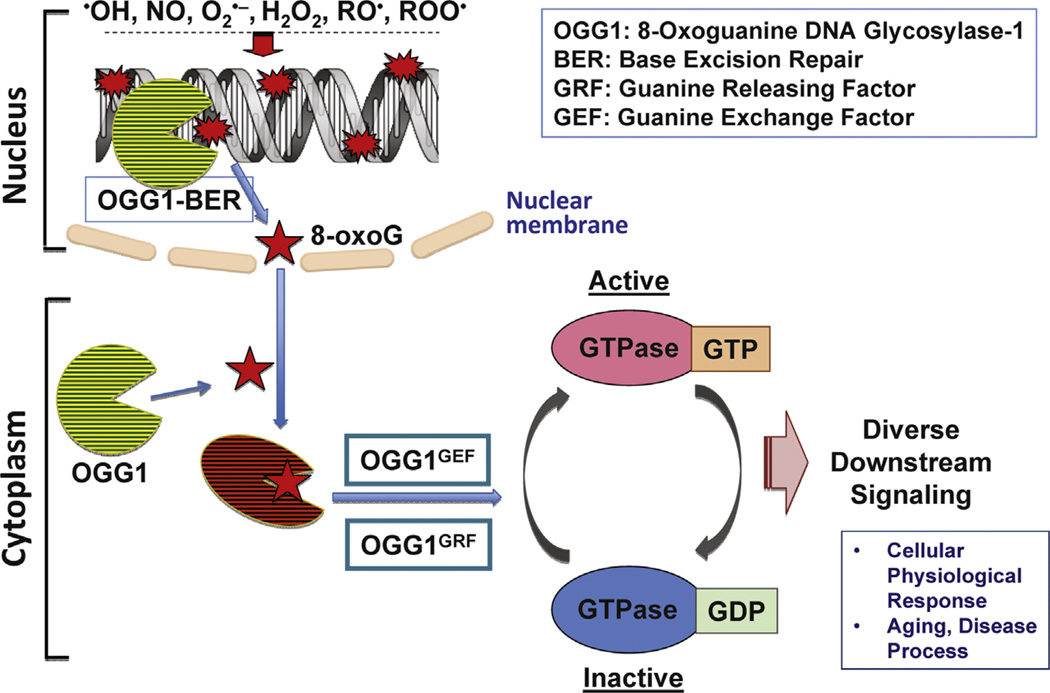
Base excision repair (BER) is the main mechanism for removing oxidized base damage from DNA, and the repair pathway is conserved from prokaryotes to mammals. Defective BER has been associated with multiple diseases and also contributes to aging, both presumably due to the accumulation of unrepaired base lesions. Now an unexpected link between 8-oxoguanine DNA glycosylase-1-initiated DNA BER (OGG1-BER) and cell signaling activation via the RAS homology small GTPase (RHO) has been established by a group of investigators led by Dr. Boldogh, which is published in this issue of Free Radical Biology & Medicine [1]. This paper is the latest in a series from the Boldogh laboratory linking OGG1-BER to increased levels of activated small GTPases, including canonical RAS family members and RAC1 [2–4].
The low reduction potential of guanine (G) among nucleic acid bases [5] makes it the predominant target for oxidation by reactive oxygen species (ROS), generating 7,8-dihydro-8-oxoguanine (8-oxoG). When unrepaired, 8-oxoG accumulates in the mammalian genome and has been linked to carcinogenesis, cellular senescence, aging, and aging-associated diseases [6]. The basis for 8-oxoG-mediated mutagenicity during carcinogenesis is well established, for example 8-oxoG adopts a syn conformation that can base pair with adenine (A), resulting in a G–C to T–A transversion. However, with numerous scientific reports implicating base damage accumulation in the pathobiology of various human diseases, it is still not clear whether base damage accumulation is the cause or a consequence of the disease process. To address this important issue, researchers have generated knockout mouse models lacking base-specific DNA glycosylases, including OGG1. Surprisingly, embryonic development of OGG1-null mice [7,8] is not impaired, the life span is unaltered, and there is only a modest predisposition to tumorigenesis despite supraphysiological levels of genomic 8-oxoG. More unexpectedly OGG1-null mice have increased resistance to inflammation [9,10]. These observations raised the possibility that it may not be the genomic level of 8-oxoG (or other oxidized guanines, such as FapyG) but the free 8-oxoG generated by BER that provides the linkage to disease/aging processes as previously proposed [11].
Although this theory is novel and attractive, the role of free 8-oxoG is not obvious. Observations indicated that OGG1 binds 8-oxoG base (OGG1–8-oxoG) with high affinity. Interestingly, the free FapyG base, as abundant as 8-oxoG in oxidatively damaged DNA, is an equally good OGG1 substrate but does not bind OGG1. Furthermore, OGG1 did not bind 8-oxodG, emphasizing the specificity of the OGG1 and 8-oxoG interaction [2]. The significance of these observations became apparent when evidence indicated that 8-oxoG induced a conformational change in OGG1 that allowed interaction with and activation of small GTPases. Specifically, in the presence of 8-oxoG, OGG1 caused displacement of K-, N-, and H-RAS-bound GDP with GTP. Interestingly, subsequent analysis showed that OGG1–8-oxoG also catalyzed the release of bound GTP, suggesting that it indiscriminately releases the nucleotide and allows rebinding [2], thus functioning as a guanine nucleotide releasing factor (GRF), similar to other RASactivating factors [12]. Increasing the cellular 8-oxoG level by adding it to cells or activating OGG1-BER in cellulo rapidly increased RAS–GTP levels, which induced phosphorylation of the MAPK kinase (MEK1/2) and extracellular signal-regulated kinase (ERK1/2) and the latter’s nuclear translocation [2,3].
The RHO family of small GTPases makes up a large portion of the RAS superfamily. The well-characterized family members are RHOA, RAC (RAC1 and its isoforms RAC2 and RAC3), and Cdc42. Because of high sequence homology among RAS and RHO family GTPases, Boldogh’s group examined whether the repair of oxidatively damaged DNA by OGG1, and subsequent formation of OGG1–8-oxoG complex, activates the RHO family member RAC1. Hajas et al. [4] reported that in addition to the canonical RAS family members, the OGG1–8-oxoG complex physically interacts with guanine nucleotide-free and GDP-bound RAC1. This interaction resulted in a rapid increase in levels of RAC1–GTP or GDP → GTP, but not GTP → GDP exchange, thus OGG1–8-oxoG functions as a prototypical guanine nucleotide exchange factor (GEF; OGG1GEF) [4]. Taken together, these results imply that the OGG1–8-oxoG complex functions as a GRF for canonical RAS family GTPases [2], whereas it has GEF activity for RAC1 [4].
In the study reported in this issue, Luo et al. provided insight into the biological consequences of OGG1-initiated repair of DNA by demonstrating that only OGG1-expressing cells displayed increased activation of RHO-GTPase owing to oxidative stress. These results are unexpected as small GTPases are redoxsensitive [1]. The effect of ROS on these GTPases is similar to that of GEFs, in that they modulate the guanine nucleotide binding of the GTPase, ensuring an increase in their GTP-bound forms [13]. Therefore, the rate-limiting role of OGG1 expression in RHO activation is extremely surprising and cannot be explained so far.
To obtain initial information on the role of OGG1 in RHO activation, Luo and colleagues first examined OGG1’s ability to bind RHOA in vitro. OGG1 physically interacts with RHOA protein, but does not require 8-oxoG, an unexpected observation that is distinct from that for 8-oxoG-dependent binding of OGG1 to RAS or RAC1 [2–4]. The authors then elegantly showed that interaction of OGG1 alone with RHO was not sufficient for GDP → GTP exchange; however, a rapid increase in RHOA-GTP levels was observed after the addition of 8-oxoG base to the in vitro reaction mixture. Moreover, their results demonstrating that exposing cells to the product of OGG-BER 8-oxoG base led to increased RHOAGTP levels, supporting the possibility that OGG1-BER is indeed coupled to RHO activation in oxidatively stressed cells.
The physiological functions of the RHO family GTPases are diverse, including modulation of cellular signaling, gene expression, cell–cell interactions, cell migration, reorganization of intracellular filaments, and regulation of the extracellular matrix. To test for the functional significance of RHOA activation by OGG1GEF, the assembly of contractile actomyosin stress fibers, a well-established consequence of RHOA activation, was investigated. Activation of RHOA resulted in polymerization of α smooth muscle actin (α-SMA) into stress fibers in both human and mouse fibroblasts, as documented by microscopic imaging and via molecular approaches. Moreover, ablation of OGG1 via siRNA significantly decreased not only RHOA activation but also α-SMA polymerization into stress fibers in oxidatively stressed or 8-oxoGchallenged cells. Importantly, challenging mouse lungs with the OGG1-BER product 8-oxoG resulted in RHOA activation and consequent α-SMA polymerization into the cytoskeleton.
The findings described in this and previous papers show an unexpected link between OGG1-intiated BER and cellular signaling initiated via the RAS and RAS homology family GTPases [2–4]. It is likely that second messengers such as 8-oxoG resulting from the OGG1-initiated repair of oxidatively damaged DNA, which occurs continuously, act synergistically with the oxidative stress-induced signaling. This paper thus documents a novel discovery—the role of OGG1 in physiological and pathophysiological cell signaling (Fig. 1). The authors speculate that decreased OGG1 activity during aging and in diseased tissues is tightly associated and could be a cellular defense against exacerbation of pathophysiological processes.
Fig. 1.
Proposed model of cellular signaling and pathophysiological processes resulting from OGG1-initiated repair of oxidatively damaged DNA.
References
- 1.Lou J, Hosoki K, Bacsi A, Radak Z, Hegde ML, Sur S, Hazra TK, Brasier AR, Ba X, Boldogh I. 8-Oxoguanine DNA glycosylase-1-mediated DNA repair is associated with Rho GTPase activation and alpha-smooth muscle actin polymerization. Free Radic Biol Med. doi: 10.1016/j.freeradbiomed.2014.03.030. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boldogh I, Hajas G, Aguilera-Aguirre L, Hegde ML, Radak Z, Bacsi A, Sur S, Hazra TK, Mitra S. Activation of ras signaling pathway by 8- oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. J. Biol. Chem. 2012;287:20769–20773. doi: 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.German P, Szaniszlo P, Hajas G, Radak Z, Bacsi A, Hazra TK, Hegde ML, Ba X, Boldogh I. Activation of cellular signaling by 8-oxoguanine DNA glycosylase-1-initiated DNA base excision repair. DNA Repair (Amsterdam) 2013;12:856–863. doi: 10.1016/j.dnarep.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajas G, Bacsi A, Aguilera-Aguirre L, Hegde ML, Tapas KH, Sur S, Radak Z, Ba X, Boldogh I. 8-Oxoguanine DNA glycosylase-1 links DNA repair to cellular signaling via the activation of the small GTPase Rac1. Free Radic. Biol. Med. 2013;61:384–394. doi: 10.1016/j.freeradbiomed.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolin Y, Cloutier JF, Shafirovich V, Geacintov NE, Dedon PC. Paradoxical hotspots for guanine oxidation by a chemical mediator of inflammation. Nat. Chem. Biol. 2006;2:365–366. doi: 10.1038/nchembio796. [DOI] [PubMed] [Google Scholar]
- 6.David SS, O'Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minowa O, Arai T, Hirano M, Monden Y, Nakai S, Fukuda M, Itoh M, Takano H, Hippou Y, Aburatani H, Masumura K, Nohmi T, Nishimura S, Noda T. Mmh/Ogg1 gene inactivation results in accumulation of 8-hydroxyguanine in mice. Proc. Natl. Acad. Sci. USA. 2000;97:4156–4161. doi: 10.1073/pnas.050404497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc. Natl. Acad. Sci. USA. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mabley JG, Pacher P, Deb A, Wallace R, Elder RH, Szabo C. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J. 2005;19:290–292. doi: 10.1096/fj.04-2278fje. [DOI] [PubMed] [Google Scholar]
- 10.Bacsi A, Aguilera-Aguirre L, Szczesny B, Radak Z, Hazra TK, Sur S, Ba X, Boldogh I. Down-regulation of 8-oxoguanine DNA glycosylase 1 expression in the airway epithelium ameliorates allergic lung inflammation. DNA Repair (Amsterdam) 2013;12:18–26. doi: 10.1016/j.dnarep.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radak Z, Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic. Biol. Med. 2010;49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosteller RD, Han J, Broek D. Identification of residues of the H-ras protein critical for functional interaction with guanine nucleotide exchange factors. Mol. Cell. Biol. 1994;14:1104–1112. doi: 10.1128/mcb.14.2.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo J. Redox control of GTPases: from molecular mechanisms to functional significance in health and disease. Antioxid. Redox Signaling. 2011;14:689–724. doi: 10.1089/ars.2009.2984. [DOI] [PubMed] [Google Scholar]