiponectin, appears to have prominent anti-fibrotic actions, and the PPAR γ effects on stellate cells are at least in part, adiponectin dependent 95. Because of its added putative beneficial role in the metabolic syndrome, adiponectin, is an attractive therapeutic target. Given the potential of PPAR γ agonists in treatment of patients with fibrosis and preliminary studies that demonstrated significant anti-fibrotic effects of the PPAR γ agonist, farglitazar, in animal models of fibrosis 96, a large multicenter randomized trial of farglitazar in patients with HCV was performed 97. This well deisgned study demonstrated that farglitazar therapy for 52 weeks failed to have an effect on stellate cell activation or fibrosis in this population.
Polyenylphosphatidylcholine contains a mixture of polyunsaturated phosphatidylcholines, extracted from soybeans. Because of its presumed cytoprotective effect, it has been examined in humans 98. Unfortunately, in a major multicenter, prospective, randomized, double-blind placebo-controlled trial study of 789 alcoholics (average alcohol intake of 16 drinks/day). comnparing either polyenylphosphatidylcholine or placebo for 2 years, there was no significant improvement in fibrosis. Of note, the majority of subjects reduced their ethanol consumption during the trial (presumably leading to an improvement in fibrosis in the control group).
Silymarin extract, derived from the milk thistle Silybum marianum (the major active component of which is silybinin), reduces lipid peroxidation and inhibits fibrogenesis in animal models 99–101. In humans with fibrosis, the compound has had mixed effects 102, 103. Thus, although silymarin appears to be safe, data supporting its use are lacking and further study is underway in patients with HCV (ClinicalTrials.gov Identifier: NCT00680342) and NASH (ClinicalTrials.gov Identifier: NCT00680407).
Ursodeoxycholic acid binds to hepatocyte membranes and appears to be cytoprotective, thereby reducing inflammation and thus fibrogenesis 104. The aggregate data suggest that ursodeoxycholic acid may impede progression of fibrosis in primary biliary cirrhosis via effects on bile ductal inflammation, particularly if given early in the disease course 105, 106. In a large randomized controlled trial of ursodeoxycholic acid in patients with non-alcoholic steatohepatitis over a 2-year course, examining 107 subjects who had paired biopsy data, there was no improvement in fibrosis 106. In aggregate, rsodeoxycholic acid is safe, and while expensive, it is this author’s belief that the available data justify its use at least in patients with primary biliary cirrhosis as an anti-fibrotic.
Vitamin E has gained a great deal of attention as a potential antifibrotic; it appears to be effective in animal models 107. In humans vitamin E has has equivocal effects in patients with HCV 108, and alcoholic hepatitis 109, 110. In patients with NASH, vitamin E led to reductions in aminotransferases, hepatic steatosis, and lobular inflammation, but failed to lead to an improvement in fibrosis 111.
A number of herbal medicines have been shown to have anti-fibrotic properties in animal models, and in some, specific mechanisms have been identified 112–115. Herbal medicines with putative anti-viral, anti-inflammatory, and anti-fibrotic effects are being used extensively in the Far East in patients with a variety of liver diseases 116. Medications containing herbs of the Salvia genus have been popular in particular as anti-fibrotics 116. Although human trials have suggested effectiveness of specific herbal medicines in some studies 116, data in peer-reviewed Western journals remains lacking. Since it is well appreciated that such herbal medicines may have significant toxicity, including hepatotoxicity 117, these medications should be used with caution.
The use of anti-tumor necrosis factor alpha (TNF-α) compounds in patients with alcoholic hepatitis is predicated on the rationale that TNF-α is upregulated after alcohol mediated hepatocellular injury (Figure 1), and thus these compounds should reduce inflammation, and resultant fibrosis. While early studies suggested an improvement in inflammation, 118–121, further larger studies revealed that their use was associated with an increase in the risk of serious infection 122 and mortality 123. Pentoxyfylline appears to reduce TNF-α expression, and may also have primary antifibrotic effects 124, 125. While data suggest an effect on certain clinical outcomes 118, 126, definitive evidence of an antifibrotic effect in humans is lacking.
Malotilate, penicillamine, methotrexate, S-adenosylmethionine, and propylthiouracil all have have been shown to exhibit some degree of anti-inflammatory and/or cytoprotective effects (presumably through their anti-oxidant properties) and as such, may have an effect on fibrogenesis 127, 128, 129. However, evidence of an effect on fibrosis is equivocal at best 130–138. It is important to emphasize that for many of these human studies, subjects with alcoholic hepatitis and liver injury were examined, and in these studies fibrosis was not typically measured as a specific outcome. Thus, it is may not be entirely appropriate to consider these agents as primary anti-fibrotics, but rather as compounds that could have secondary effects on fibrogenesis due to other properties.
Novel Approaches
A number of novel approaches to treat liver fibrosis exist. This includes novel mechanisms of targeting the liver, such as the use of siRNA 139, 140 or specific targeting systems 69, 141. For example, TGF-β is well known to play a central role in the fibrogenic cascade and therefore is an important therapeutic target. Multiple proof of concept studies have demonstrated that its inhibition (through use of specific antibodies that immobilize active TGF-β or receptor antagonists) is likely to be effective in fibrosis 38, 142, 143. However, given its important role in regulation of cell growth, global inhibition of TGF-β, or similar agents that have widespread biological effects such as PDGF or endothelin-1 could be potentially harmful. Thus, it will likley be critical to localize biological effects to fibrogenic effector cells. Early studies have provided proof of concept of this approach for stellate cells 144.
Previous and exciting new pathophysiologic studies point to further translational opportunities to treat fibrosis (Table 4). Given the central role of inflammation in chronic hepatic injury and the ensuing wound-healing process (Figure 1), it follows that bacterial products, particularly LPS may be important pathogenically. New evidence suggests that the microbiota may be important in the pathogenesis of liver inflammation 145, fibrosis 146, and even development of hepatocellular cancer 147. In quiescent stellate cells, TLR4 (a major LPS receptor) activation not only upregulates chemokine secretion (further driving inflammation), but it also downregulates the transforming growth factor (TGF)-β pseudoreceptor Bambi, which in turn sensitizes stellate cells to TGF-β-induced signaling 148. In another study, liver injury was associated with early onset of increased intestinal permeability and bacterial translocation that preceded changes in the microbiota 149. Changes in the microbiota have also been associated with fibrosis progression 146. As such, manipulation of the intestinal flora may be an innovative approach to anti-fibrotic therapy.
Table 4.
Potential Anti-Fibrotic Targets
| Agent or System | Mechanism |
|---|---|
| Intestinal microbiota/TLR4 | TLR4 on multiple cells types, including stellate cells activates inflammatory pathways |
| NRF2 | Transcription factor whose downstream target genes play an important role in cellular anti-oxidant defense |
| Loxl2 | Enzyme ca thetalyzes first step in the formation of crosslinks in collagens and elastin |
| Adiponectin | 244-amino-acid-long polypeptide regulating glucose levels as well as fatty acid breakdown that has direct effects on stellate cell fibrogenesis |
| Angiostatin/Endostatin | Endogenous angiogenesis inhibitors |
| Endothelin | 21 aa potent vasoconstrictor, that also stimulates stellate cell activation |
TLR4 = Toll like receptor 4
NRF2 = Nuclear factor (erythroid-derived 2)-like 2
LOXL2 = Lysyl oxidase homolog 2
MicroRNAs (miRs) have become recognized as being important in gene regulation and recent evidence suggests that a number of miRs are invovled in the pathogenesis of different forms of organ fibrosis 150 and in stellate cell function and liver fibrosis and 151, 152, and therefore may represent novel therapeutic targets.
A variety of other systems are also attractive. Among these include those related to collagen synthesis, such as the lysyl oxidase system; inhibition of this copper-dependent extracellular enzyme that catalyzes lysine-derived cross-links in collagen and elastin, could abrogate tissue fibrosis 153, 154. Angiogenic pathways appear to be important in fibrosis, including the liver, and thus, interruption of this pathway could be an effective treatment approach. For example, a short peptide derived from endostatin, a naturally-occurring 20-kDa C-terminal fragment derived from type 18 collagen, appeared to have potent anti-fibrotic activity in skin and pulmonary fibrosis in vivo 155. Nuclear factor (erythroid-derived 2)-like 2 (nrf2), a transcription factor that appears to active a number of genes involved in oxidative stress reponse appears to have protective effects for fibrosis 156, 157. Additionaly, compounds such as pirfenidone 158, and 5′-lipoxygenase inhibitors 159 appear to have direct effects on stellate cells and/or in vivo effects in hepatic fibrogenesis. While there has been much interest in manipulating the balance between matrix synthesis and degradation via stimulation of collagen degrading metalloproteases, or dampening the effect of metalloprotease inhibitors, this area remains largely open.
Vascular biologic systems are intriguing because they could potentially have benefical effects both for fibrosis and for portal hypertnesion. Stellate cells express angiotensin II and endothelin receptors and stimulation of these receptors with their cognate ligands leads to prominent stellate cell effects 45.
Challenges in Developing Anti-fibrotic Therapy
Currently, a potent and effective anti-fibrotic drug or agent is not available. This is likely the result of several factors, highlighted below. Additionally, in order to develop a highly effective anti-fibrotic agent(s), several key features - as highlighted - will be important.
1. Diagnosis/Monitoring of Hepatic Fibrosis and Cirrhosis
Perhaps one of the most difficult challenges in the field of development of antifibrotic medications is monitoring the effectiveness of putative compounds. An ideal test would be one that is non-invasive and simple to perform, yet inexpensive. Currently, liver biopsy is considered to be the gold standard test for determining the extent and progression of fibrosis 160. A quantitative measure of collagen content can be made by colorimetric assay of sirius red in liver tissue or by image analytic quantitation of collagen containing tissue 6. Additionally, scoring systems have been developed 161–163 to quantitate fibrosis and to help standardize the interpretation of biopsies amongst different centers; such systems are most useful for standardization and comparison of fibrosis in studies.
Unfortunately, liver biopsy, while considered the gold standard tool to assess fibrosis, is inexact. Not only is liver biopsy subject to inter-observer variability, but sampling error may be important, as evidenced by studies examining liver samples from different regions of the liver 164. Additionally, liver biopsy is also associated with significant potential morbidity, including a significant risk of death 160 Thus, noninvasive measures that can monitor fibrogenesis would be ideal 165. Noninvasive tools used to assess fibrosis include radiographic tests 166, combinations of routine laboratory tests 167, 168, and specific serum markers 169. In particular, serum marker panels, including several that utilize mathematical algorithms 167, 168, 170, have been emphasized. Although some of these may even have predictive clinical value 171, 172, they have generally proven to be of limited clinical utility.
Finally, the field of molecular imaging is emerging, and with it, it is possible that effectors cells such as stellate cells may be imaged in order to more precisely quantitate their activity and or fibrogenic features 173, 174.
2. Cell Specific Targeting
As emphasized above, it would be ideal to localize therapy to only effector cells. This is particularly imporant for the targeting of systems involving systems that have widespread biological effects such as TGF-β, PDGF or endothelin-1 for example. TGF-β, in particular, is an attractive target since it appears to be the most potent stimulator of fibrogenesis. However, given its important role in regulation of cell growth, and neoplasia, it is highly likely that its global inhibition would have undesirable effects. A number of studies have provided proof of concept that at least stellate cells can be specifically targeted; by taking advantage of the expression of the mannose 6-phosphate/insulin-like growth factor II (M6P/IGF-II) receptor on stellate cells, it has been elegantly demonstrated that M6P-modified albumins conjugated to specific inhibitors or toxins reduced stellate cell mediated fibrogenesis 144, 175. Alternatively, it is possible that physical properties of activated stellate cells may be taken advantage of, and that stellate cells could be targeted with specialized liposomes or similar compounds 176–178.
3. Length of Therapy
As emphasized above, fibrogenesis is a dynamic process that occurs over a period of time; advanced fibrosis typically develops over prolonged periods of time. Thus, it is likely that reversion of fibrosis would be expected to also occur over more prlonged periods of time. Most of the trials examining novel agents have been performed over relatively short periods of time, typically over 6 or 12 months. To see meaningful regression of fibrosis, it is likely that a trial will require longer than 1 year, and perhaps longer than 2 years.
3. Endpoints
The most appropriate endpoint for a novel treatment is a signal that the compound has antifibrotic effects. Notwithstanding this point, trials to date have used histologic assessment. This means that it is likely that the agent to be tested must be effective enough to cause a change in histology. It may be more appropriate to use a marker or set of markers that detect a fibrogenic signal. For example, serum markers assessed over time may be acceptable. Additionally, some have suggested that an anti-fibrotic agent should have an effect on clinical outcomes. This would require a prolonged treatment, which would make the likelihood of developing an effective agent difficult.
Summary and Future Directions
The pathogenesis of hepatic fibrogenesis is now better understood than ever before. The central event in fibrogenesis appears to be activation of effector cells, most prominently hepatic stellate cells. Stellate cell activation is characterized by many important features including prominently, enhanced matrix synthesis and a contractile phenotype. The activation process is complex, leading to multiple potential sites for therapeutic interventions. A further critical concept is that the fibrogenic lesion, in particular, the extracellular matrix, is a dynamic structure; even advanced fibrosis may be reversible. These data have helped spawn interest in development of therapeutic antifibrotics. Notwithstanding, the most effective therapy for hepatic fibrogenesis is removal of the underlying disease process. While a number of challenges exist, including in the area of cell specific targeting, fibrosis monitoring, and execution of suitable clinical trials, the prospects for translation of the basic pathophysiology to therapy are bright. As for specific therapy directed primarly at the fibrotic lesion, the most effective therapies will most likely be directed fibrogenic effectors, in most cases hepatic stellate cells. In aggregate, although specific, effective, safe, and inexpensive anti-fibrotic therapies are not yet currently available, multiple potential targets have been identified, and one or more will likely emerge.
Acknowledgments
This work was supported by the NIH (Grant R01 DK 57830).
Footnotes
Author contributions:
Don Rockey - drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Disclosures: The author certifies that he has no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jarnagin WR, Rockey DC, Koteliansky VE, et al. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC. The cell and molecular biology of hepatic fibrogenesis. Clinical and therapeutic implications. Clin Liver Dis. 2000;4:319–55. doi: 10.1016/s1089-3261(05)70113-6. [DOI] [PubMed] [Google Scholar]
- 4.Rockey DC. Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3:95–107. doi: 10.1016/s1542-3565(04)00445-8. [DOI] [PubMed] [Google Scholar]
- 5.Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–49. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98:1381–8. doi: 10.1172/JCI118925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group [see comments] N Engl J Med. 1998;339:61–8. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–13. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 9.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–7. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 10.Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56:1171–80. doi: 10.1016/j.jhep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Bruno S, Crosignani A, Facciotto C, et al. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069–76. doi: 10.1002/hep.23528. [DOI] [PubMed] [Google Scholar]
- 12.Mallet V, Gilgenkrantz H, Serpaggi J, et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399–403. doi: 10.7326/0003-4819-149-6-200809160-00006. [DOI] [PubMed] [Google Scholar]
- 13.Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646–52. doi: 10.1016/j.jhep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–31. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 15.Hammel P, Couvelard A, O’Toole D, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344:418–23. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 16.Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- 17.Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981–5. doi: 10.7326/0003-4819-127-11-199712010-00006. [DOI] [PubMed] [Google Scholar]
- 18.Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10:459–79. vii–viii. doi: 10.1016/j.cld.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Dranoff JA, Chan EP, et al. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–56. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 20.Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–38. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaimori A, Potter J, Kaimori JY, et al. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007;282:22089–101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 23.Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–353. doi: 10.1016/s0074-7696(08)61977-4. [DOI] [PubMed] [Google Scholar]
- 24.Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991;5:271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- 25.Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockey DC, Boyles JK, Gabbiani G, et al. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193–203. [PubMed] [Google Scholar]
- 27.Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol. 2006;85:175–81. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 29.Aleffi S, Petrai I, Bertolani C, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–48. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 30.Santamato A, Fransvea E, Dituri F, et al. Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clin Sci (Lond) 2011;121:159–68. doi: 10.1042/CS20110002. [DOI] [PubMed] [Google Scholar]
- 31.Gentilini A, Rombouts K, Galastri S, et al. Role of the stromal-derived factor-1 (SDF-1)-CXCR4 axis in the interaction between hepatic stellate cells and cholangiocarcinoma. J Hepatol. 2012;57:813–20. doi: 10.1016/j.jhep.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Bonacchi A, Petrai I, Defranco RM, et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060–76. doi: 10.1016/s0016-5085(03)01194-6. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson N, Factor V, Nagy P, et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92:2572–6. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellerbrand C, Stefanovic B, Giordano F, et al. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. Journal of Hepatology. 1999;30:77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 35.Schnabl B, Kweon YO, Frederick JP, et al. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;34:89–100. doi: 10.1053/jhep.2001.25349. [DOI] [PubMed] [Google Scholar]
- 36.Friedman SL, Maher JJ, Bissell DM. Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. Hepatology. 2000;32:1403–8. doi: 10.1053/jhep.2000.20243. [DOI] [PubMed] [Google Scholar]
- 37.Bissell DM, Wang SS, Jarnagin WR, et al. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447–55. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yata Y, Gotwals P, Koteliansky V, et al. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology. 2002;35:1022–30. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- 39.Khimji AK, Shao R, Rockey DC. Divergent transforming growth factor-beta signaling in hepatic stellate cells after liver injury: functional effects on ECE-1 regulation. Am J Pathol. 2008;173:716–27. doi: 10.2353/ajpath.2008.071121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang G, Brigstock DR. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci. 2012;17:2495–507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]
- 41.Tong Z, Chen R, Alt DS, et al. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50:939–47. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saxena NK, Saliba G, Floyd JJ, et al. Leptin induces increased alpha2(I) collagen gene expression in cultured rat hepatic stellate cells. J Cell Biochem. 2003;89:311–20. doi: 10.1002/jcb.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bataller R, Schwabe RF, Choi YH, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–94. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bataller R, Gabele E, Parsons CJ, et al. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046–55. doi: 10.1002/hep.20665. [DOI] [PubMed] [Google Scholar]
- 45.Rockey DC. Vascular mediators in the injured liver. Hepatology. 2003;37:4–12. doi: 10.1053/jhep.2003.50044. [DOI] [PubMed] [Google Scholar]
- 46.Oben JA, Roskams T, Yang S, et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut. 2004;53:438–45. doi: 10.1136/gut.2003.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rockey DC, Chung JJ. Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med. 1994;42:660–70. [PubMed] [Google Scholar]
- 48.Inagaki Y, Nemoto T, Kushida M, et al. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38:890–9. doi: 10.1053/jhep.2003.50408. [DOI] [PubMed] [Google Scholar]
- 49.Kamada Y, Tamura S, Kiso S, et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 50.Ueki T, Kaneda Y, Tsutsui H, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–30. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 51.Kawada N, Kristensen DB, Asahina K, et al. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem. 2001;276:25318–23. doi: 10.1074/jbc.M102630200. [DOI] [PubMed] [Google Scholar]
- 52.Friedman SL, Roll FJ, Boyles J, et al. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989;264:10756–10762. [PubMed] [Google Scholar]
- 53.Gaca MDA, Georges P, Jammey P, et al. Matrix compliance determines hepatic stellate cell phenotype. Hepatology. 2003;38:776A. [Google Scholar]
- 54.Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–8. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Racine-Samson L, Rockey DC, Bissell DM. The role of alpha1beta1 integrin in wound contraction. A quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem. 1997;272:30911–7. doi: 10.1074/jbc.272.49.30911. [DOI] [PubMed] [Google Scholar]
- 56.Shafiei MS, Rockey DC. The role of integrin-linked kinase in liver wound healing. J Biol Chem. 2006;281:24863–72. doi: 10.1074/jbc.M513544200. [DOI] [PubMed] [Google Scholar]
- 57.Shafiei MS, Rockey DC. The function of integrin-linked kinase in normal and activated stellate cells: implications for fibrogenesis in wound healing. Lab Invest. 2012;92:305–16. doi: 10.1038/labinvest.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–9. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 59.Han YP, Zhou L, Wang J, et al. Essential role of matrix metalloproteinases in interleukin-1-induced myofibroblastic activation of hepatic stellate cell in collagen. J Biol Chem. 2004;279:4820–8. doi: 10.1074/jbc.M310999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21 (Suppl 3):S88–91. doi: 10.1111/j.1440-1746.2006.04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Banuelos J, Siller-Lopez F, Miranda A, et al. Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors. Evidence of cirrhosis reversion. Gene Ther. 2002;9:127–34. doi: 10.1038/sj.gt.3301647. [DOI] [PubMed] [Google Scholar]
- 62.Xu G, Rockey DC. Regulation of smooth muscle myosin heavy chain isoforms in hepatic myofibroblasts during liver injury. Hepatology. 1999;30:492A. [Google Scholar]
- 63.Pinzani M, Failli P, Ruocco C, et al. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992;90:642–646. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iizuka M, Murata T, Hori M, et al. Increased contractility of hepatic stellate cells in cirrhosis is mediated by enhanced Ca2+-dependent and Ca2+-sensitization pathways. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1010–21. doi: 10.1152/ajpgi.00350.2010. [DOI] [PubMed] [Google Scholar]
- 66.Xu H, Zhou Y, Lu C, et al. Salvianolic acid B lowers portal pressure in cirrhotic rats and attenuates contraction of rat hepatic stellate cells by inhibiting RhoA signaling pathway. Lab Invest. 2012;92:1738–48. doi: 10.1038/labinvest.2012.113. [DOI] [PubMed] [Google Scholar]
- 67.Trebicka J, Hennenberg M, Schulze Probsting A, et al. Role of beta3-adrenoceptors for intrahepatic resistance and portal hypertension in liver cirrhosis. Hepatology. 2009;50:1924–35. doi: 10.1002/hep.23222. [DOI] [PubMed] [Google Scholar]
- 68.Zou WL, Yang Z, Zang YJ, et al. Inhibitory effects of prostaglandin E1 on activation of hepatic stellate cells in rabbits with schistosomiasis. Hepatobiliary Pancreat Dis Int. 2007;6:176–81. [PubMed] [Google Scholar]
- 69.van Beuge MM, Prakash J, Lacombe M, et al. Reduction of fibrogenesis by selective delivery of a Rho kinase inhibitor to hepatic stellate cells in mice. J Pharmacol Exp Ther. 2011;337:628–35. doi: 10.1124/jpet.111.179143. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe A, Sohail MA, Gautam S, et al. Adenine induces differentiation of rat hepatic stellate cells. Dig Dis Sci. 2012;57:2371–8. doi: 10.1007/s10620-012-2183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J, Kuruba R, Wilson A, et al. Inhibition of endothelin-1-mediated contraction of hepatic stellate cells by FXR ligand. PLoS One. 2010;5:e13955. doi: 10.1371/journal.pone.0013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wright MC, Issa R, Smart DE, et al. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–98. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- 73.Canbay A, Taimr P, Torok N, et al. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–63. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 74.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–30. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thoen LF, Guimaraes EL, Grunsven LA. Autophagy: a new player in hepatic stellate cell activation. Autophagy. 2012;8:126–8. doi: 10.4161/auto.8.1.18105. [DOI] [PubMed] [Google Scholar]
- 77.Thoen LF, Guimaraes EL, Dolle L, et al. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55:1353–60. doi: 10.1016/j.jhep.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 78.Hernndez-Gea V, Friedman SL. Autophagy fuels tissue fibrogenesis. Autophagy. 2012;8:849–50. doi: 10.4161/auto.19947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramond MJ, Poynard T, Rueff B, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507–12. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 80.Spahr L, Rubbia-Brandt L, Pugin J, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol. 2001;35:582–9. doi: 10.1016/s0168-8278(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 81.Powell LW, Kerr JF. Reversal of “cirrhosis” in idiopathic haemochromatosis following long-term intensive venesection therapy. Australas Ann Med. 1970;19:54–7. doi: 10.1111/imj.1970.19.1.54. [DOI] [PubMed] [Google Scholar]
- 82.Blumberg RS, Chopra S, Ibrahim R, et al. Primary hepatocellular carcinoma in idiopathic hemochromatosis after reversal of cirrhosis. Gastroenterology. 1988;95:1399–402. doi: 10.1016/0016-5085(88)90379-4. [DOI] [PubMed] [Google Scholar]
- 83.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, et al. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–17. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 84.Rodriguez L, Cerbon-Ambriz J, Munoz ML. Effects of colchicine and colchiceine in a biochemical model of liver injury and fibrosis. Arch Med Res. 1998;29:109–16. [PubMed] [Google Scholar]
- 85.Kaplan MM, Alling DW, Zimmerman HJ, et al. A prospective trial of colchicine for primary biliary cirrhosis. N Engl J Med. 1986;315:1448–54. doi: 10.1056/NEJM198612043152304. [DOI] [PubMed] [Google Scholar]
- 86.Kershenobich D, Vargas F, Garcia-Tsao G, et al. Colchicine in the treatment of cirrhosis of the liver. N Engl J Med. 1988;318:1709–13. doi: 10.1056/NEJM198806303182602. [DOI] [PubMed] [Google Scholar]
- 87.Rambaldi A, Gluud C. Colchicine for alcoholic and non-alcoholic liver fibrosis or cirrhosis. Liver. 2001;21:129–36. doi: 10.1034/j.1600-0676.2001.021002129.x. [DOI] [PubMed] [Google Scholar]
- 88.Morgan TR, Weiss DG, Nemchausky B, et al. Colchicine treatment of alcoholic cirrhosis: a randomized, placebo-controlled clinical trial of patient survival. Gastroenterology. 2005;128:882–90. doi: 10.1053/j.gastro.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 89.Rockey DC, Maher JJ, Jarnagin WR, et al. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776–784. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- 90.Muir AJ, Sylvestre PB, Rockey DC. Interferon gamma-1b for the treatment of fibrosis in chronic hepatitis C infection. J Viral Hepat. 2006;13:322–8. doi: 10.1111/j.1365-2893.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 91.Rockey DC. Current and future anti-fibrotic therapies for chronic liver disease. Clin Liver Dis. 2008;12:939–62. xi. doi: 10.1016/j.cld.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–22. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 93.Hazra S, Xiong S, Wang J, et al. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J Biol Chem. 2004;279:11392–401. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- 94.Yang L, Chan CC, Kwon OS, et al. Regulation of peroxisome proliferator-activated receptor-gamma in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G902–11. doi: 10.1152/ajpgi.00124.2006. [DOI] [PubMed] [Google Scholar]
- 95.Shafiei MS, Shetty S, Scherer PE, et al. Adiponectin regulation of stellate cell activation via PPARgamma-dependent and -independent mechanisms. Am J Pathol. 2011;178:2690–9. doi: 10.1016/j.ajpath.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang L, Stimpson SA, Chen L, et al. Effectiveness of the PPARgamma agonist, GW570, in liver fibrosis. Inflamm Res. 2010;59:1061–71. doi: 10.1007/s00011-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 97.McHutchison J, Goodman Z, Patel K, et al. Farglitazar lacks antifibrotic activity in patients with chronic hepatitis C infection. Gastroenterology. 2010;138:1365–73. 1373e1–2. doi: 10.1053/j.gastro.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Lieber CS, Weiss DG, Groszmann R, et al. II. Veterans Affairs Cooperative Study of Polyenylphosphatidylcholine in Alcoholic Liver Disease. Alcohol Clin Exp Res. 2003;27:1765–72. doi: 10.1097/01.ALC.0000093743.03049.80. [DOI] [PubMed] [Google Scholar]
- 99.Boigk G, Stroedter L, Herbst H, et al. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643–9. doi: 10.1002/hep.510260316. [DOI] [PubMed] [Google Scholar]
- 100.Jia JD, Bauer M, Cho JJ, et al. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen alpha1(I) and TIMP-1. J Hepatol. 2001;35:392–8. doi: 10.1016/s0168-8278(01)00148-9. [DOI] [PubMed] [Google Scholar]
- 101.Lieber CS, Leo MA, Cao Q, et al. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37:336–9. doi: 10.1097/00004836-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 102.Ferenci P, Dragosics B, Dittrich H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–13. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 103.Pares A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial [see comments] J Hepatol. 1998;28:615–21. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 104.Nava-Ocampo AA, Suster S, Muriel P. Effect of colchiceine and ursodeoxycholic acid on hepatocyte and erythrocyte membranes and liver histology in experimentally induced carbon tetrachloride cirrhosis in rats. Eur J Clin Invest. 1997;27:77–84. doi: 10.1046/j.1365-2362.1997.910615.x. [DOI] [PubMed] [Google Scholar]
- 105.Poupon RE, Lindor KD, Pares A, et al. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39:12–6. doi: 10.1016/s0168-8278(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 106.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 107.Brown KE, Poulos JE, Li L, et al. Effect of vitamin E supplementation on hepatic fibrogenesis in chronic dietary iron overload. Am J Physiol. 1997;272:G116–23. doi: 10.1152/ajpgi.1997.272.1.G116. [DOI] [PubMed] [Google Scholar]
- 108.Houglum K, Venkataramani A, Lyche K, et al. A pilot study of the effects of d-alpha-tocopherol on hepatic stellate cell activation in chronic hepatitis C. Gastroenterology. 1997;113:1069–73. doi: 10.1053/gast.1997.v113.pm9322499. [DOI] [PubMed] [Google Scholar]
- 109.Mezey E, Potter J, Rennie-Tankersley L, et al. A randomized placebo controlled trial of vitamin E in alcoholic hepatitis. J Hepatol. 2004;40:40–6. doi: 10.1016/s0168-8278(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 110.Stewart S, Prince M, Bassendine M, et al. A trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. Journal of Hepatology. 2002;36 (S):16. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 111.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sakaida I, Tsuchiya M, Kawaguchi K, et al. Herbal medicine Inchin-ko-to (TJ-135) prevents liver fibrosis and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. J Hepatol. 2003;38:762–9. doi: 10.1016/s0168-8278(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 113.Shimizu I, Ma YR, Mizobuchi Y, et al. Effects of Sho-saiko-to, a Japanese herbal medicine, on hepatic fibrosis in rats [see comments] Hepatology. 1999;29:149–60. doi: 10.1002/hep.510290108. [DOI] [PubMed] [Google Scholar]
- 114.Zhang XL, Liu L, Jiang HQ. Salvia miltiorrhiza monomer IH764-3 induces hepatic stellate cell apoptosis via caspase-3 activation. World J Gastroenterol. 2002;8:515–9. doi: 10.3748/wjg.v8.i3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen ZX, Zhang SJ, Lao SX, et al. He Jie Tang in the treatment of chronic hepatitis B patients. World J Gastroenterol. 2005;11:6638–43. doi: 10.3748/wjg.v11.i42.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol. 2000;15 (Suppl):E67–70. doi: 10.1046/j.1440-1746.2000.02100.x. [DOI] [PubMed] [Google Scholar]
- 117.Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195–206. doi: 10.1055/s-2002-30104. [DOI] [PubMed] [Google Scholar]
- 118.Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–48. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 119.Spahr L, Rubbia-Brandt L, Frossard JL, et al. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37:448–55. doi: 10.1016/s0168-8278(02)00230-1. [DOI] [PubMed] [Google Scholar]
- 120.Tilg H, Jalan R, Kaser A, et al. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. 2003;38:419–25. doi: 10.1016/s0168-8278(02)00442-7. [DOI] [PubMed] [Google Scholar]
- 121.Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol. 2004;99:255–60. doi: 10.1111/j.1572-0241.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- 122.Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–7. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 123.Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–60. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hernandez E, Bucio L, Souza V, et al. Pentoxifylline downregulates alpha (I) collagen expression by the inhibition of Ikappabalpha degradation in liver stellate cells. Cell Biol Toxicol. 2008;24:303–14. doi: 10.1007/s10565-007-9039-5. [DOI] [PubMed] [Google Scholar]
- 125.Zhang D, Jiang H, Wang Y, et al. Pentoxifylline inhibits hepatic stellate cells proliferation via the Raf/ERK pathway. APMIS. 2012;120:572–81. doi: 10.1111/j.1600-0463.2011.02868.x. [DOI] [PubMed] [Google Scholar]
- 126.Sidhu SS, Goyal O, Singla M, et al. Pentoxifylline in severe alcoholic hepatitis: a prospective, randomised trial. J Assoc Physicians India. 2012;60:20–2. [PubMed] [Google Scholar]
- 127.Schaff Z, Lapis K, Szende B, et al. The effect of D-penicillamine on CCl4-induced experimental liver cirrhosis. Exp Pathol. 1991;43:111–20. doi: 10.1016/s0232-1513(11)80156-8. [DOI] [PubMed] [Google Scholar]
- 128.Lu SC, Tsukamoto H, Mato JM. Role of abnormal methionine metabolism in alcoholic liver injury. Alcohol. 2002;27:155–62. doi: 10.1016/s0741-8329(02)00226-4. [DOI] [PubMed] [Google Scholar]
- 129.Aithal GP, Haugk B, Das S, et al. Monitoring methotrexate-induced hepatic fibrosis in patients with psoriasis: are serial liver biopsies justified? Aliment Pharmacol Ther. 2004;19:391–9. doi: 10.1046/j.1365-2036.2004.01819.x. [DOI] [PubMed] [Google Scholar]
- 130.Anonymous. The results of a randomized double blind controlled trial evaluating malotilate in primary biliary cirrhosis. A European multicentre study group. J Hepatol. 1993;17:227–35. doi: 10.1016/s0168-8278(05)80043-1. [DOI] [PubMed] [Google Scholar]
- 131.Bodenheimer HC, Jr, Schaffner F, Sternlieb I, et al. A prospective clinical trial of D-penicillamine in the treatment of primary biliary cirrhosis. Hepatology. 1985;5:1139–42. doi: 10.1002/hep.1840050613. [DOI] [PubMed] [Google Scholar]
- 132.Dickson ER, Fleming TR, Wiesner RH, et al. Trial of penicillamine in advanced primary biliary cirrhosis. N Engl J Med. 1985;312:1011–5. doi: 10.1056/NEJM198504183121602. [DOI] [PubMed] [Google Scholar]
- 133.Kaplan MM, DeLellis RA, Wolfe HJ. Sustained biochemical and histologic remission of primary biliary cirrhosis in response to medical treatment. Ann Intern Med. 1997;126:682–8. doi: 10.7326/0003-4819-126-9-199705010-00002. [DOI] [PubMed] [Google Scholar]
- 134.Hendrickse MT, Rigney E, Giaffer MH, et al. Low-dose methotrexate is ineffective in primary biliary cirrhosis: long-term results of a placebo-controlled trial [see comments] Gastroenterology. 1999;117:400–7. doi: 10.1053/gast.1999.0029900400. [DOI] [PubMed] [Google Scholar]
- 135.Bach N, Bodian C, Bodenheimer H, et al. Methotrexate therapy for primary biliary cirrhosis. Am J Gastroenterol. 2003;98:187–93. doi: 10.1111/j.1572-0241.2003.07173.x. [DOI] [PubMed] [Google Scholar]
- 136.Kaplan MM, Cheng S, Price LL, et al. A randomized controlled trial of colchicine plus ursodiol versus methotrexate plus ursodiol in primary biliary cirrhosis: ten-year results. Hepatology. 2004;39:915–23. doi: 10.1002/hep.20103. [DOI] [PubMed] [Google Scholar]
- 137.Mato JM, Camara J, Fernandez de Paz J, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–9. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 138.Rambaldi A, Gluud C. Meta-analysis of propylthiouracil for alcoholic liver disease--a Cochrane Hepato-Biliary Group Review. Liver. 2001;21:398–404. doi: 10.1034/j.1600-0676.2001.210606.x. [DOI] [PubMed] [Google Scholar]
- 139.Novobrantseva TI, Akinc A, Borodovsky A, et al. Delivering silence: advancements in developing siRNA therapeutics. Curr Opin Drug Discov Devel. 2008;11:217–24. [PubMed] [Google Scholar]
- 140.Vaishnaw AK, Gollob J, Gamba-Vitalo C, et al. A status report on RNAi therapeutics. Silence. 2011;1:14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bisht S, Khan MA, Bekhit M, et al. A polymeric nanoparticle formulation of curcumin (NanoCurc) ameliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab Invest. 2011;91:1383–95. doi: 10.1038/labinvest.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Isaka Y, Brees DK, Ikegaya K, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nature Medicine. 1996;2:418–23. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 143.George J, Roulot D, Koteliansky VE, et al. In vivo inhibition of rat stellate cell activation by soluble TGF beta type II receptor: a potential new therapy for hepatic fibrosis. Proceedings of the National Academy of Sciences:USA. 1999;96:12719–12724. doi: 10.1073/pnas.96.22.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gonzalo T, Beljaars L, van de Bovenkamp M, et al. Local inhibition of liver fibrosis by specific delivery of a platelet-derived growth factor kinase inhibitor to hepatic stellate cells. J Pharmacol Exp Ther. 2007;321:856–65. doi: 10.1124/jpet.106.114496. [DOI] [PubMed] [Google Scholar]
- 145.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447–58. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gomez-Hurtado I, Santacruz A, Peiro G, et al. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4-induced fibrosis. PLoS One. 2011;6:e23037. doi: 10.1371/journal.pone.0023037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 149.Fouts DE, Torralba M, Nelson KE, et al. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–92. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jiang X, Tsitsiou E, Herrick SE, et al. MicroRNAs and the regulation of fibrosis. FEBS J. 277:2015–21. doi: 10.1111/j.1742-4658.2010.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sekiya Y, Ogawa T, Yoshizato K, et al. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412:74–9. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 152.Lakner AM, Steuerwald NM, Walling TL, et al. Inhibitory effects of microRNA 19b in hepatic stellate cell-mediated fibrogenesis. Hepatology. 2012;56:300–10. doi: 10.1002/hep.25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barry-Hamilton V, Spangler R, Marshall D, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 154.Lopez B, Gonzalez A, Hermida N, et al. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart Circ Physiol. 2010;299:H1–9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 155.Yamaguchi Y, Takihara T, Chambers RA, et al. A peptide derived from endostatin ameliorates organ fibrosis. Sci Transl Med. 2012;4:136ra71. doi: 10.1126/scitranslmed.3003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu W, Hellerbrand C, Kohler UA, et al. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab Invest. 2008;88:1068–78. doi: 10.1038/labinvest.2008.75. [DOI] [PubMed] [Google Scholar]
- 157.Kim IH, Kim DG, Hao P, et al. Anti-fibrotic effects of L-2-oxothiazolidine-4-carboxylic acid via modulation of nuclear factor erythroid 2-related factor 2 in rats. BMB Rep. 2012;45:348–53. doi: 10.5483/bmbrep.2012.45.6.276. [DOI] [PubMed] [Google Scholar]
- 158.Zhao XY, Zeng X, Li XM, et al. Pirfenidone inhibits carbon tetrachloride- and albumin complex-induced liver fibrosis in rodents by preventing activation of hepatic stellate cells. Clin Exp Pharmacol Physiol. 2009;36:963–8. doi: 10.1111/j.1440-1681.2009.05194.x. [DOI] [PubMed] [Google Scholar]
- 159.Titos E, Claria J, Planaguma A, et al. Inhibition of 5-lipoxygenase induces cell growth arrest and apoptosis in rat Kupffer cells: implications for liver fibrosis. Faseb J. 2003;17:1745–7. doi: 10.1096/fj.02-1157fje. [DOI] [PubMed] [Google Scholar]
- 160.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology. 2009;49:1017–44. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 161.Pilette C, Rousselet MC, Bedossa P, et al. Histopathological evaluation of liver fibrosis: quantitative image analysis vs semi-quantitative scores. Comparison with serum markers. J Hepatol. 1998;28:439–46. doi: 10.1016/s0168-8278(98)80318-8. [DOI] [PubMed] [Google Scholar]
- 162.O’Brien MJ, Keating NM, Elderiny S, et al. An assessment of digital image analysis to measure fibrosis in liver biopsy specimens of patients with chronic hepatitis C. Am J Clin Pathol. 2000;114:712–8. doi: 10.1309/D7AU-EYW7-4B6C-K08Y. [DOI] [PubMed] [Google Scholar]
- 163.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 164.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–8. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 165.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43:S113–20. doi: 10.1002/hep.21046. [DOI] [PubMed] [Google Scholar]
- 166.Colli A, Fraquelli M, Andreoletti M, et al. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89–94. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- 167.Imbert-Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–75. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 168.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 169.Callewaert N, Van Vlierberghe H, Van Hecke A, et al. Noninvasive diagnosis of liver cirrhosis using DNA sequencer-based total serum protein glycomics. Nat Med. 2004;10:429–34. doi: 10.1038/nm1006. [DOI] [PubMed] [Google Scholar]
- 170.Parkes J, Guha IN, Roderick P, et al. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol. 2006;44:462–74. doi: 10.1016/j.jhep.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 171.Mayo MJ, Parkes J, Adams-Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008;48:1549–57. doi: 10.1002/hep.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Parkes J, Roderick P, Harris S, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245–51. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 173.Li F, Song Z, Li Q, et al. Molecular imaging of hepatic stellate cell activity by visualization of hepatic integrin alphavbeta3 expression with SPECT in rat. Hepatology. 2011;54:1020–30. doi: 10.1002/hep.24467. [DOI] [PubMed] [Google Scholar]
- 174.Wang QB, Han Y, Jiang TT, et al. MR Imaging of activated hepatic stellate cells in liver injured by CCl4 of rats with integrin-targeted ultrasmall superparamagnetic iron oxide. Eur Radiol. 2011;21:1016–25. doi: 10.1007/s00330-010-1988-z. [DOI] [PubMed] [Google Scholar]
- 175.Hagens WI, Beljaars L, Mann DA, et al. Cellular targeting of the apoptosis-inducing compound gliotoxin to fibrotic rat livers. J Pharmacol Exp Ther. 2008;324:902–10. doi: 10.1124/jpet.107.132290. [DOI] [PubMed] [Google Scholar]
- 176.Park K, Hong SW, Hur W, et al. Target specific systemic delivery of TGF-beta siRNA/(PEI-SS)-g-HA complex for the treatment of liver cirrhosis. Biomaterials. 2011;32:4951–8. doi: 10.1016/j.biomaterials.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 177.Poelstra K, Schuppan D. Targeted therapy of liver fibrosis/cirrhosis and its complications. J Hepatol. 2011;55:726–8. doi: 10.1016/j.jhep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 178.Li Q, Yan Z, Li F, et al. The improving effects on hepatic fibrosis of interferon-gamma liposomes targeted to hepatic stellate cells. Nanotechnology. 2012;23:265101. doi: 10.1088/0957-4484/23/26/265101. [DOI] [PubMed] [Google Scholar]