Abstract
Graft-versus-host disease (GVHD) represents the most serious and challenging complication of allogeneic haematopoietic stem-cell transplantation (HSCT). New insights on the role of regulatory T cells, T cells, and antigen presenting cells have led to improved understanding of the pathophysiology of GVHD. However, little progress has been made since the introduction of calcineurin-inhibitor-based regimens in the mid-1980s. Despite standard prophylaxis with these regimens, GVHD still develops in approximately 40–60% of recipients. Thus, there is a need for developing newer approaches to mitigate GVHD, which may facilitate the use of allogeneic HCT for the treatment of a wider range of haematological cancers. We will discuss the rationale, clinical evidence, and outcomes of current (and widely employed) strategies for GVHD prophylaxis, namely calcineurin-inhibitor-based regimen (such as cyclosporine or tacrolimus) combined with methotrexate or mycophenolate mofetil. We assess the clinical evidence for emerging approaches in the prevention of GVHD, including therapies targeting T cells or B cells, mesenchymal stem cells, the use of chemo-cytokine antagonists (such as maraviroc, TNF-α inhibitor, IL-2 receptor antagonist, IL-6 inhibitor), and the use of novel molecular regulators that target multiple cell types simultaneously (such as atorvastatin, bortezomib, and epigenetic modulators).
INTRODUCTION
Graft-versus-host disease (GVHD) is the major complication associated with allogeneic haematopoietic stem-cell transplantation (HSCT), which significantly impacts on non-relapse mortality.1 Based on the timeframe and type of organ involvement, GVHD can be characterized as acute or chronic.2 Prevention strategies have almost exclusively been directed at reducing acute GVHD, which is the most important risk factor for chronic GVHD.3 These strategies have evolved from the early use of single-agent methotrexate to combination calcineurin-inhibitor (CNI)-based. Currently, the most widely used regimens are based on CNI, although practices continue to vary between centres.4 Based on improved biological insights on the role of B cells, natural killer cells, regulator T cells, and antigen presenting cells, newer approaches, that target different cells of the immune system, such as T-cells and B-cells, are being tested to optimize treatment and overall duration of therapy. These new approaches showed promising results in terms of GVHD prevention in early clinical trials, however, they still need to be validated in randomized controlled trials (RCTs). It is also important to understand the impact of such approaches on relapse, infection, and late complications. In this Review, we critically assess standard therapies currently used in the prevention of GVHD and highlight novel and promising regimens on the basis of the results of several phase I and II clinical trials. Many of the therapies discussed here can also be used for curative treatment; however, the focus of this Review will primarily be in the prophylaxis setting.
Standard therapies
Calcineurin inhibitors
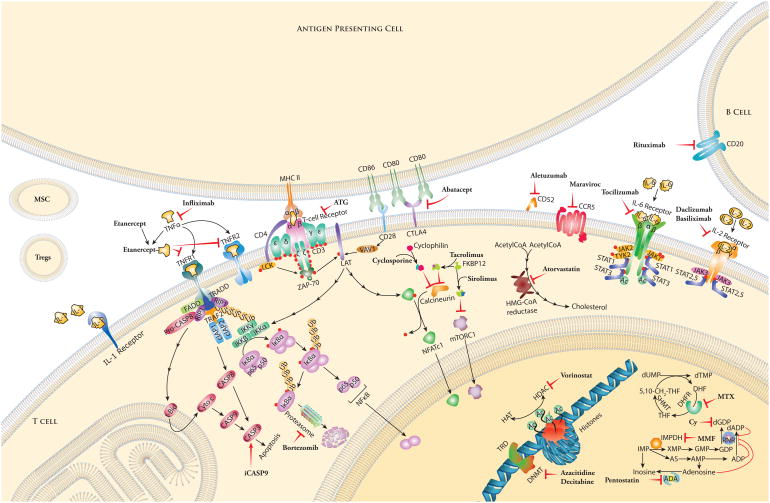
The introduction in the 1980s of two new immunosuppressive agents, cyclosporine and tacrolimus, which prevented T-cell activation by inhibiting calcineurin, has dramatically improved allograft survival rates. Furthermore, in 1986, the first studies reporting the superior outcomes of calcineurin inhibitor (CNI)-based regimens with notable reduction in GVHD and improved survival as a result of combination therapy (such as cyclosporine plus methotrexate) compared to either agent alone, were published.5 CNI-based therapies have, therefore, been considered the standard-of-care for GVHD prevention.4 Cyclosporine was originally isolated from fungi and was noted to have immunosuppressive effects. This observation led to its use in the prevention of allograft solid organ rejection and GVHD after allogeneic HCT.6 Although cyclosporine and tacrolimus are structurally distinct, their mechanisms of action are similar. Cyclosporine binds to the cytosolic protein Peptidyl prolyl cis-trans isomerase A (also known as cyclophilin), whereas tacrolimus binds to the Peptidyl-prolyl cis-trans isomerase FKBP12, and these complexes (cyclosporine–cyclophilin or tacrolimus–FKBP12) inhibit calcineurin, thereby blocking the dephosphorylation of nuclear factor of activated T cells (NFAT) and its nuclear translocation.7 These events prevent NFAT from exerting its transcriptional function, resulting in the inhibition of transcription of IL-2 and of other cytokines and ultimately leading to a reduced function of T-cells (Figure 1).7
Figure 1. Standard and emerging therapies for the prevention of acute graft-versus-host disease (GVHD).
Medications and their targets against B and T cells. Mesenchymal stem cell (MSC) and regulatory T cell (Treg) infusions are depicted extracellularly.
Acetyl CoA: Acetyl Coenzyme A; ATG: anti-thymocyte globulin; CLTA4: Cytotoxic T lymphocyte antigen 4; CCR5: C-C chemokine receptor 5; FKBP12: FK506 binding protein 12; HMG CoA reductase: 3-hydroxy-3-methyl-glutaryl Coenzyme A reductase; iCasp9: Inducible caspase 9; IκB: Nuclear factor of kappa light polypeptide gene enhance in B cells inhibitor; IL: Interleukin; MHC II: Major histocompatibility class II; mTORC: Mammalian target of rapamycin complex; NFATc: Nuclear factor of activated T cells, cytoplasmic; TNFR: Tumor necrosis factor receptor
Two multicentre, randomized, prospective trials conducted in the mid-1990s demonstrated decreased incidence of acute GVHD with the tacrolimus and methotrexate combination compared to cyclosporine and methotrexate, but overall survival was not significantly different.8, 9 These findings led some centres to favour the tacrolimus and methotrexate combination. Nonetheless, a recent survey estimated a much higher proportion of centres using cyclosporine over tacrolimus-based regimens.4 Given the practice variation in both dosing and duration, further studies are needed to compare the efficacy of the different schedules, to assess outcomes of GVHD and mortality between the two combination therapies. Such studies are unlikely, however, owing to the tremendous resources required and the perceived lack of novelty.
Methotrexate
Methotrexate is a cytotoxic drug, which at low doses, exerts its anti-inflammatory effect by attenuating T-cell activation.10 At higher doses, common adverse effects include hematopoietic, renal, hepatic, and gastrointestinal mucosal toxicity.5 Following preclinical studies that investigated its efficacy in GVHD prevention,11 it was used as monotherapy.12 However, the combination of cyclosporine and methotrexate has demonstrated superiority over single agent use,5 making the combination therapy the most commonly used GVHD prophylaxis regimen.4 The dosing schedule for a short-course of methotrexate on days 1, 3, 6 and 11 was developed as a consequence of concerns for gastrointestinal (GI) toxicity, and this schedule has remained largely unchanged over time (15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11). Nonetheless, a mini-dose of methotrexate given on days 1, 3, 6, and 11 at 5 mg/m2 is now commonly used since its introduction in patients with an unrelated donor for HSCT.10
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is an anti-metabolite and the prodrug of mycophenolic acid, which selectively inhibits inosine monophosphate dehydrogenase in T cells. The combination of MMF and any CNI has shown synergistic activity for GVHD prophylaxis.13 Although this regimen is used widely after nonmyeloablative transplants and cord blood transplants,14 it has never been formally tested in RCTs. MMF is not routinely administered after myeloablative transplants because its efficacy following transplant is not well-established. Phase I and II clinical trials, comparing the combination of cyclosporine and MMF to MMF alone, have reported less mucositis and faster neutrophil engraftment with the combination regimen, but without improvements in incidence of grade 2–4 acute GVHD.15
EMERGING STRATEGIES
T-cell depletion
T cells are absolutely essential for causing GVHD. Therefore, pharmacologic manipulation of T cells remains the mainstay of GVHD prophylaxis, even among the new strategies. T-cell depletion is the most effective means of prevention of GVHD. However, this effect can be offset by increased risks of delayed immune reconstitution, leading to life-threatening infection, graft failure, and disease recurrence.16 Nonetheless, reduction in T cell numbers by T-cell depletion of the donor graft has been attempted. The ex vivo depletion of T cells through physical separation techniques have now largely been replaced by in vivo depletion strategies, as detailed below.
Anti-thymocyte globulin
Anti-thymocyte globulins (ATG) are polyclonal immunoglobulins produced by immunizing rabbits (rabbit ATG or thymoglobulin) with the T-lymphoblastic Jurkat cell line (ATG-Fresenius, ATG-F), or immunizing horses (equine ATGAM) with human thymus lymphocytes.17 These cytotoxic antibodies, ATG or ATGAM, are directed against antigens expressed on human T lymphocytes (Figure 1), resulting in T-cell depletion through cell lysis.18
In some centres, the ATG approach has been included in routine prophylaxis for patients undergoing unrelated donor HCTs or receiving mismatched donor grafts, to prevent GVHD and, as part of the conditioning regimen, to decrease the risk of graft rejection. In an early randomized phase II study comparing patients who received methotrexate alone with those who received the methotrexate-ATGAM-prednisone combination, the incidence of acute GVHD was significantly lower in the combination arm (21% versus 48%, P = 0.001).12 Subsequent prospective studies using the ATG approach for T-cell depletion showed decreased incidence of GVHD and encouraging survival rates.19 The impact of the dose of ATG administered was assessed in two sequential phase III studies. In the first trial, patients with haematological malignancies, undergoing bone marrow transplants from unrelated donors, were randomly assigned to receive no ATG or a low dose (7.5 mg/kg/day) of ATG.20 In the second trial, the dose of ATG was doubled to 15 mg/kg/day.20 The administration of a high dose of rabbit ATG (15 mg/kg/day) was associated with reduced grade 3–4 acute GVHD compared with the no ATG arm. This reduction was, however, not observed in the low-dose trial. Nevertheless, the high-dose ATG trial was associated with an increased risk of lethal infections, resulting in comparable rates for survival and non-relapse mortality with the low-dose ATG trial.20
The administration of a different type of ATG, anti-Jurkat ATG-F, has also been studied in a randomized phase III trial comparing the standard GVHD prophylaxis with cyclosporin and methotrexate alone or in combination with ATG-F for patients undergoing a myeloablative conditioning HSCT from matched unrelated donors.15. The ATG-F-based regimen, compared with standard prophylaxis, decreased grade 2–4 acute GVHD (33% in the ATG-F arm versus 51% in the standard arm, P = 0.011) and chronic GVHD (30.9% versus 58.8%, P <0.0001). However, this did not result in a statistically significant survival outcome (59.2% versus 51.9%, P = 0.47). Furthermore, the ATG-F regimen was not associated with an increase in infectious disease or relapse mortality. However, patients treated with ATG-F exhibited a delayed neutrophil and platelet engraftment, and a higher incidence of EBV post-transplant lymphoproliferative disease.21 The specific role of ATG administration in allogeneic HSCT remains unclear. Of note, the two sequential randomized studies of ATG were performed when no molecular HLA typing was available and the source of transplant cells was either bone marrow or peripheral blood stem cells,20 whereas the ATG-F study could benefit from molecular HLA typing and the source of transplant cells was peripheral blood stem cells.21 The importance of the different source of transplant cells (bone marrow versus peripheral blood stem cells) with or without ATG has not been previously tested. Currently, a large phase III study of ATG-F is being conducted in adult acute myeloid leukemia, acute lymphoid leukemia, and myelodysplastic syndrome patients undergoing bone marrow or peripheral blood stem cell transplantation from unrelated donors. While the primary outcome measure is the first occurrence of moderate or severe chronic GVHD or death from any cause, acute GVHD will be assessed as ATG-F will be given three days prior to transplantation. It will be interesting to observe the outcomes in terms of GVHD prevention and adverse effects (ClinicalTrials.gov number NCT01295710).
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody directed against the CD52 receptor that depletes B and T lymphocytes by complement fixation and antibody-dependent cell-mediated cytotoxicity mechanisms.22 (Figure 1). Early phase II studies of treatment with alemtuzumab prior to allogeneic HSCT have shown decreased incidence and severity of GVHD, and reduced mortality.22 However, the perceived benefit was offset by the increased graft failure, disease recurrence, and delayed immune reconstitution.16 Due to prolonged lymphopenia observed in the post-HSCT setting, lower doses of alemtuzumab have been studied. Although no standard dose has been established when in combination with various conditioning regimens, doses lower than a fixed total dose of 20 mg have been associated with increased risk of severe GVHD and post-transplant lymphoproliferative disease.23 Although alemtuzumab is used widely in the nonmyeloablative setting on the basis of reports that suggested low incidence of acute GVHD (10–20%),24 it has never been formally tested in an RCT. Subsequent studies have explored the efficacy of alemtuzumab in HLA-mismatched unrelated HCTs.25 Although graft rejection was higher in mismatched compared to matched unrelated donor HCTs (8% versus 0%, P <0.01), the incidences of acute or chronic GVHD, and overall survival, were not significantly different, suggesting a role for alemtuzumab in high-risk mismatched HSCTs.25
Alemtuzumab has also recently been incorporated in the conditioning regimen for non-malignant diseases.26 No difference in the incidence of graft failure was observed, however, a decreased GVHD in aplastic anaemia and an improved long-term engraftment in sickle-cell disease was observed.27 Despite the extensive use of alemtuzumab, the optimal dose of this drug to achieve minimal immunosuppression and infection monitoring remains undefined.23
Suicide gene therapy
A safety switch system was initially developed as a promising cellular therapy in allogeneic HSCT by expressing thymidine kinase enzyme from the herpes simplex 1 virus (HS-tk).28 Ganciclovir was required as a prodrug for cell elimination, which functioned to inhibit HS-tk-expressing T cells if subsequent GVHD developed.28 A phase I–II multicentre non-randomized trial was conducted to investigate the infusion of HS-tk cells after haploidentical HSCT to prevent GVHD.29 In this trial, 50 patients received haploidentical stem-cell transplants for high-risk leukaemia, and only 10 patients developed grade 1–4 acute GVHD, demonstrating that the therapy was feasible and potentially efficacious.29 However, the concerns that HS-tk was a virus-derived agent, and potentially immunogeneic, led to the development of an alternative suicide gene therapy by engineering human T cells through the fusion of an inducible human caspase 9 gene (iCasp9) to the human FKBP12 (Figure 1).30 This iCasp9-suicide strategy was tested in a clinical study of five paediatric recipients, aged 3–17 years, who had haploidentical HSCT. The patients received infusions of genetically modified iCasp9-expressing T cells from haploidentical donors. Skin GVHD developed in four patients between 14 and 42 days after the infusion and concomitant liver GVHD occurred in one patient.31 Each of these four patients received a single infusion of the dimerizer drug AP1903, which induced the suicide of the activated-engineered T cells.31 GVHD resolved within 24 hours of the AP1903 infusion. This strategy is very promising; however, its efficacy on GVHD, infection, and relapse incidences will need to be established in larger, prospective RCTs.
CTLA4-Ig
CTLA4-Ig (also known as abatacept), is a soluble fusion protein of the Fc portion of human immunoglobulin G1 (IgG1) fused with CTLA4, and functions as a co-stimulation blocking agent that inhibits T cells (Figure 1).32 It is approved by the Food and Drug administration for use in rheumatoid arthritis in adults and in children older than 6 years.33 The experience with abatacept from three RCT indicates that is a safe agent.34–36 Abatacept was not associated with any hematologic, renal, cardiac, pulmonary, hepatic, or neurologic abnormalities. However, chronically-treated patients experienced increased risk of infections.37 Preclinical studies using CTLA4-Ig in murine and non-human primate models showed amelioration of GVHD.38 The feasibility of adding abatacept to cyclosporine and methotrexate therapy for GVHD prevention following unrelated donor HSCT has recently been investigated in a pilot trial involving 10 patients.39 Only two patients developed grade 2–4 acute GVHD, but seven patients showed cytomegalovirus (CMV) or Epstein-Barr virus (EBV) reactivation.27 Building on this experience, a phase II multicentre, randomized, double-blind RCT of abatacept combined with CNI and methotrexate versus placebo following unrelated donor HCT is currently underway (ClinicalTrials.gov NCT01743131).
Regulatory T cells
Regulatory T cells, TREGS, are characterized by the expression of the transcription factor forkhead box P3 (Foxp3).40 Several preclinical murine studies have shown that CD4+CD25+ TREGS are important regulators of self-tolerance and are critical in immune tolerance to alloantigen, decreased graft rejection, and decreased incidence and severity of GVHD (Figure 1).41, 42 TREGS have been shown to suppress the early expansion of alloreactive donor T cells and limit the capacity to induce GVHD without minimizing the graft-versus-leukaemia (GVL) effect.43 Given these preclinical data, human TREG infusions are being tested in clinical trials for GVHD prevention. The safety and efficacy of TREG infusions was evaluated in a phase I clinical trial. In this trial, TREGS were isolated from a partially HLA-matched umbilical cord unit and expanded ex vivo in culture before infusion.. The incidence of grade 2–4 acute GVHD in patients treated with TREG infusion was 43% compared with 61% in historical controls.44 A major challenge of the study was the fact that 25% of patients received less than the targeted TREGS dose.44 In another study, which evaluated the role of donor TREGS co-infused with conventional T cells in the HLA-haploidentical setting, 26 of the 28 enrolled patients achieved sustained donor engraftment, and lethal GVHD was minimized.33 No cases of chronic GVHD were reported at a median follow-up of 11.2 months. However, four cases of lethal infections (such as aspergillosis) were described.45 Despite the challenges with TREG purity and manufacturing at a large scale, these two trials showed feasibility. Hopefully, the development of new strategies to overcome these technical limitations will contribute to a greater use of TREG infusions in clinical trials and eventually in clinical practice. Another way to enhance TREG expansion might be through administration of low-dose IL-2, as has been studied in the context of chronic GVHD.46, 47 Daily IL-2 therapy for 8 weeks increased the proliferation of peripheral TREG and increased generation of thymic TREG, which correlated with clinical improvement in manifestations of chronic GVHD and reduction of glucocorticosteroid dose.46, 47
Molecular targets in T cells
Donor-derived T cell are the major effector cells mediating acute GVHD.1 Therapeutic strategies have therefore targeted cells involved in T cell activation in response to alloantigen signals.
Sirolimus
Sirolimus (also known as rapamycin) is a macrocyclic lactone produced by the actinomycete Streptomyces hygorscopicus and was originally developed as an anti-fungal agent.48 Similar in structure to tacrolimus, sirolimus binds to the intracellular protein FKBP12.49 However, unlike the tacrolimus–FKBP12 complex that inhibits calcineurin, the sirolimus–FKBP12 complex inhibits the mammalian target of the rapamycin (mTOR) pathway (Figure 1), which blocks IL-2 mediated signal transduction and prevents cell-cycle progression in naïve T cells.50 Preclinical studies investigating the use of sirolimus showed that it was effective in preventing GVHD-induced lethality, which led to its use in GVHD prophylaxis.51, 52
In a phase I–II trial, 41 patients received sirolimus combined with tacrolimus and methotrexate.53 This study showed feasibility and activity of sirolimus in lowering the incidence of grade 2–4 acute GVHD in patients conditioned with a myeloablative regimen followed by unrelated and mismatched donor grafts compared with historical controls.53 In an effort to omit methotrexate and thereby minimize potential complications, the sole combination of tacrolimus and sirolimus was tested in a related donor setting.54 In this phase II study, feasibility and encouraging incidences of grade 2–4 acute GVHD, neutrophil recovery, and overall survival at 1-year follow up were reported.54 These initial findings have been followed by other single-institution studies with mixed reports. For example, a prospective clinical trial that assessed sirolimus in combination with a CNI plus methotrexate in patients undergoing HSCT from an unrelated donor resulted in a high incidence of grade 2–4 acute GVHD.55 However, the results from a randomized trial conducted on 74 patients evaluating tacrolimus in combination with sirolimus or methotrexate demonstrated a significant reduction in grade 2–4 acute GVHD at day 100 post-HSCT in those patients who received the tacrolimus and sirolimus combination compared with the tacrolimus and methotrexate regimen (43% versus 89%, respectively, P <0.001).56 These findings were observed for patients with matched sibling donors and those with matched unrelated donors.56 Randomization was stratified for age (≥50 versus <50 years) and donor type (sibling versus unrelated). Overall survival did not differ significantly between the treatment arms.56 This result highlights the challenge of direct comparisons given the heterogeneity in the patient populations in relation to donor types (unrelated and related donor), conditioning regimens (such as myeloablative and reduced intensity conditioning, diseases treated (malignant and non-malignant), age of the recipients (≥18 years and <18 years), and timing and dose of sirolimus administered. The reduction in GVHD is sometimes offset by the occurrence of adverse complications (such as veno-occlusive disease, thrombotic microangiopathy or effusions), resulting in no significant difference in overall survival. In order to definitively establish the role of sirolimus for GVHD prevention, an open-label, multicentre, phase III RCT was conducted in patients undergoing HSCTs from a related donor.43 This study, sponsored by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN), completed its target accrual of 304 patients in October 2011 There was no difference in the incidence of grade 2–4 acute GVHD at day 114 post-HSCT between the tacrolimus- and sirolimus and tacrolimus and methotrexate groups (26% versus 34%, respectively, P = 0.17). There was a trend toward increased incidences of endothelial injury syndromes, including veno-occlusive disease (11% versus 5%, P = 0.06) and thrombotic microangiopathy (5% versus 1%, P = 0.09), as well as chronic GVHD (53% versus 45%, P = 0.06) for tacrolimus and sirolimus compared with tacrolimus and methotrexate. However, neutrophil engraftment was more rapid (14% versus 16%, P < 0.001) and mucositis was less severe (Oral Mucosistis Assessment Scale score 0.76 versus 0.96, P < 0.001). The primary end points of the trial, 114-day GVHD-free survival, and overall survival at 2-year follow up were not statistically different between groups.57 Nonetheless, tacrolimus and sirolimus could be considered in patients at high risk for oral mucositis or infectious complications and who may benefit from faster neutrophil engraftment.
Cyclophosphamide
Cyclophosphamide is a nitrogen mustard alkylating agent used in the treatment of various cancers and autoimmune conditions because of its potent immunosuppressive properties.58 Experimental studies have shown that the use of cyclophosphamide post-transplantation induced stable mixed chimerism (where both host and donor bone marrow-derived elements coexist in the recipient)59 across major histocompatibility complex (MHC) and mismatched bone marrow transplantation following non-myeloablative conditioning and abrogated GVHD.60 Two similar phase II studies that accrued 68 patients at two different institutions following non-myeloablative conditioning in haploidentical HSCT showed an incidence of grade 2–4 acute GVHD of 34% with post-transplantation treatment comprised of cyclophosphamide in combination with tacrolimus-MMF. The median time to neutrophil engraftment was 15 days and graft rejection occurred in 9 of the 66 patients (13%). The 1-year non-relapse mortality and relapse incidence rates were 15% and 55%, respectively.61 In an effort to eliminate the use of CNIs, a phase I–II study was conducted to determine the efficacy of post-transplantation high-dose cyclophosphamide alone (on days 3 and 4) for GVHD prevention after myeloablative conditioning and HLA-matched related or unrelated donor HSCT. The incidence of grade 2–4 and grade 3–4 acute GVHD were 43% and 10%, respectively.62 At 2-years, the incidence of chronic GVHD and non-relapse mortality were appreciably low at 10% and 17%, respectively. Relapse mortality and overall survival were 44% and 55%, respectively. The GVHD-protective mechanism obtained through post-transplantation treatment with cyclophosphamide might be a consequence of TREG preservation, achieved through the expression of aldehyde dehydrogenase.62
Similar results were observed in a study of 50 patients with high-risk haematological malignancies who underwent haploidentical HSCT followed by myeloablative conditioning and post-transplantation cyclophosphamide combined with cyclosporine-MMF. The incidence of grade 2–4 acute GVHD by day 100 was 12% and chronic GVHD was 10%. The 1-year non-relapse mortality and relapse incidence were 18% and 26%, respectively, which translated into an overall survival of 62% at 18 months.63 These findings are in contrast with a recent phase II study of single-agent post-transplantation cyclophosphamide used in the reduced intensity conditioning setting. A higher incidence of acute GVHD was observed compared with matched controls who received tacrolimus and methotrexate.64 Further studies are, therefore, warranted to more clearly define the optimal setting of post-transplant cyclophosphamide for GVHD prevention.
Pentostatin
Pentostatin was initially developed as an anti-cancer chemotherapeutic agent to treat hairy cell leukaemia.65 It inhibits adenosine deaminase and blocks the metabolism of 2′-deoxyadenosine. Thus, it induces lymphocyte apoptosis and impairs T-cell function, leading to prolonged immunosuppression.66 Pretreatment of mice with 2′ deoxycoformycin, in experimental BMT models, resulted in a reduction of GVHD.67 Recently, a phase I–II controlled study was performed where patients received pentostatin doses of 0, 0.5, 1, 1.5 and 2 mg/m2 on days 8, 15, 22, and 30 post-HSCT combined with tacrolimus and methotrexate for GVHD prevention.68 Patients with haematological malignancies undergoing matched unrelated, related or mismatched related donor HSCT were eligible. The incidence of grade 2–4 acute GVHD in pentostatin-treated patients was 43.9% compared with 55.6% in the control arm of tacrolimus and methotrexate.68 The lowest incidence of acute GVHD (35.7%) was observed in patients who received the 1.5 mg/m2 dose.68 There did not seem to be any significant difference in infectious complications between the different subgroups. An active multicentre trial is assessing the safety and efficacy of pentostatin combined with cyclosporine (Table 1).
Therapy targeting B cells
B cells have been implicated in the pathogenesis of GVHD.69 The effect of rituximab on chronic GVHD has been studied,70, 71 and is now being explored in the prevention of acute GVHD.
Rituximab
Rituximab is a chimeric monoclonal antibody targeted against CD20+ B lymphocytes (Figure 1).72 B-cell depletion by rituximab for GVHD prevention has been evaluated in several retrospective, single-institution analyses and through registry data. Patients with CD20+ non-Hodgkin lymphoma (NHL) who received rituximab pre-transplant as part of the conditioning regimen (n=13) or post-transplant as prophylaxis for disease control (n=4) were compared with patients who did not receive rituximab.73 In this brief report, patients who received both ATG and rituximab did not develop any GVHD compared with patients who received ATG alone, implicating a possible role for pre-transplant or peri-transplant rituximab treatment in GVHD prevention. In addition, patients with CD20+ malignancies who received rituximab within 3 months of HSCT experienced a reduction in grade 2–4 acute GVHD compared with those patients who were not previously treated with rituximab. This effect was more pronounced in a subgroup of patients who received ATG in combination with rituximab as part of the conditioning regimen.55 Furthermore, a large analysis of 435 patients with B-cell lymphomas—reported in the Center for International Blood and Marrow Transplant Research (CIBMTR) database—was conducted to assess the impact of rituximab on the incidence of acute GVHD.74 In this study, prior exposure to rituximab correlated with significantly decreased acute GVHD and improved survival. These findings are encouraging; however, additional prospective studies are warranted to better define the role of rituximub in GVHD prevention.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are a heterogeneous population of multipotent mesenchymal stromal cells with fibroblastic-like morphology that can differentiate into bone, cartilage, and fat cells.75 MSCs have been shown to possess non-specific immunosuppressive activities and immunomodulatory effects. Early feasibility studies showed safety with no evidence of adverse events associated with infusion of autologous human MSCs.76 A recent study assessing the role of ex vivo expanded, third party, MSC co-infused with transplantation in high-risk settings, such as mismatched unrelated donor HCT, suggests that MSCs may reduce life-threatening GVHD.77 However, in patients who received HLA-identical sibling matched HCT co-transplanted with MSCs for haematological malignancies, acute GVHD was lower, but the incidence of relapse was considerably higher.78 The results from these human HCT trials have shown that MSC infusions are well-tolerated, but long-term safety data have yet to be established along with the production of guidelines for how to culture and expand MSCs. More knowledge is needed about the biology and clinical role of MSCs in GVHD prevention.
Chemo-cytokine antagonists
Chemokines and their receptors are involved in both the innate and adaptive immune responses and play an important role in T-cell migration.79
Maraviroc
The C-C chemokine receptor type 5, also known as CCR5, is a receptor for chemokines on the surface of white blood cells. CCR5 was discovered in 1996 as a human immunodeficiency virus (HIV)-1 co-receptor and since then has been studied widely in allograft rejection, autoimmunity, tumour immune surveillance, and clearance of pathogens.79 In murine models of GVHD, CCR5 has been shown to mediate GVHD pathogenesis through its role in lymphocyte migration to target tissues (Figure 1).80, 81
Maraviroc is a CCR5-receptor antagonist, approved for use in the USA in combination with other antiretroviral agents for the treatment of patients with multidrug-resistant CCR5-tropic HIV-1.82 The role of maraviroc combined with tacrolimus and methotrexate for GVHD prophylaxis has been investigated.83 In total, 38 patients with high-risk haematological malignancies undergoing reduced intensity conditioning HSCT were enrolled in a phase I and II single-institution clinical trial.83 Cumulative incidences of grade 2–4 acute GVHD at day 100 and day 180 were 14.7% and 23.6%, respectively, and overall survival at 2-year post-treatment was 57%. Although the incidence of acute GVHD was low in maraviroc-treated patients, relapse at 1-year was 55.9%.65 Taken together, these results suggest that maraviroc might be efficacious in the reduction of GVHD. The role of this drug in the unrelated donor HCT setting is currently being explored (Table 1).
TNF-α inhibition
Studies from murine models as well as human transplants indicate TNF-α is an important molecule in the induction of experimental GVHD.84, 85 Previous reports have shown that patients with higher levels of TNF-α during the conditioning regimen had higher incidence of acute GVHD (90%) and mortality (>70%) than those patients with lower levels.86 Clinical studies investigating the role of TNF-α monoclonal antibodies in the prevention of GVHD reported a significant decrease in the release of TNF-α during the conditioning regimen, delayed onset of acute GVHD, and produced responses in target organs.87 Etanercept consists of two recombinant human TNF receptor (TNFR, p75) monomers fused to the Fc portion of human immunoglobulin G1 that binds to TNF-α and renders it inactive.88 In a phase II single-arm clinical trial conducted in patients undergoing myeloablative HSCT from an unrelated donor, the addition of etanercept to standard GVHD prophylaxis consisting of tacrolimus and methotrexate did not seem to affect the overall risk of grade 2–4 acute GVHD by day 100.89 Patients who received a non-radiation-based conditioning regimen experienced low TNFR1 ratios by day 7 and an encouraging 1-year survival (69%). However, these findings were not observed in those patients who received a radiation-based conditioning regimen, cautioning the use of etanercept in this context.89
Infliximab is a murine-human chimerized IgG1 monoclonal antibody that binds to TNF-α (Figure 1) and blocks the interaction with the TNF receptor. In a small prospective study of 19 patients undergoing myeloablative HSCT, the addition of infliximab to standard GVHD prophylaxis, consisting of cyclosporine and methotrexate, did not lower the risk of GVHD.90
Interleukin-2 receptor antagonist
Interleukin 2 (IL-2) is a cytokine signalling molecule that is essential for the proliferation and differentiation of T cells. Daclizumab is a humanized IgG1 monoclonal antibody and basiliximab is a human-mouse chimeric monoclonal antibody directed against the α-subunit of IL-2 receptor (IL-2R, or CD25) (Figure 1). The use of up-front daclizumab combined with steroids for the treatment of acute GVHD was found to be deleterious and the randomized trial was halted after a planned interim analysis showed inferior 100-day survival compared with the steroid plus placebo arm.91 However, a retrospective analysis evaluating the impact of basiliximab or daclizumab combined with standard immunoprophylaxis for prevention of GVHD in patients with haematological malignancies undergoing unrelated donor HSCT was reported.92 All patients were engrafted; the incidence of acute GVHD for the study population was 35% (grade 2–4) and 15.9% (grade 3–4). The researchers did not observe any significant difference in the incidence of acute GVHD between the use of basiliximab or daclizumab. However, chronic GVHD was significantly lower in basiliximab-treated patients compared with those patients who received daclizumab.92 CMV reactivation and bacterial infections were observed in 47.6% and 48.8% of patients, respectively. The deaths related to infections were 3.7%, which was favorable compared with 7% of patients reported with 7.5 mg/kg ATG treatment.20 The study was limited by its retrospective analysis. Further prospective evaluations should be performed.
A recent study was conducted to evaluate an intensive GVHD prophylaxis regimen that included basiliximab, ATG-F, cyclosporine, and methotrexate, as previously described,93 in patients with high-risk malignancies who received unmanipulated G-CSF primed bone marrow grafts from haploidentical family members.94 Sixty-four patients received a myeloablative conditioning regimen and 16 patients received a reduced-intensity conditioning regimen. The findings were encouraging with incidences of grade 2–4 acute GVHD at day 100, chronic GVHD at 2-year, and overall survival at 3-year of 24%, 6%, and 54%, respectively, and suggest that haploidentical family donors can be regarded as potential alternatives when transplants are urgently needed.94 Based on early preclinical allodepletion studies,95 the feasibility of depleting alloreactive T cells responsible for GVHD in an ex vivo approach with a CD25 specific immunotoxin, RFT5-dgA, has also been evaluated.96 These allodepleted T cells were infused back into 15 paediatric patients, 15 and 47 days after haploidentical and unrelated donor HSCT.96 The criteria for infusion included engraftment of donor cells and absence of GVHD. The study showed safety and efficacy. No cases of severe GVHD were reported and in three patients who had either CMV, EBV, or both at the time of allodepleted T cell infusion, the infections subsequently cleared, suggesting a role for these cells in immune reconstitution.96 This concept was also shown to be feasible in older patients with a median age of 65 years (range, 51–73 years) undergoing related donor HCT.97 In order to eliminate alloactivated donor T cells that potentially increase the risk of severe acute GVHD in the haploidentical setting another study used an anti-CD25 immunotoxin and studied two different doses of allodepleted T cells that were added back, both of which were associated with a low incidence of GVHD and improved T-cell recovery. In fact, there seemed to be accelerated recovery of viral-specific immunity in patients treated with the higher dose level.98 These studies collectively showed the feasibility of adding back allodepleted donor T cells without causing severe, life-threatening GVHD. However, in these conditions, relapse remained a major concern that will need to be addressed.
Interleukin-6 inhibition
Interleukin (IL)-6 plays an essential role in inflammation and immune regulation and has been implicated in a variety of immune-mediated inflammatory diseases.99 IL-6 signals through the IL-6R and the signal transducing component gp130 (CD130). In murine models of BMT, IL-6 and IL-6R levels are increased during GVHD, and IL-6 blockade reduces GVHD severity.100, 101 Building on these preclinical observations, early blockade of IL-6 (Figure 1) after allogeneic HCT is currently being tested in a clinical trial of GVHD prevention.102 Interim results of the phase I–II study showed that in 36 evaluable patients who received the human neutralizing monoclonal antibody against IL-6R on day 1 of myeloablative HSCT, the incidence of grade 2–4 acute GVHD was 11.1%.102 These early findings are encouraging and further evaluations are underway, including a planned multicentre study.
Novel molecular regulators
Several novel molecules have been shown to regulate the responses of donor T-cell and also of other immune cell subsets, such as antigen presenting cells (APCs) and TREGS transcriptional and translational regulation, including the use of storvastatin, bortezomib, and epigenetic modulators (such as histone deacetylase [HDAC] inhibitors and hypomethylating agents).
Atorvastatin
The inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, statins, have been shown to possess immunomodulatory and anti-inflammatory properties by inducing T-cell hyporesponsiveness, TREG expansion, T helper cells Type 2 polarization, and down-regulating APC function.103, 104 In a preclinical model of MHC-mismatched allogeneic BMT, acute GVHD-related mortality was significantly reduced when donor or recipient mice were treated with the inhibitor atorvastatin.103 A small retrospective analysis of human recipients who received statins at the time of HSCT has also shown decreased risk of grade 2–4 acute GVHD.105 By contrast, a larger study where recipients were treated with any statin agent at the time of HSCT showed no effect on the incidence of acute GVHD or overall survival, but reported a decreased incidence of chronic GVHD and increased recurrence of malignancy.106 Interestingly, any statin treatment in donors alone or both donor and recipients was associated with significantly reduced grade 3–4 acute GVHD. This effect was limited to recipients treated with cyclosporine-based GVHD prophylaxis.107 Given these data, atorvastatin treatment is currently being explored prospectively in several single-institutional studies (Table 1).
Bortezomib
Bortezomib, a dipeptide boronic acid, is the first proteasome inhibitor approved in the USA for treating multiple myeloma and mantle-cell lymphoma. Preclinical studies have shown that bortezomib blocks NF-kappa β activation (Figure 1) and augments the apoptotic response to chemotherapy.108 Accordingly, NF-kappa β blockade has been shown to block T-cell activation, proliferation, and survival within alloreactive T cells and abrogate GVHD.109–111 Bortezomib can control GVHD in clinical studies.112 However, its delayed administration has resulted in increased GI-related GVHD mortality, implying that timing might be important.113 Recently, a phase I–II prospective trial of bortezomib combined with tacrolimus-methotrexate for GVHD prophylaxis was conducted in patients with haematological malignancies undergoing reduced-intensity conditioning HLA-mismatched unrelated donor HSCT.114 Bortezomib-treated patients experienced encouraging outcomes with cumulative incidence of grade 2–4 at day 180, acute GVHD of 22%, 1-year chronic GVHD of 29%, and 2-year non-relapse mortality, relapse, and overall survival rates of 11%, 38%, and 64%, respectively.114 These favourable results, particularly in the setting of high-risk HCT with HLA-mismatched donor grafts, suggest that bortezomib might have a role in GVHD prevention. Bortezomib combined with either tacrolimus-methotrexate or tacrolimus-sirolimus is currently being compared with tacrolimus-methotrexate (Table 1).
Epigenetic modulators
Histone deacetylase inhibition
Histone acetylation regulates transcriptional activation. Lysine residues at the amino-terminus of histone H3 and H4 tails are acetylated by histone acetyltransferase enzymes (HATs) or deacetylated by HDACs (Figure 2), leading to modifications in DNA accessibility. HDAC inhibition results in the accumulation of hyperacetylated histones, thereby altering the patterns of gene expression (Figure 2). In recent years, HDAC inhibitors have gained wide attention in cancer therapy.115 Emerging data have shown that HDAC inhibitors at lower and noncytotoxic concentrations possess anti-inflammatory and immunoregulatory effects.116
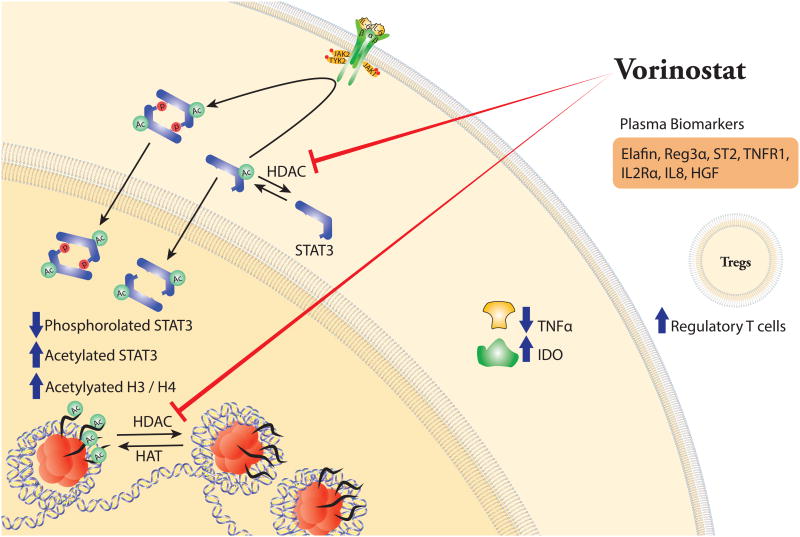
Figure 2. Effects of histone deacetylase (HDAC) inhibition in the prevention of acute graft-versus-host disease (GVHD).
Vorinostat significantly increases acetylation of histones (H3/H4) and signal transducer and activator of transcription 3 (STAT3) by inhibiting HDACs. Vorinostat reduces phosphorylated STAT3 and levels of tumor necrosis factor alpha (TNFα), but enhances indoleamine-2,3-dioxygenase (IDO) mRNA expression and regulatory T cells (Treg).
Studies in murine models of BMT have shown that HDAC inhibitors suppress pro-inflammatory cytokine production, reduce GVHD, and preserve GVL by modulating indoleamine-2,3-dioxygenase dependent innate immune and allo-stimulating functions of APCs in a STAT-3-dependent manner.117, 118 HDAC inhibitors also enhance natural TREG functions (Figure 2).119 On this basis, Choi et al.100 investigated the use of the HDAC inhibitor vorinostat, along with tacrolimus-MMF in a phase I–II first-in-human clinical trial of reduced-intensity conditioning HSCT. This prospective, multicentre, single-arm study recruited adult patients, aged ≥18 years, with high-risk haematological malignancies and an available 8/8 or 7/8-HLA matched related donor. The primary end point of the trial was the cumulative incidence of grade 2–4 acute GVHD by day 100 post-HSCT. The 22% incidence was lower than the pre-specified limit (25%) and that reported in the literature (38–60%).120, 121 The relapse incidence and overall survival rates at 2 years were 16% and 73%, respectively.122 Although limited by the single-arm design, these findings are encouraging and further studies in larger RCTs are warranted. Currently, there is an active study of vorinostat combined with tacrolimus-methotrexate to assess safety and efficacy in myeloablative, unrelated donor HSCT (Table 1), ClinicalTrials.gov number NCT00810602.
Hypomethylating agents
Hypomethylating agents, such as azacitidine and 5-aza-deoxycitidine, have shown efficacy in the treatment of patients with myelodysplastic syndrome and recurrent or refractory leukaemia. DNA methylation is an epigenetic modification used by the cell to control processes, such as gene transcription. The hypomethylating agents are incorporated into DNA and act as DNMT inhibitors.123 In murine models of BMT, these agents have been shown to induce FoxP3 expression in CD4+CD25-T cells,124 and inhibit activation and proliferation of alloreactive donor T cells thereby reducing GVHD.125 Interestingly, in a phase I–II study assessing the administration of azacitidine in the reduced-intensity conditioning HSCT setting, reported a significantly increased number of CD4+CD25+CD127−FoxP3+ T cells in azacitidine-treated patients compared with controls.126 Clinically, in the 27 evaluable patients, only three patients developed grade 2 acute GVHD and no cases of grade 3–4 acute GVHD were reported. Two patients developed limited chronic GVHD.126 These results are promising and suggest the need to assess the role of hypomethylating agents prospectively for GVHD prophylaxis. Two studies have investigated low-dose azacitidine in the post-HCT setting to maximize the GVL effect with encouraging results, including decreased incidence of GVHD.127, 128
Conclusions
Developing safer strategies to prevent and treat GVHD will expand the therapeutic modality of alloreactivity to higher-risk transplant populations, including older patients and those with higher co-morbidities or advanced diseases. CNIs remain the mainstay of immunosuppression in mitigating the incidence of GVHD after allogeneic HSCT. With improved understanding of the biology of GVHD coupled with better technological methods, newer approaches are being evaluated. The majority of published results reflect single-institution, phase I–II or phase II studies. The number of ongoing studies being performed on an international-scale indicates active investigation of novel GVHD prophylaxis regimens (Table 1). Another layer of complexity to consider in the study design is inclusion of heterogeneous patient populations, disease conditions, donor sources, and degree of HLA-match in efforts to generalize findings. The next several years will be exciting times with rapid advances being made from the translation of preclinical studies into clinical implementation. As newer therapies emerge, planning and executing well-designed multicentre RCTs will be imperative. This will require large-scale commercialization or availability of drugs or modified cell products, consideration of different practices across centres, transplantation of heterogeneous patient populations, and efforts to study these newer strategies in a rigorous, hypothesis-driven, evidence-based manner. Proposals to use some of these newer therapeutic approaches were recently submitted to the BMT CTN, and the ‘GVHD State-of-the-Science Committee’ conducted an analysis comparing matched controls from the CIBMTR database under these regimens. As a result of this analysis, the upcoming BMT CTN Prophylaxis Trial Design will consist of a phase II RCT of adult patients aged >18 years comparing post-transplant tacrolimus-MMF-cyclophosphamide, tacrolimus-methotrexate-bortezomib, and tacrolimus-methotrexate-maraviroc in recipients of HLA-matched or one-antigen mismatched unrelated donor RIC transplant with a prospectively matched cohort of patients included in the CIBMTR database. Furthermore, studies that use HDAC inhibitors or hypomethylating agents could allow for eventual randomized studies aimed at identifying the best possible therapeutic alternative to prevent GVHD among these newer approaches.
Supplementary Material
Acute graft-versus-host disease (GVHD) remains a significant barrier to the wider application of allogeneic hematopoietic stem cell transplantation (HSCT).
Clinically significant acute GVHD develops in approximately 40–60% of patients undergoing allogeneic HSCT.
The most widely used approach to prevent life-threatening acute GVHD is comprised of a calcineurin inhibitor-based prophylaxis regimen typically administered during the first 180 days of HSCT.
Based on improved biological insights of the pathophysiology of GVHD, newer approaches that target different cells (T cells and B cells) of the immune system are being tested in clinical trials.
Newer agents that target multiple relevant pathways and cellular subsets coupled with a clearer understanding of the precise molecular and cellular interactions that mediate GVHD are required in order to develop effective strategies that prevent this complication without causing other adverse effects.
Biographies
Dr. Sung Won Choi received her MD in 1999 from Wayne State University, Michigan, USA. Dr. Choi is an Assistant Professor of Pediatrics and Communicable Diseases at the University of Michigan. She holds an MS in Clinical Research Design and Statistical Analysis from the University of Michigan School of Public Health in Ann Arbor, MI, USA. Her research interests are in HSCT clinical trials, specifically related to the prevention and treatment of acute GVHD. Since 2009, Dr. Choi has focused on the clinical application of histone deacetylase inhibition for prevention of GVHD.
Dr. Pavan Reddy gained his MD in 1994 from Osmania Medical College, India. Currently, Dr. Reddy is the Moshe Talpaz Professor Oncology and Co-Director of the Hematological Malignancies and BMT Program at the University of Michigan. He is a member of the American Society of Clinical Investigation and American Clinical and Climatological Association and has received a Scholar Award from the Leukemia and Lymphoma Society. He has significant experience in animal models and translational studies of GVHD. He has chaired a number of national and international committees related to the immunobiology of allogeneic HSCT, including the American Society of Hematology Scientific Affairs Committee, the American Society of Blood and Marrow Transplantation Scientific Committee, and Leukemia and Lymphoma Society.
References
- 1.Choi S, Reddy P. Graft-versus-host disease. Panminerva Med. 2010;52:11124. [PubMed] [Google Scholar]
- 2.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–33. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 4.Ruutu T, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.107. [DOI] [PubMed] [Google Scholar]
- 5.Storb R, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–35. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 6.Powles RL, et al. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet. 1980;1:327–9. doi: 10.1016/s0140-6736(80)90881-8. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 8.Ratanatharathorn V, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–14. [PubMed] [Google Scholar]
- 9.Nash RA, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–8. [PubMed] [Google Scholar]
- 10.Przepiorka D, et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after matched unrelated donor marrow transplantation. Blood. 1996;88:4383–9. [PubMed] [Google Scholar]
- 11.Lochte HL, Jr, Levy AS, Guenther DM, Thomas ED, Ferrebee JW. Prevention of delayed foreign marrow reaction in lethally irradiated mice by early administration of methotrexate. Nature. 1962;196:1110–1. doi: 10.1038/1961110a0. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay NK, et al. A randomized study of the prevention of acute graft-versus-host disease. N Engl J Med. 1982;306:392–7. doi: 10.1056/NEJM198202183060703. [DOI] [PubMed] [Google Scholar]
- 13.Brunstein CG, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McSweeney PA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 15.Nash RA, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 17.Raefsky EL, Gascon P, Gratwohl A, Speck B, Young NS. Biological and immunological characterization of ATG and ALG. Blood. 1986;68:712–9. [PubMed] [Google Scholar]
- 18.Martin PJ, et al. Effects of in vitro depletion of T cells in HLA-identical allogeneic marrow grafts. Blood. 1985;66:664–72. [PubMed] [Google Scholar]
- 19.Finke J, Schmoor C, Lang H, Potthoff K, Bertz H. Matched and mismatched allogeneic stem-cell transplantation from unrelated donors using combined graft-versus-host disease prophylaxis including rabbit anti-T lymphocyte globulin. J Clin Oncol. 2003;21:506–13. doi: 10.1200/JCO.2003.03.129. [DOI] [PubMed] [Google Scholar]
- 20.Bacigalupo A, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–7. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 21.Finke J, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 22.Hale G, Cobbold S, Waldmann H. T cell depletion with CAMPATH-1 in allogeneic bone marrow transplantation. Transplantation. 1988;45:753–9. doi: 10.1097/00007890-198804000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Chakraverty R, et al. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116:3080–8. doi: 10.1182/blood-2010-05-286856. [DOI] [PubMed] [Google Scholar]
- 24.Kottaridis PD, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyeloablative stem cell transplantation. Blood. 2000;96:2419–25. [PubMed] [Google Scholar]
- 25.Mead AJ, et al. HLA-mismatched unrelated donors are a viable alternate graft source for allogeneic transplantation following alemtuzumab-based reduced-intensity conditioning. Blood. 2010;115:5147–53. doi: 10.1182/blood-2010-01-265413. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh MM, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–17. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Besien K, et al. Patterns and kinetics of T-cell chimerism after allo transplant with alemtuzumab-based conditioning: mixed chimerism protects from GVHD, but does not portend disease recurrence. Leuk Lymphoma. 2009;50:1809–17. doi: 10.3109/10428190903200790. [DOI] [PubMed] [Google Scholar]
- 28.Tiberghien P, et al. Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood. 2001;97:63–72. doi: 10.1182/blood.v97.1.63. [DOI] [PubMed] [Google Scholar]
- 29.Ciceri F, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 30.Straathof KC, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–54. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–8. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 33.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–8. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Kremer JM, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 35.Genovese MC, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 36.Ruperto N, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–91. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 37.Simon TA, et al. Infections requiring hospitalization in the abatacept clinical development program: an epidemiological assessment. Arthritis Res Ther. 2010;12:R67. doi: 10.1186/ar2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815–25. [PubMed] [Google Scholar]
- 39.Koura DT, et al. In vivo T cell Costimulation Blockade With Abatacept for Acute Graft-versus-Host Disease Prevention: A First In Disease Trial. Biol Blood Marrow Transplant. 2013;19:1638–49. doi: 10.1016/j.bbmt.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–8. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 43.Edinger M, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 44.Brunstein CG, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Ianni M, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 46.Koreth J, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuoka K, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5:179ra43. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–6. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 49.Fruman DA, Burakoff SJ, Bierer BE. Immunophilins in protein folding and immunosuppression. FASEB J. 1994;8:391–400. doi: 10.1096/fasebj.8.6.7513288. [DOI] [PubMed] [Google Scholar]
- 50.Terada N, et al. Rapamycin blocks cell cycle progression of activated T cells prior to events characteristic of the middle to late G1 phase of the cycle. J Cell Physiol. 1993;154:7–15. doi: 10.1002/jcp.1041540103. [DOI] [PubMed] [Google Scholar]
- 51.Blazar BR, Taylor PA, Snover DC, Sehgal SN, Vallera DA. Murine recipients of fully mismatched donor marrow are protected from lethal graft-versus-host disease by the in vivo administration of rapamycin but develop an autoimmune-like syndrome. J Immunol. 1993;151:5726–41. [PubMed] [Google Scholar]
- 52.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sehgal S, Vallera DA. In vivo inhibition of cytokine responsiveness and graft-versus-host disease mortality by rapamycin leads to a clinical-pathological syndrome discrete from that observed with cyclosporin A. Blood. 1996;87:4001–9. [PubMed] [Google Scholar]
- 53.Antin JH, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102:1601–5. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 54.Cutler C, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:328–36. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 55.Furlong T, et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant. 2008;14:531–7. doi: 10.1016/j.bbmt.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pidala J, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica. 2012;97:1882–9. doi: 10.3324/haematol.2012.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cutler C, et al. Less severe acute GVHD but equivalent long-term outcomes using tacrolimus/sirolimus vs. tacrolimus/methotrexate after HLA-matched, related donor hematopoietic stem cell transplantation: results of BMT CTN 0402. Blood (ASH Annual Meeting Abstracts) 2012;120:739. [Google Scholar]
- 58.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–47. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 59.Sykes M, Sachs DH. Mixed chimerism. Philos Trans R Soc Lond B Biol Sci. 2001;356:707–26. doi: 10.1098/rstb.2001.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–64. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 61.Luznik L, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanakry CG, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5:211ra157. doi: 10.1126/scitranslmed.3006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raiola AM, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117–22. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 64.Alousi AM, et al. A matched controlled analysis of post-transplant cyclophosphamide (Cy) versus tacrolimus and mini-dose methotrexate in matched sibling and unrelated donor transplant recipients receiving reduced-intensity conditioning: post-transplant Cy is associated with higher rates of acute GVHD. Blood (ASH Annual Meeting Abstracts) 2012;120:4200. [Google Scholar]
- 65.Flinn IW, et al. Long-term follow-up of remission duration, mortality, and second malignancies in hairy cell leukemia patients treated with pentostatin. Blood. 2000;96:2981–6. [PubMed] [Google Scholar]
- 66.Kraut EH, Neff JC, Bouroncle BA, Gochnour D, Grever MR. Immunosuppressive effects of pentostatin. J Clin Oncol. 1990;8:848–55. doi: 10.1200/JCO.1990.8.5.848. [DOI] [PubMed] [Google Scholar]
- 67.Epstein J, et al. Prevention of graft-versus-host disease in allogeneic bone marrow transplantation by pretreatment with 2′-deoxycoformycin. Exp Hematol. 1986;14:845–9. [PubMed] [Google Scholar]
- 68.Parmar S, et al. Prophylaxis of graft-versus-host disease in unrelated donor transplantation with pentostatin, tacrolimus, and mini-methotrexate: a phase I/II controlled, adaptively randomized study. J Clin Oncol. 2011;29:294–302. doi: 10.1200/JCO.2010.30.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114:4919–27. doi: 10.1182/blood-2008-10-161638. [DOI] [PubMed] [Google Scholar]
- 70.Arai S, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–54. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cutler C, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–7. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reff ME, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 73.Christopeit M, et al. Rituximab reduces the incidence of acute graft-versus-host disease. Blood. 2009;113:3130–1. doi: 10.1182/blood-2009-01-200527. [DOI] [PubMed] [Google Scholar]
- 74.Ratanatharathorn V, et al. Prior rituximab correlates with less acute graft-versus-host disease and better survival in B-cell lymphoma patients who received allogeneic peripheral blood stem cell transplantation. Br J Haematol. 2009;145:816–24. doi: 10.1111/j.1365-2141.2009.07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 76.Koc ON, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 77.Baron F, et al. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2010;16:838–47. doi: 10.1016/j.bbmt.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 78.Ning H, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–9. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 79.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–9. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palmer LA, et al. Chemokine receptor CCR5 mediates alloimmune responses in graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:311–9. doi: 10.1016/j.bbmt.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi SW, et al. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood. 2007;110:3447–55. doi: 10.1182/blood-2007-05-087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gulick RM, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reshef R, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–45. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piguet PF, Grau GE, Allet B, Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987;166:1280–9. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Symington FW, Pepe MS, Chen AB, Deliganis A. Serum tumor necrosis factor alpha associated with acute graft-versus-host disease in humans. Transplantation. 1990;50:518–21. doi: 10.1097/00007890-199009000-00033. [DOI] [PubMed] [Google Scholar]
- 86.Holler E, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–6. [PubMed] [Google Scholar]
- 87.Levine JE, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–5. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000;34:161–4. doi: 10.1345/aph.19126. [DOI] [PubMed] [Google Scholar]
- 89.Choi SW, et al. TNF-inhibition with etanercept for graft-versus-host disease prevention in high-risk HCT: lower TNFR1 levels correlate with better outcomes. Biol Blood Marrow Transplant. 2012;18:1525–32. doi: 10.1016/j.bbmt.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamadani M, et al. Addition of infliximab to standard acute graft-versus-host disease prophylaxis following allogeneic peripheral blood cell transplantation. Biol Blood Marrow Transplant. 2008;14:783–9. doi: 10.1016/j.bbmt.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee SJ, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–64. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 92.Fang J, et al. Prophylactic effects of interleukin-2 receptor antagonists against graft-versus-host disease following unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:754–62. doi: 10.1016/j.bbmt.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Ji SQ, et al. Anti-CD25 monoclonal antibody (basiliximab) for prevention of graft-versus-host disease after haploidentical bone marrow transplantation for hematological malignancies. Bone Marrow Transplant. 2005;36:349–54. doi: 10.1038/sj.bmt.1705046. [DOI] [PubMed] [Google Scholar]
- 94.Di Bartolomeo P, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121:849–57. doi: 10.1182/blood-2012-08-453399. [DOI] [PubMed] [Google Scholar]
- 95.Cavazzana-Calvo M, et al. Specific elimination of alloreactive T cells by an anti-interleukin-2 receptor B chain-specific immunotoxin. Transplantation. 1990;50:1–7. doi: 10.1097/00007890-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 96.Andre-Schmutz I, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360:130–7. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 97.Solomon SR, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106:1123–9. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amrolia PJ, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–26. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 100.Chen X, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tawara I, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clin Cancer Res. 2011;17:77–88. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kennedy GA, et al. The Addition of Interleukin-6 Inhibition to Standard GVHD Prophylaxis Prevents Acute GVHD: Interim Results of a Phase I/II Clinical Study. Blood (ASH Annual Meeting Abstracts) 2013;122:908. [Google Scholar]
- 103.Zeiser R, et al. Preemptive HMG-CoA reductase inhibition provides graft-versus-host disease protection by Th-2 polarization while sparing graft-versus-leukemia activity. Blood. 2007;110:4588–98. doi: 10.1182/blood-2007-08-106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimabukuro-Vornhagen A, Liebig T, von Bergwelt-Baildon M. Statins inhibit human APC function: implications for the treatment of GVHD. Blood. 2008;112:1544–5. doi: 10.1182/blood-2008-04-149609. [DOI] [PubMed] [Google Scholar]
- 105.Hamadani M, Awan FT, Devine SM. The impact of HMG-CoA reductase inhibition on the incidence and severity of graft-versus-host disease in patients with acute leukemia undergoing allogeneic transplantation. Blood. 2008;111:3901–2. doi: 10.1182/blood-2008-01-132050. [DOI] [PubMed] [Google Scholar]
- 106.Rotta M, et al. Impact of recipient statin treatment on graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1463–6. doi: 10.1016/j.bbmt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rotta M, et al. Donor statin treatment protects against severe acute graft-versus-host disease after related allogeneic hematopoietic cell transplantation. Blood. 2010;115:1288–95. doi: 10.1182/blood-2009-08-240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cusack JC, Jr, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–40. [PubMed] [Google Scholar]
- 109.Sun K, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004;101:8120–5. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blanco B, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–83. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 111.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–34. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kroger N, et al. Bortezomib after dose-reduced allogeneic stem cell transplantation for multiple myeloma to enhance or maintain remission status. Exp Hematol. 2006;34:770–5. doi: 10.1016/j.exphem.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 113.Sun K, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–9. doi: 10.1182/blood-2004-11-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koreth J, et al. Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30:3202–8. doi: 10.1200/JCO.2012.42.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choi S, Reddy P. HDAC inhibition and graft versus host disease. Mol Med. 2011;17:404–16. doi: 10.2119/molmed.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leoni F, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reddy P, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–73. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun Y, et al. Cutting edge: Negative regulation of dendritic cells through acetylation of the nonhistone protein STAT-3. J Immunol. 2009;182:5899–903. doi: 10.4049/jimmunol.0804388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 120.Mielcarek M, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–62. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 121.Sandmaier BM, Mackinnon S, Childs RW. Reduced intensity conditioning for allogeneic hematopoietic cell transplantation: current perspectives. Biol Blood Marrow Transplant. 2007;13:87–97. doi: 10.1016/j.bbmt.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choi SW, et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 124.Choi J, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116:129–39. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanchez-Abarca LI, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115:107–21. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]
- 126.Goodyear OC, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML) Blood. 2012;119:3361–9. doi: 10.1182/blood-2011-09-377044. [DOI] [PubMed] [Google Scholar]
- 127.de Lima M, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–31. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jabbour E, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.