Abstract
Background
Research on contingency management to treat excessive alcohol use is limited due to feasibility issues with monitoring adherence. This study examined the effectiveness of using transdermal alcohol monitoring as a continuous measure of alcohol use to implement financial contingencies to reduce heavy drinking.
Methods
Twenty-six male and female drinkers (from 21–39 years old) were recruited from the community. Participants were randomly assigned to one of two treatment sequences. Sequence 1 received 4 weeks of no financial contingency (i.e., $0) drinking followed by 4 weeks each of $25 and then $50 contingency management; Sequence 2 received 4 weeks of $25 contingency management followed by 4 weeks each of no contingency (i.e., $0) and then $50 contingency management. During the $25 and $50 contingency management conditions, participants were paid each week when the Secure Continuous Remote Alcohol Monitor (SCRAM-II™) identified no heavy drinking days.
Results
Participants in both contingency management conditions had fewer drinking episodes and reduced frequencies of heavy drinking compared to the $0 condition. Participants randomized to Sequence 2 (receiving $25 contingency before the $0 condition) exhibited less frequent drinking and less heavy drinking in the $0 condition compared to participants from Sequence 1.
Conclusions
Transdermal alcohol monitoring can be used to implement contingency management programs to reduce excessive alcohol consumption.
Keywords: Transdermal alcohol monitoring, Contingency management, Excessive alcohol use
1. INTRODUCTION
Contingency management provides financial incentives to clients to achieve targeted behaviors, such as moderation or elimination of substance use (Griffith et al., 2000; Higgins and Silverman, 2008; Lussier et al., 2006; Prendergast et al., 2006; Roll et al., 2013; Stitzer and Petry, 2006). Incentives typically depend on objective measures (e.g., blood or urine testing) to verify compliance, by measuring the presence of metabolites of drugs of abuse (e.g., marijuana, cocaine, opiates) that remain in the body for days after use (e.g., Budney et al., 2000; Higgins et al., 2000; Petry et al., 2005a).
In contrast to other drugs of abuse, biological markers for identifying alcohol use are not as straightforward for the use of financial contingencies. Biological markers for alcohol use are either direct (i.e., ethanol itself or analytes of ethanol metabolism) or indirect (i.e., toxic or nontoxic effects of alcohol). Direct biological markers of alcohol have short half-lives, so without excessive monitoring, verification of true abstinence is difficult. For example, breath alcohol concentration (BrAC) or blood alcohol concentration (BAC) is present only for a few hours, whereas urinary ethyl glucuronide and urinary ethyl sulfate are present for a few days (Maenhout et al., 2013; McDonell et al., 2011). While there is a more promising direct marker, phosphatidylethanol, that may better detect alcohol consumption over longer periods of time, its pharmacokinetics require more study before assessing its utility (Hahn et al., 2011; Helander et al., 2012). Indirect markers of alcohol use have longer half-lives, measured in weeks or months (e.g., liver enzymes such as γ-glutamyltransferase or carbohydrate-deficient transferrin), but they are not specific to alcohol use and may result in false positives (Helander et al., 2014; Maenhout et al., 2013; Marques et al., 2010; Marques, 2012). In short, implementation of biomarkers in contingency management procedures for alcohol use is difficult.
Nonetheless, several studies indicate that contingency management procedures may effectively reduce excessive alcohol use (e.g., Alessi et al., 2007; Alessi and Petry, 2013; Barnett et al., 2011; Hagedorn et al., 2013; Hunt and Azrin, 1973; Kaffarnus et al., 2011; McDonell et al., 2012; Miller, 1975; Miller et al., 1974a, 1974b; Petry et al., 2000, 2005b). However, to verify abstinence, most studies measured overt signs of intoxication, BAC, and/or BrAC at intervals ranging from daily to once a week. Because alcohol remains in the body only for several hours after the last use, BAC or BrAC readings ideally would be measured multiple times daily; even this may not ensure adherence to contingency management programs (Alessi and Petry, 2013). McDonell et al. (2012), verified abstinence by measuring urinary ethyl glucuronide twice weekly during a four-week contingency management procedure. However, urinary ethyl glucuronide is present only for up to two days (Maenhout et al., 2013; McDonell et al., 2011) and would need to be measured every other day to ensure adherence. With infrequent monitoring, a drinker can time alcohol consumption to prevent a positive screening; with frequent monitoring, procedures become burdensome and invasive.
Accurate transdermal alcohol monitoring devices create new opportunities for both research and treatment, including use in contingency management procedures. They detect alcohol excreted through the skin (Swift, 2003) and provide a continuous measure of transdermal alcohol concentration (TAC) over time (Swift, 2000, 2003). Recent methods for converting TAC data to more clinically meaningful outcomes (i.e., peak BrAC and number of standardized units of alcohol consumed; Dougherty et al., 2012, in review; Hill-Kapturczak et al., 2014) make their use even more compelling.
To our knowledge, only one study (Barnett et al., 2011) examined the feasibility of using transdermal alcohol monitoring devices in a contingency management procedure. This study included 13 heavy drinkers (men who consumed ≥15 drinks and women who consumed ≥8 drinks per week, including 2 or more heavy drinking episodes per week) who expressed interest in reducing or stopping drinking. Most had either a lifetime diagnosis of alcohol dependence or alcohol abuse. Participants wore a transdermal alcohol monitor for three weeks. In the first week, participants were told to drink as usual. During the subsequent two weeks, participants were told not to drink and received financial reinforcement (on an escalating scale) if their TAC reading did not exceed .02 g/dl. Average TAC readings (compared to baseline) were reduced by 72%, and 63% self-reported that they reduced drinking to below the national recommended weekly limit. Nonetheless, participants did not reduce the number of drinks they consumed when they did drink.
The present study sought to determine whether transdermal alcohol monitors could be used effectively to implement contingency management in non-treatment-seeking drinkers, with different drinking patterns, for a longer intervention period. Our goals were to: (1) reduce problematic patterns of drinking (not abstinence); (2) determine whether incentive magnitude affected drinking outcomes; and (3) determine any carryover effect of contingency management after the incentive was removed.
2. METHODS
2.1. Participants and criteria
We recruited 29 healthy participants from the community (n = 20 men and n = 9 women) aged 21–39 who reported patterns of drinking episodes that met National Institute on Alcohol Abuse and Alcoholism (2010) "at-risk" drinking criteria (daily limits of >3 drinks for women and >4 drinks for men) on 3 or more days within the prior 28 days. Individuals responded to newspaper, radio, and flyer advertisements. They underwent an initial phone screening about psychiatric/medical health and current drinking behavior to determine eligibility. Those who passed this initial prescreen were invited to the laboratory to complete a more extensive 3-hour screening. Exclusion criteria included an IQ less than 70, a current Axis I psychiatric disorder, pregnancy, current serious medical condition (e.g., diabetes, uncontrolled hypertension), history of substance dependence, and a positive urine drug test for the metabolites of drugs of abuse (cocaine, opiates, methamphetamines, barbiturates, benzodiazepines, or THC).
Additional screening included a detailed substance abuse history, review of alcohol consumption patterns during the prior 28 days using the Timeline Followback procedure (Sobell and Sobell, 1992), psychiatric screening using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders: Research Version, Non-Patient Edition (SCID-I/NP; First et al., 2001), intelligence screening using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), urine drug and pregnancy tests, and a medical history and physical examination by a physician or physician’s assistant. The Institutional Review Board at The University of Texas Health Science Center at San Antonio reviewed and approved the protocol.
2.2. Procedures
2.2.1. Study design
The study was divided into three 4-week experimental conditions: where $0 (no contingency; drinking as usual), $25, or $50 was provided when TAC readings did not exceed 0.03 g/dl on any day during the experimental week. Based on our earlier work (Dougherty et al. 2012; Hill-Kapturczak et al., 2014), this TAC level corresponded to light to moderate drinking (1–2 beers), but was generally exceeded with drinking 3 or more beers. Participants exceeded the criteria if three or more consecutive TAC readings achieved or exceeded 0.03 g/dl during a positive TAC event confirmed by Alcohol Monitoring Systems (AMS, Littleton, CO). Participants were randomly assigned to Sequence 1 – 4 weeks of $0 (no contingency) followed by 4 weeks of $25 contingency management, or Sequence 2 – 4 weeks of $25 contingency management followed by 4 weeks each of $0 (no contingency). During the $0 contingency conditions, participants received no directions regarding alcohol consumption. Conditions were counterbalanced to explore whether reductions in drinking during the $25 incentive condition persisted after the incentive was removed. After completing either sequence, the weekly incentive was increased to $50 for 4 weeks to determine whether increased payment resulted in further suppression of drinking. Weekly $25 or $50 incentive payments were delivered only when the TAC level criterion was not exceeded on any day that week. Weekly incentives were used to reduce burden on participants visiting the laboratory, and to parallel usual treatment. All participants received $10 per day for wearing the monitor and an additional $15 for each weekly clinic visit.
2.2.2. Transdermal alcohol monitoring
TAC was measured continuously using a tamper-resistant Secure Continuous Remote Alcohol Monitor (SCRAM-II™, Alcohol Monitoring Systems Inc., Highlands Ranch, CO). Each participant was fitted with a device and wore it for 12 weeks. The SCRAM-II measured TAC approximately every 30 minutes until removal of the device. Infrared signals and temperature were also recorded to ensure that no tampering or device disruption occurred. Data were retrieved weekly in our clinic using SCRAM Direct Connect™, which connects the transdermal alcohol monitor to a computer via a USB cable. Data were then uploaded to a web-based application for download and export.
2.2.3. Timeline Followback (TLFB; Sobell and Sobell, 1992)
Incentives were delivered based solely on TAC monitoring data to prevent bias. A TLFB assessment was completed only after the incentive was (or was not) delivered. The quantity of alcohol consumed each day during the 7 days before each laboratory visit was recorded. Following standard convention (National Institute on Alcohol Abuse and Alcoholism, 2010), heavy drinking on any given day was defined as ≥4 standard units for women and ≥5 for men. These data were used to determine the level of correspondence between the TAC monitoring criteria and participants’ self-reported alcohol use.
2.3. Data analysis
The characteristics of the participants were summarized using descriptive statistics. Differences between men and women and between the two treatment sequences were examined using t-tests or chi-squared tests for continuous and categorical variables, respectively.
The analysis for this 3-treatment 2-sequence crossover design utilized a simple first-order carryover effect model (Hedayat and Stufken, 2003), which is a special case of a mixed-effect model to account for all three phases of the contingency and the four weeks within a phase. The analytic model considered fixed effects such as the direct treatment effect (i.e., $0 vs. $25 vs. $50) while simultaneously examining the treatment sequence/group effect (i.e., whether participants were in the group that received $25 contingency first or second, an inter-subjects factor), period effect (i.e., 12 weeks over 3 different contingencies), and simple first-order carryover effect (i.e., the treatment effect from the previous period that does not interact with the direct treatment effect in the current period), along with random subject effects and random measurement errors. Analyses considering the period, sequence, direct treatment, and first-order carryover effects were conducted for the percent of participants exceeding criteria, proportion of days with any drinking, and the proportion of days with heavy drinking (i.e., using TAC data to estimate peak BrAC, see below). These analyses yielded significant findings only for the proportion of days with heavy drinking. Sensitivity analyses of this measure examined the difference between $25 contingency vs. $0 contingency using the first 4 weeks of data (i.e., before crossover) and from the weeks 5 to 8 (i.e., after crossover) separately. All further analyses were collapsed across randomized groups and examined the 4 weeks for each contingency without further consideration of order effects. For the continuous outcomes (e.g., proportion of days with heavy drinking per week), both repeated-measures ANOVA and mixed-effects models were used. For the binary outcome (i.e., whether or not exceeding contingency for each day of the week), mixed-effects logistic regression was used. All statistical tests were conducted at a 2-sided significance level of 0.05 and all analyses were performed using Stata/SE (Version 13, College Station, TX).
TAC-generated estimates of heavy drinking were determined by estimating peak BrAC (eBrAC) using TAC-measured parameters in an equation previously reported and validated (Hill-Kapturczak et al., in press). That equation is: eBrAC = 0.02158 + 0.3940 *peak TAC + 0.000149 * time-to-peak TAC − 0.00366 * sex – 0.1887 * peak TAC * sex. In this equation, sex was coded as Men = 1, Women = 0. To account for instances where TAC values were zero, eBrAC was also estimated as zero. Heavy drinking was defined as eBrAC ≥ 0.08%. TAC data included peak TAC (the highest TAC value recorded during a drinking episode), and time-to-peak TAC (the time in minutes from the last 0.000 g/dl TAC recording to the peak TAC recording in a drinking episode). To account for the fact that heavy drinking events generally occur in the evening hours, and TAC readings are delayed by 2–3 hours after drinking, we defined a day as noon-to-noon. Thus, if a drinking event started in the evening on one day and TAC levels were recorded after midnight, this drinking event is attributed to the day it started. Because the contingency was designed to reduce moderate to heavy drinking but permit low-level drinking, we used the TAC data to classify different levels of drinking. Four levels of drinking were created: a) no drinking (TAC = 0); b) low drinking (peak TAC > 0 but below the < 0.03 g/dl criterion used for the contingency); c) moderate drinking (peak TAC ≥ 0.03 g/dl but < 0.08%; and d) heavy drinking (eBrAC ≥ 0.08%).
3. RESULTS
Three participants withdrew before the end of the study (2 for reasons unknown, 1 due to incarceration). Characteristics of the final sample (N = 26) are shown in Table 1. Men and women did not differ in body mass index (BMI), level of education, number of drinks consumed per week, or how often they exceeded the National Institute on Alcohol Abuse and Alcoholism daily drinking guidelines (i.e., at-risk drinking) in the month before the study began. The number of at-risk drinking days during the 28 days prior to study entry ranged from 3 to 21 (M = 7.6) for women and 3 to 20 (M = 8.9) for men. Eleven (42%) participants were randomly assigned to treatment sequence 1 and 15 (58%) participants to treatment sequence 2. No significant differences were observed between treatment sequence groups (all p > .20).
Table 1.
Demographic Characteristics
| M | SD | |
|---|---|---|
| Age (years) | 28.5 | 5.2 |
| Education (years) | 12.1 | 3.0 |
| Body Mass Index | 28.4 | 4.2 |
| Height (inches) | 67.0 | 3.7 |
| Weight (pounds) | 180.9 | 30.2 |
| Drinks per Week | 20.4 | 12.0 |
| At-Risk Drinking Days† | 8.4 | 5.0 |
| Ethnicity* (AA/C/H/Other) | 1/3/13/9 | |
Note.
Number of days participants exceeded the NIAAA daily at-risk drinking guidelines during the prior 28 days (≥3 units for women or ≥4 units for men).
Ethnicity is represented as the frequency of individuals in each group: African-American (AA), Caucasian (C), Hispanic (H), or Other (O).
3.1. Participants exceeding the contingency criteria (across all weeks)
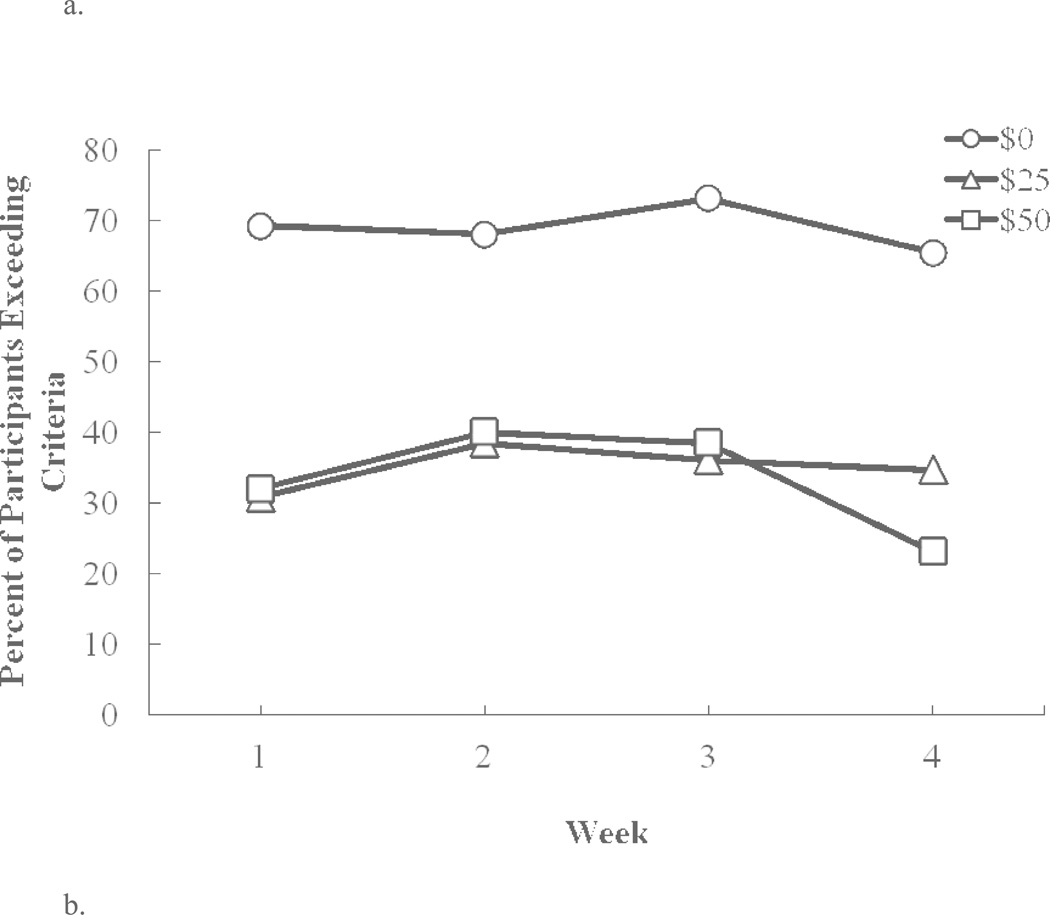
Figure 1a shows the percentage (%) of subjects who exceeded the threshold TAC criteria (i.e., at least three consecutive readings of ≥ 0.03 g/dl TAC) across each week of each contingency condition. Data were consolidated across different sequence conditions and a logistic regression model examining the binary outcome (i.e., exceed vs. not exceed) was used to analyze the fixed effects of group, contingency, and week and all possible interactions. Only the main effect of contingency was significant [χ2(2) = 36.96, p < 0.001]. Under the $0 contingency condition, about 70% of subjects exceeded the TAC contingency on any given week. However, compared with $0 contingency, $25 contingency decreased the odds of exceeding contingency criteria by 87% (OR = 0.13, 95% CI: 0.06 to 0.28), and $50 contingency decreased the odds of exceeding contingency criteria by 89% (OR = 0.11, 95% CI: 0.05 to 0.24). The difference between $25 contingency and $50 contingency was not significant (p = 0.679).
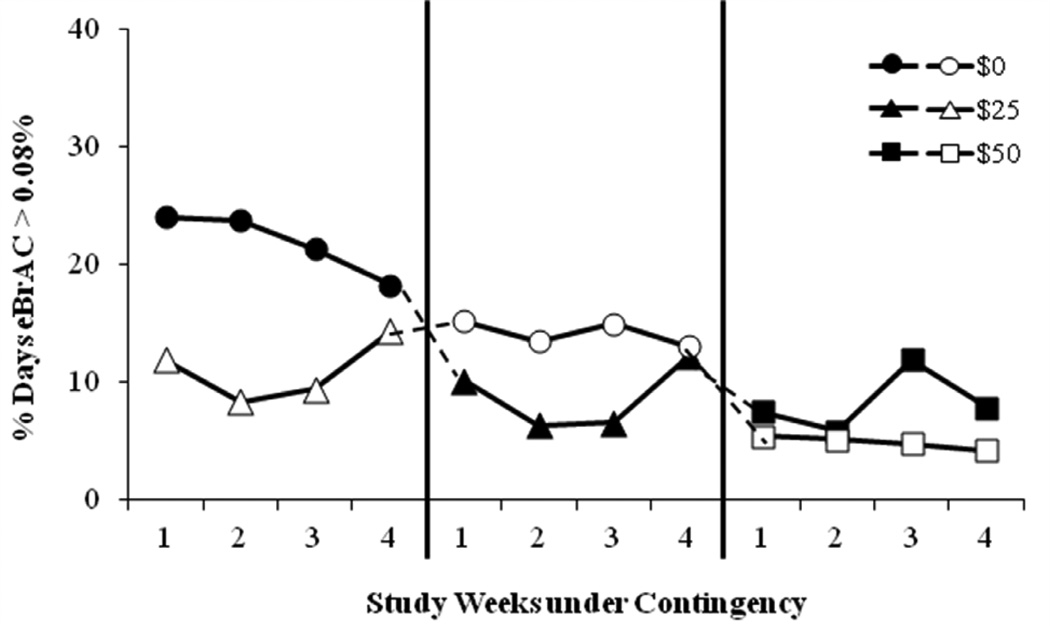
Fig. 3.
Sequence and carryover effect on percent days per week that participants had estimated peak BrAC (eBrAC) ≥ 0/08% BAC under: $0 (circles), $25 (triangles), and $50 (squares) contingency management conditions. Sequence 1 participants = closed symbols; Sequence 2 participants = open symbols.
3.2. Percent days of any drinking (across all weeks)
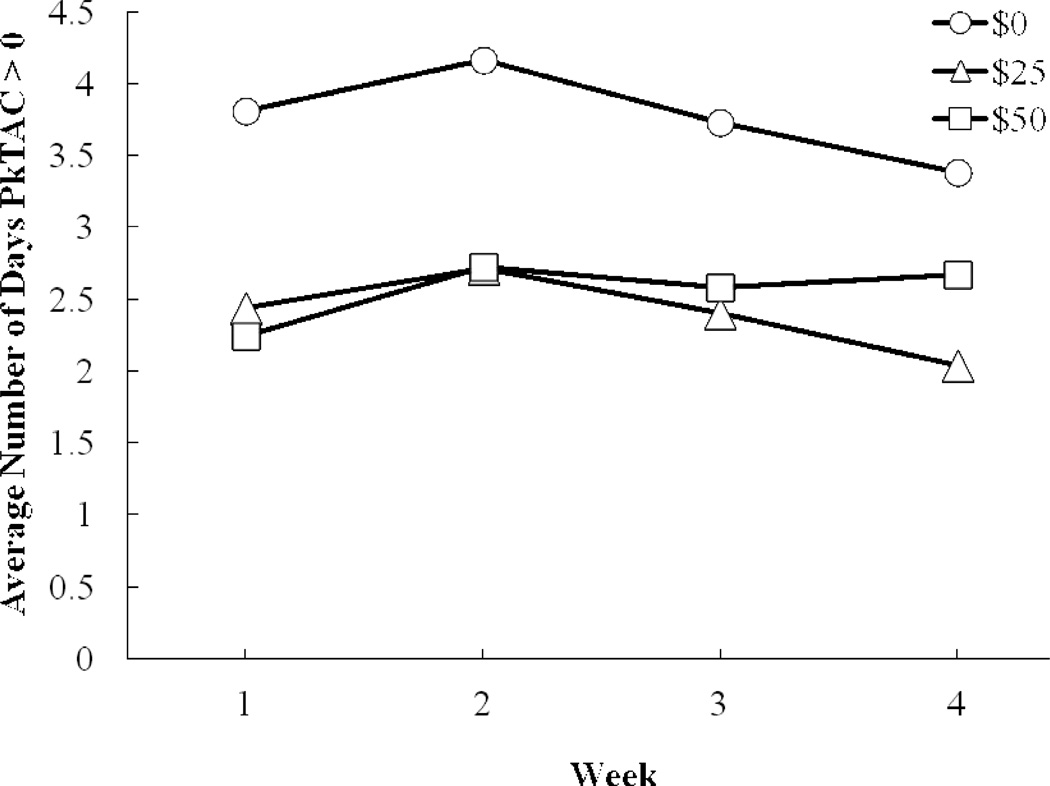
Because financial contingencies were set to permit low-level drinking, but discourage moderate and heavy drinking, a repeated-measures ANOVA examining the effects of group, contingency condition, and week was used to characterize the frequency of any drinking detected by any positive TAC readings (peak TAC > 0). Again, the main effect of contingency was highly significant [F(2, 48) = 16.61, p < 0.0001; Figure 1b], but no other main effect or interaction was significant. On average, compared with $0 contingency, $25 contingency decreased the percent days of drinking by 19.7% (95% CI: 12.4–27.0%); and $50 contingency decreased the percent days of drinking by 17.4% (95% CI: 10.1% to 24.7%). There was no significant difference between $25 and $50 contingency (p = 0.533).
Fig. 1.
(a) Percent of participants exceeding contingency criteria (TAC ≥ 0.03 g/dl) and (b) average number of days per week that participants had peak TAC (PkTAC) > 0, under: $0 (circles), $25 (triangles), and $50 (squares) contingency management conditions.
3.3. Self-reported drinking measured by TLFB
Self-report data (not shown) paralleled the TAC-based analyses. Main effects of contingency were significant both for proportion of days of heavy drinking [F(2, 29) = 8.7, p < 0.001] and for reports of any drinking [F(2, 47) = 16.3, p < 0.0001]. Under the different contingency conditions, the percentage of days of self-reported heavy drinking decreased from 28.4% on $0 contingency to 14.3% under $25 and 9.1% under the $50 contingencies. Likewise, the percentage of days of any drinking decreased from 46.2% to 33.6% and to 25.3% under the $0, $25, and $50 contingencies, respectively. Self-reported data verified the correct denial of contingent payments: on 87.1% of the weeks that TAC criteria were exceeded, participants also self-reported consuming three or more standard drinks on at least one day of that week.
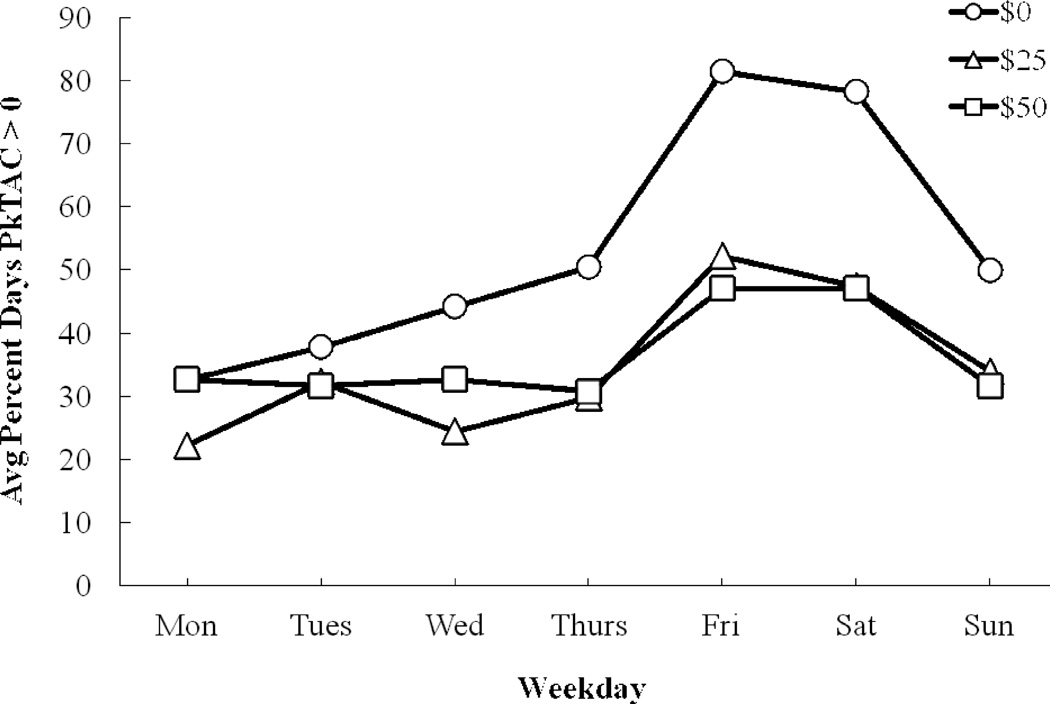
3.4. Percent of days with heavy drinking (by day of the week)
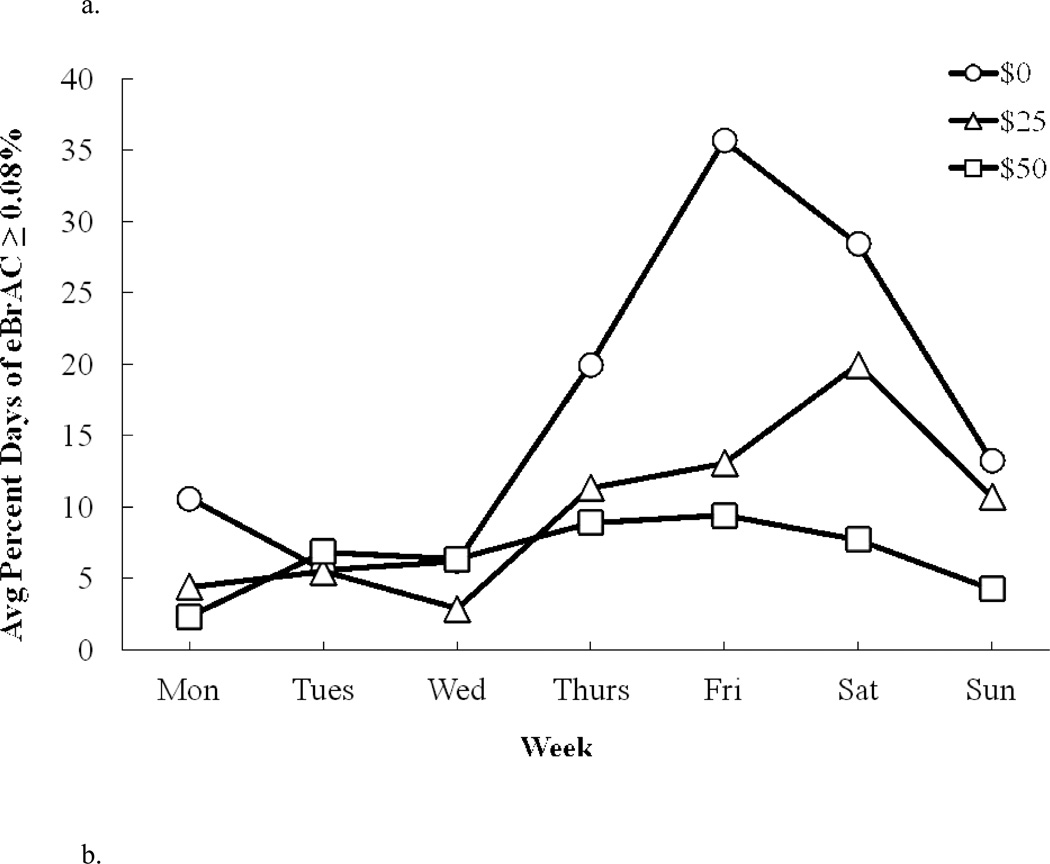
We examined the frequency of heavy drinking (i.e., eBrAC exceeding 0.08% BAC) as a function of days of the week (Figure 2a). The main effect of contingency management on the proportion of days of heavy drinking was highly significant [F(2, 50) = 14.23, p < 0.0001] as was the main effect of weekday [F(6, 150) = 11.98, p < 0.0001], and the interaction between contingency and weekday [F(12, 300) = 4.19, p < 0.0001]. Post-hoc contrasts showed that compared to the $0 condition, heavy drinking was significantly less frequent on Friday (p < 0.001) and Saturday (p = 0.01) in the $25 contingency condition, and less frequent on Thursday through Sunday in the $50 contingency condition (all p < 0.04). Furthermore, heavy drinking on Saturday was significantly (p = 0.007) less frequent in the $50 contingency condition than in the $25 condition.
Fig. 2.
Average percent days (by days of the week) that participants had (a) estimated peak BrAC (eBrAC) ≥ 0.08% BAC and (b) peak TAC (PkTAC) > 0, under: $0 (circles), $25 (triangles), and $50 (squares) contingency management conditions.
3.5. Any drinking (by day of the week)
The frequency of any drinking (i.e., peak TAC > 0) by day of the week was examined by repeated-measures ANOVA. The main effect of contingency was significant [F(2, 50) = 16.74, p < 0.0001] (Figure 2b), as was the weekday effect [F(6, 150) = 17.23, p < 0.0001] and the interaction between contingency and weekday [F(12, 300) = 2.15, p = 0.014]. Compared to the $0 condition, drinking was significantly less frequent on Wednesday through Sunday in the $25 condition (all p < 0.02), and significantly less frequent on Thursday through Sunday in the $50 contingency condition (all p < 0.007). However, there was no significant difference in drinking between the $25 and $50 contingency conditions on any day.
3.6. Sequence and carryover effects
To determine if there were carryover effects of contingency management when the contingency changed (i.e., Sequence 1: $0 to $25 to $50 and Sequence 2: $25 to $0 to $50) a 3-treatment, 2-sequence, 12-period crossover analysis was conducted. Only the percentage of days of heavy drinking was statistically significant, where there was a significant simple first-order carryover effect (R2 = 45.65%). There also was a significant main effect of contingency [F(2, 269) =9.34, p < 0.001]. The main effects of group and period were not significant. On average, compared with $0 contingency, the percent days of heaving drinking was decreased in the $25 contingency by 8.4% (95% CI: 3.5% to 13.3%), and in the $50 contingency by 15.3% (95% CI: 7.2% to 23.4%). The difference between $25 contingency and $50 contingency was marginal and not significant (p = 0.085). The $0 and $25 contingencies were different from one another before crossover (Phase 1), but not after crossover (Phase 2). Within Phase 1, the main effect of contingency was significant [F(1, 24) = 6.89, p = 0.015]; the $25 contingency decreased heavy drinking by 14.9% (95% CI: 3.8% to 25.9%) compared to the $0 contingency. However, within Phase 2, the contingency effect was not significant [F(1, 24) = 0.25, p = 0.62] and the $25 contingency decreased the % days of heavy drinking by only 2.7% (95% CI: −13.0% to 7.7%) compared with the $0 contingency.
3.7. Levels of drinking measured by TAC across contingency conditions
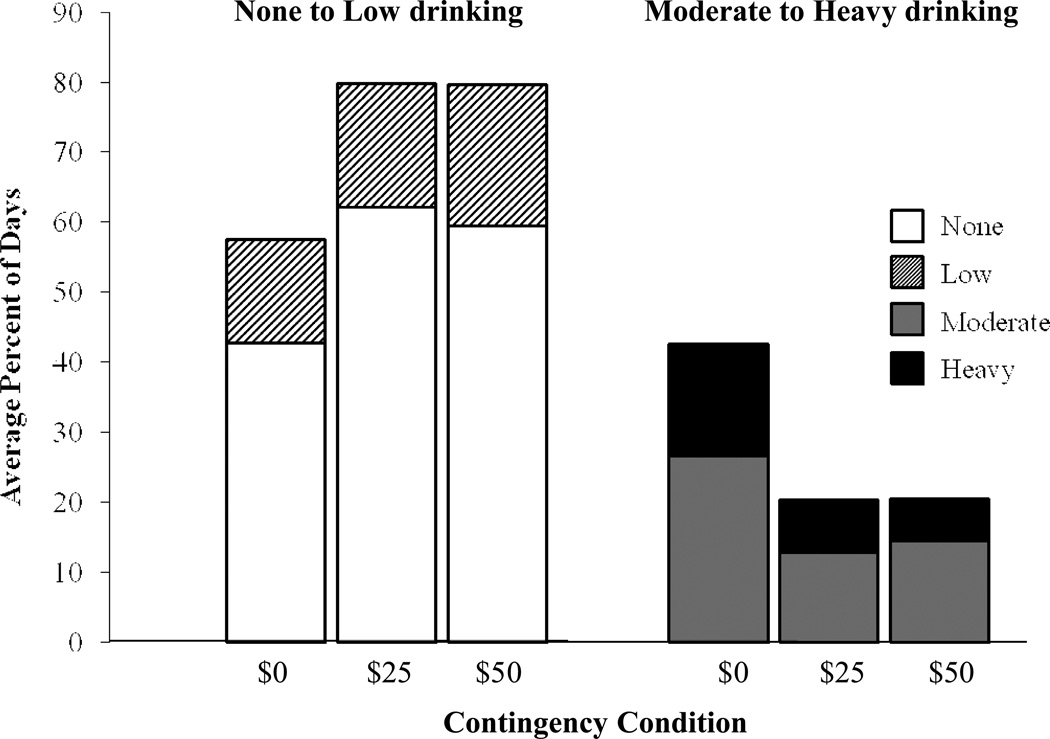
We found a significant [F(2, 50) = 13.2, p < 0.0001] main effect of contingency on the frequency of no and low drinking days, both below the 0.03 TAC criterion (Figure 4). Most of that effect was due to increases in the no drinking category for the $25 and $50 contingencies; there was no difference (p > 0.1) between the $25 and $50 contingency. Figure 4 also displays reciprocally-related reductions in the moderate and heavy drinking categories, both above the 0.03 TAC criterion.
Fig. 4.
Average percent days that participants were in high (eBrAC ≥ .08% BAC), moderate (TAC ≥ .03 g/dl and eBrAC < 08% BAC), low (TAC > 0 but < .03 g/dl), and no drinking level categories, under: $0, $25, and $50 contingency management conditions.
4. DISCUSSION
The results of this study indicate transdermal alcohol monitors can be used effectively to implement contingency management treatment to reduce heavy drinking patterns of alcohol consumption. The contingency management procedure reduced: (a) the number of days where drinking exceeded low-level drinking (TAC readings ≥ 0.03 g/dl) across 4 weeks; (b) the average number of days where any drinking occurred (TAC > 0), sustained across 4 weeks, with the greatest reductions in drinking on weekends; (c) the frequency of moderate to heavy drinking overall and heavy drinking (eBrAC ≥ 0.08%) on weekends. The procedure increased instances of no or low levels of drinking. The incentive ($25 versus $50) did not affect some outcomes (i.e., exceeding criteria, any drinking, heavy drinking), but the larger incentive was more effective in reducing heavy weekend drinking on Saturdays. Lastly, among participants who began in the $25 incentive condition, reductions in heavy drinking persisted when this incentive was removed (Figure 3b). Collectively, these data lend support for using transdermal alcohol monitoring in contingency management procedures aimed at harm reduction (i.e., achieving moderation in drinking as opposed to abstinence; Rosenberg and Davis, 2002).
Similar to Barnett and colleagues (2011), our results showed that contingencies may be used to reduce the frequency of problematic alcohol use. They reported that participants who were heavy drinkers did not reduce the number of drinks they consumed on days they drank. Although our participants had similar average drinking levels before contingency management (Table 1), we saw a shift from moderate and heavy drinking to no or low levels of drinking, despite using lower incentives than in the previous study ($25 or $50/week compared to a possible $77/week). Our participants were paid a fixed amount if they did not exceed the contingency criterion, whereas in the study by Barnett et al (2011), participants were paid on an escalating scale, with payment re-set when alcohol consumption was detected. Additionally, participants in our study were non-treatment seeking and reported wide variations in drinking patterns.
Our study demonstrated that participants who received the $25 contingency condition before the $0 contingency condition persisted in less frequent heavy drinking compared to those who experienced the $0 contingency condition first. These data suggest that behavior may have changed as a result of the contingency period, and thus benefits of the contingency management procedure may persist after the intervention.
Another study of the effects of contingency management on alcohol consumption (McDonell et al., 2012) found a significant reduction in alcohol use during the intervention, but even higher alcohol use post-intervention than pre-intervention. However, these participants were alcohol dependent, and the contingency criteria required abstinence rather than reduction of harmful drinking. Persistent effects of the contingency management procedure beyond the intervention have been reported in studies of cocaine-dependent outpatients (e.g., Higgins et al., 1995; 2000; 2006). Increasing the value of contingent incentives early in treatment appeared to be effective for increasing during-treatment and longer-term (i.e., after treatment) cocaine abstinence (Higgins et al., 2006).
Potential carryover effect was of particular interest to us because this study included non-treatment seeking problematic drinkers, an understudied population for whom the use of contingencies to modify a pattern of harmful behavior might be most meaningful. We also examined whether subjects who failed the criteria on one day of the week then persisted in heavy drinking on other days, but we found no detectable trend for this effect (data not reported).
The current study had strengths and limitations. Transdermal alcohol monitoring can be an effective intervention for reducing heavy drinking of alcohol. However, this device is primarily associated with the criminal justice system. Although no participants reported withdrawing from the study due to feeling stigmatized by the monitors, their use could less acceptable for those not receiving compensation. Future research should continue to assess tolerability of the device while systemically examining different contingency management variables (e.g., longer durations of contingency management, booster reinforcement, etc.). Longer follow-ups after contingency management also are needed to better understand possible enduring effects of contingency management. Finally, future research could examine the use of transdermal alcohol monitoring with other alcohol treatment approaches (e.g., medications, motivational techniques, and relapse prevention). For example, TAC data could be used to characterize individual drinking behavior and used as feedback to educate problem drinkers, by concretely demonstrating their alcohol consumption and motivating behavior change.
We randomly assigned non-treatment seekers to two contingency management sequences.
Those in contingency management had fewer drinking episodes than $0 contingency.
Both contingency conditions had reduced heavy drinking compared to $0 condition.
Acknowledgements
The authors appreciate the supportive functions performed for by our valued colleagues: Cameron Hunt and Krystal Shilling.
Role of Funding Source
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health [R01AA14988]. The research was also supported in part by the National Institute of Drug Abuse [T32DA031115] for postdoctoral training for Dr. Karns, Dr. Lake, and Dr. Mullen. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Dougherty also gratefully acknowledges support from a research endowment, the William and Marguerite Wurzbach Distinguished Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors significantly contributed to this manuscript and have read and approved the final manuscript.
Conflicts of Interest
None of the authors have conflicting interests concerning this manuscript.
REFERENCES
- Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: Delivering incentives partially in group therapy. Exp. Clin. Psychopharmacol. 2007;15:293–300. doi: 10.1037/1064-1297.15.3.293. [DOI] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction. 2013;108:900–909. doi: 10.1111/add.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R, Colby SM. Contingency management for alcohol use reduction: a pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011;118:391–399. doi: 10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J. Consult. Clin. Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Charles NE, Acheson A, John S, Furr RM, Hill-Kapturczak N. Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Exp. Clin. Psychopharmacol. 2012;20:373–381. doi: 10.1037/a0029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Walters CJ, Cates SE, Karns T, Roache JD. Predicting standard alcohol units consumed using transdermal alcohol concentration readings. Addict. Disord. Their Treat. Under review. 2014 [Google Scholar]
- First MB, Spitzer RL, Givvon M, Williams JBW. Biometrics Research. New York: New York State Psychiatric Institute; 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug. Alcohol Depend. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Hagedorn HJ, Noorbaloochi S, Simon AB, Bangerter A, Stitzer ML, Stetler CB, Kivlahan D. Rewarding early abstinence in Veterans Health Administration addiction clinics. J. Subst. Abuse Treat. 2013;45:109–117. doi: 10.1016/j.jsat.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Hahn JA, Woolf-King SE, Muyindike W. Adding fuel to the fire: alcohol's effect on the HIV epidemic in Sub-Saharan Africa. Curr. HIV/AIDS Rep. 2011;8:172–180. doi: 10.1007/s11904-011-0088-2. [DOI] [PubMed] [Google Scholar]
- Hedayat AS, Stufken J. Optimal and efficient crossover designs under different assumptions about the carryover effects. J. Biopharm. Stat. 2003;13:519–528. doi: 10.1081/BIP-120022771. [DOI] [PubMed] [Google Scholar]
- Helander A, Jaeken J, Matthijs G, Eggertsen G. Asymptomatic phosphomannose isomerase deficiency (MPI-CDG) initially mistaken for excessive alcohol consumption. Clin. Chim. Acta. 2014;431:15–18. doi: 10.1016/j.cca.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Helander A, Peter O, Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552–557. doi: 10.1093/alcalc/ags065. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Ogden D, Badger GJ. Outpatient behavioral treatment for cocaine dependence: one-year outcome. Exp. Clin. Psychopharmacol. 1995;3:205–212. [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2006;102:271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K. Contingency Management. Arlington, VA: American Psychiatric Publishing; 2008. [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DEH, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J. Consult. Clin. Psychol. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Roache JD, Liang YY, Karns TE, Cates SE, Dougherty DM. Accounting for sex-related differences in the estimation of breath alcohol levels using transdermal alcohol monitoring. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3644-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GM, Azrin NH. A community-reinforcement approach to alcoholism. Behav. Res. Ther. 1973;11:91–104. doi: 10.1016/0005-7967(73)90072-7. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Wong CJ, Diemer K, Needham M, Hampton J, Fingerhood M, Svikis DS, Bigelow GE, Silverman K. A randomized clinical trial of a therapeutic workplace for chronically unemployed, homeless, alcohol-dependent adults. Alcohol Alcohol. 2011;46:561–569. doi: 10.1093/alcalc/agr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Maenhout TM, De Buyzere ML, Delanghe JR. Non-oxidative ethanol metabolites as a measure of alcohol intake. Clin. Chim. Acta. 2013;415:322–329. doi: 10.1016/j.cca.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Marques P, Tippetts S, Allen J, Javors M, Alling C, Yegles M, Pragst F, Wurst F. Estimating driver risk using alcohol biomarkers, interlock blood alcohol concentration tests and psychometric assessments: Initial descriptives. Addiction. 2010;105:226–239. doi: 10.1111/j.1360-0443.2009.02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques PR. Levels and types of alcohol biomarkers in DUI and clinic samples for estimating workplace alcohol problems. Drug Test. Anal. 2012;4:76–82. doi: 10.1002/dta.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Howell H, McPherson S, Cameron JM, Srebnik D, Roll JM, Ries RK. Voucher-based reinforcement for alcohol abstinence using the ethyl-glucuronide alcohol biomarker. J. Appl. Behav. Anal. 2012;45:161–165. doi: 10.1901/jaba.2012.45-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell MG, Srebnik D, Angelo F, Sugar AM, Howell D, Rainey C, Roll J, Short R, Reis R. Evaluation of ethyl glucuronide immunoassay urinalysis in five alcohol-dependent outpatients. Am. J. Addict. 2011;20:482–484. doi: 10.1111/j.1521-0391.2011.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM. A behavioral intervention program for chronic public drunkenness offenders. Arch. Gen. Psychiatry. 1975;32:915–918. doi: 10.1001/archpsyc.1975.01760250107012. [DOI] [PubMed] [Google Scholar]
- Miller PM, Hersen M, Eisler RM. Relative effectiveness of instructions, agreements, and reinforcement in behavioral contracts with alcoholics. J. Abnorm. Psychol. 1974a;83:548–553. doi: 10.1037/h0037098. [DOI] [PubMed] [Google Scholar]
- Miller PM, Hersen M, Eisler RM, Watts JG. Contingent reinforcement of lowered blood/alcohol levels in an outpatient chronic alcoholic. Behav. Res. Ther. 1974b;12:261–263. doi: 10.1016/0005-7967(74)90125-9. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Rethinking Drinking. NIH Publication No. 13-3770. Washington, DC: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: contingency management treatment of substance abusers in community settings. J. Consult. Clin. Psychol. 2005a;73:1005–1014. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: contingency management for treatment of alcohol dependence. J. Consult. Clin. Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F., Jr Prize reinforcement contingency management for cocaine dependence: integration with group therapy in a methadone clinic. J. Consult. Clin. Psychol. 2005b;73:354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Roll JM, Chudzynski J, Cameron JM, Howell DN, McPherson S. Duration effects in contingency management treatment of methamphetamine disorders. Addict. Behav. 2013;38:2455–2462. doi: 10.1016/j.addbeh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H, Davis MA. Differences in the acceptability of non-abstinence goals by type of drug among American substance abuse clinicians. J. Subst. Abuse Treat. 2014;46:214–218. doi: 10.1016/j.jsat.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RX, editors. Measure Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu. Rev. Clin. Psychol. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- Swift R. Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcohol. Clin. Exp. Res. 2000;24:422–423. [PubMed] [Google Scholar]
- Swift R. Direct measurement of alcohol and its metabolites. Addiction. 2003;98:73–80. doi: 10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- Weschler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation, Harcourt Brace and Company; 1999. [Google Scholar]