Abstract
Neurons in mouse V1 increase their response to visual stimulation during locomotion. In this issue of Neuron, Lee et al. (2014) show that optogenetic stimulation of a locomotion area in the brainstem can mimic the effect of locomotion on sensory processing.
Sensory experience depends critically on behavioral state. However, the neural mechanisms that mediate state-dependent changes in perception are poorly understood. In previous work, Niell and Stryker established a mouse model for studying state-dependent changes in sensory responses(Niell and Stryker, 2010). They demonstrated that, for head-fixed mice on a spherical treadmill, locomotion and quiet wakefulness correspond to two distinct brain states in primary visual cortex (V1): during movement, power in the LFP shifts from low to high frequencies and visual responses are amplified by two-fold, similar to the physiological correlates of attention in primates (Harris and Thiele, 2011).
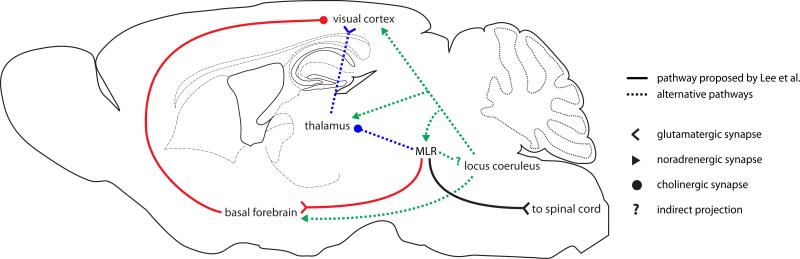
What circuits mediate the modulation of cortical processing during locomotion? Electrical stimulation of a region in the brainstem, the mesencephalic locomotor region (MLR), has been shown to elicit locomotion with short latencies in several species(Grillner, 2003). This functionally defined nucleus has gone byseveral names, including the parabrachial nucleus and the pedunculopontine nucleus, and includes both a descending motor component and an ascending modulatory component, which forms part of the reticular activating system(Grillner et al., 2008). Apart from its effects on locomotion, stimulation of this region has been shown to desynchronize the EEG and increase alertness(Moruzzi and Magoun, 1949). However, because electrical stimulation recruits local cells and axons indiscriminately, it was unclear whether the circuits promoting arousal and locomotion were distinct.In this issue of Neuron, Lee et al. (2014) show that optogenetic stimulation of glutamatergic cells in the MLR elicits locomotion. Interestingly, the authors further demonstrate that stimulation of these cells at frequencies that do not elicit locomotion is still sufficient to desynchronize the LFP in V1 and enhance visual responses.These data support an elegant model in which glutamatergic cells in the MLR promote both arousal and locomotion through ascending and descending projections, respectively (Figure 1, solid lines). It should be noted however, that whether the same MLR cells affect both visual responses and locomotion remains unclear.
Figure 1.
Possible anatomical pathways through which the MLR may influence cortical activity
The authors next attempted to identify what downstream circuits couple MLR activation to visual cortical modulation. Recent work has demonstrated that stimulation of cholinergic cells in the basal forebrain (BF)desynchronizes the LFP and enhances visual responses, suggesting that the basal forebrain may mediate the effects of MLR activation(Pinto et al., 2013). Interestingly, the authors find that stimulating axons from the MLR in the basal forebrain reproduces the effects of direct MLR stimulation on cortical activity and occludes the changes observed during spontaneous locomotion. Together, these data suggest an MLR→BF→V1 circuit that links locomotion to modulation of visual responses (Figure 1, solid lines).
Though this circuit is parsimonious, other subcortical circuits involving the MLR are not ruled out by their data. Importantly, whether release of ACh in V1 is necessary for the effects of MLR stimulation was not tested. Indeed, photostimulation in the BF may activate fibers of passage and axon collaterals to other brain nuclei. Moreover, the BF contains not only cholinergic, but also glutamatergic and GABAergic projection neurons (Henny and Jones, 2008), and the specific cell types activated by MLR axons were not identified. In the future it will be interesting to test whether cholinergic antagonists in the cortex block the effects of MLR stimulation.
The MLR is embedded in the reticular activating system, which contains several interconnected nuclei including the locus coeruleus (LC), the main source of noradrenaline in cortex. Release of noradrenaline during locomotion has been shown to depolarize pyramidal cells in V1 and likely contributes to modulation of visual responses (Polack et al., 2013). Moreover, both the MLR and the LC innervate the thalamus and may thus affect the propagation of sensory information to cortex. However, the contribution of these pathways to modulation of cortical activity during locomotion remains unclear (Figure 1, dotted lines).
Despite these caveats, several studies suggest that modulation of visual responses during locomotion may be mediated by release of ACh in V1.Fu et al.(Fu et al., 2014) recently demonstrated that nicotinic activation of vasointestinal peptide-expressing (VIP) interneurons during locomotion may facilitate pyramidal cell responses by selectively inhibiting somatostatin-expressing (SST) interneurons [but see (Polack et al., 2013)]. Indeed, activation of VIP cells appears necessary and sufficient for the visual response gain increase during locomotion. These results are consistent with two other findings:surround suppression, which has been shown to be mediated in part by SST interneurons (Adesnik et al., 2012), is reduced (Ayaz et al., 2013) during locomotion, and the E/I balance for visual responses is shifted toward excitation (Bennett et al., 2013).
Interestingly, the effects of locomotion on cortical activity vary across sensory cortices. In the visual cortex, the membrane potential of pyramidal neurons is depolarized during locomotion, and subthreshold visual responses are amplified due to larger visually-evoked conductances. However, in the auditory cortex, pyramidal cells are hyperpolarized, sensory responses are suppressed, and sensory-evoked conductances are reduced (Zhou et al., 2014). Lee et. al. (2014) provide a starting point from which we can begin to trace the circuits that link behavioral states and the modulation of sensory processing across cortical areas.
The authors demonstrate that subthreshold activation of glutamatergic cells in the MLR produces electrophysiological correlates of arousal in sensory cortex, raising several important questions. In the future, it will be interesting to determine whether the activity of these cells is ever uncoupled from locomotion during natural behavior and whether they play a necessary role in generating active cortical states. Moreover, investigating the local circuitry of the MLR will elucidate how other cell types, including cholinergic neurons projecting to the thalamus, interact with the glutamatergic cells stimulated in this study,and whether they also contribute to the effects of MLR stimulation. It will also be necessary to conclusively establish that the BF mediates the effects of subthreshold MLR stimulation on cortical processing, and, if so, what BF cell-types are activated by MLR afferents.It was previously demonstrated that both stimulation of cholinergic axons from the BF (Pinto et al., 2013) and locomotion (Bennett et al., 2013) enhance performance on visual tasks. Ultimately, it will be important to test how subthreshold stimulation of the MLR affects perception and behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz A, Saleem AB, Scholvinck ML, Carandini M. Locomotion controls spatial integration in mouse visual cortex. Current biology : CB. 2013;23:890–894. doi: 10.1016/j.cub.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nature reviews Neuroscience. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallen P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates--an overview. Brain research reviews. 2008;57:2–12. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Harris KD, Thiele A. Cortical state and attention. Nature reviews Neuroscience. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. The European journal of neuroscience. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalography and clinical neurophysiology. 1949;1:455–473. [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature neuroscience. 2013;16:1857–1863. doi: 10.1038/nn.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nature neuroscience. 2013;16:1331–1339. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Liang F, Xiong XR, Li L, Li H, Xiao Z, Tao HW, Zhang LI. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nature neuroscience. 2014;17:841–850. doi: 10.1038/nn.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]