1. OPTIMIZING PROSTATE BIOPSY IN CLINICAL PRACTICE – CORE NUMBER AND LOCATION
a. Cancer Detection Rate
Optimizing prostate cancer detection rates in clinical practice translates into defining the ideal number and location of biopsy cores to maximize clinically significant cancer detection, minimize insignificant cancer detection, and reduce the necessity for repetitive re-biopsy. The recently published AUA recommendations on the optimal technique of prostate biopsy and specimen handling1 along with an accompanying review article2 recommended the use of an extended 12 core biopsy strategy, incorporating far lateral and apical samples, for initial prostate biopsy. Historically, comparison of cancer detection rate (CDR) between sextant biopsy protocols and extended-core biopsy protocols (involving 10–12 cores) have demonstrated a trend of increasing CDR with greater core number (Table 1).3 Although increasing the cores from 6 to 12 results in a significant increased in CDR, increasing the number of cores to 18 or 21 (saturation biopsy) as an initial biopsy strategy does not appear to result in a similar increase.4 De La Taille et al. (n=303) found that the CDRs using sextant, extended 12-core, 18-core, and 21-core biopsy schemes were 22.7%, 28.3%, 30.7%, and 31.3%, respectively.5 Diagnostic yield improved by 24.7% when the number of cores increased from 6 to 12, but only by 10.6% when the number of cores increased from 12 to 21. In their review of the diagnostic value of systematic prostate biopsies, Eichler et al noted taking more than 12 cores did not significantly improve cancer yield.6
Table 1.
Cancer detection rates by number of prostate biopsy cores
| Cancer Detection Rate | |||||
|---|---|---|---|---|---|
| No. prostate biopsy cores | 6 | 10–12 | 18 | 20–21 | 24 |
| Study | |||||
| Eskew et al | 26.1% | 40.3%* | |||
| Naughton et al | 26% | 27% | |||
| Presti et al | 33.5% | 39.7% | |||
| Babaian et al | 20% | 30% | |||
| Elabbady et al | 24.8% | 36.4% | |||
| Gore et al | 31% | 43% | |||
| Philip et al | 23% | 32% | |||
| Shim et al | 22% | 28% | |||
| Scattoni et al | 38.5% | 39.9% | |||
| De La Taille et al | 22.7% | 28.3% | 30.7% | 31.3% | |
| Pepe et al | 39.8% | 39.8% | 49.0%** | ||
| Jones et al | 52% | 45% | |||
| Guichard et al | 38.7% | 41.5% | 42.5% | ||
| Ploussard et al | 32.5% | 40.4% | 43.3% | ||
With regard to core location, the AUA white paper highlights the need to sample both apical and far-lateral regions as these appear to increase CDR, but notes that transition-zone sampling does not improve prostate CDR at initial extended biopsy. In a study by Babaian et al. evaluating an 11-core biopsy strategy in 362 patients, the CDR was 34% among 85 men undergoing primary biopsy.3 Among 9 cancers identified uniquely at non-sextant sites, 7 were identified by anterior-horn (far lateral) biopsies and 2 by transition-zone biopsies. Because the entire apex is composed of peripheral zone, biopsies performed at the apex or lateral apex might not sample the anterior apex. Biopsy cores directed at the anterior apex exclusively contribute to cancer detection in 4–6% of men.7 Moreover, additional extreme anterior apical cores (one on each side) have achieved the highest rate of unique cancer detection (p=0.011).8 Transition-zone biopsies, as part of an initial diagnostic strategy, have generally demonstrated a low rate of exclusive cancer detection (2.9%) 9, although in some series CDR did improve with transition zone sampling (p=0.023).4
b. Likelihood of clinically significant/insignificant prostate cancer
Among the growing concerns of prostate cancer over-detection, a potential drawback of increasing core numbers at the time of initial biopsy is the increased likelihood of detecting insignificant prostate cancers. Few reports have shown a higher detection rate of clinically insignificant prostate cancer with extended biopsy schemes as compared to sextant,10 while the majority of studies found no significant differences in the detection rate of insignificant cancers between sextant and extended biopsy schemes.11 In a large database study (n=4,072), Meng and colleagues found that increasing the number of biopsy cores did not result in the identification of a disproportionate number of lower-risk tumors.11 However, increasing the number of cores beyond the extended biopsy strategy does appear to increase the rate of indolent cancer detection. Haas et al. showed that an extended-biopsy 18-core strategy increased the detection rate of insignificant prostate cancers by 22%.12 Far lateral and apical directed biopsy cores do not appear to increase the detection of insignificant cancers while the cancer detection rate of the transition zone sample is already low.2
c. Negative predictive value/avoidance of repeat biopsy
Sextant biopsies have false-negative rates of 15–34% based on CDR at the time of repeat biopsy and computer simulation.13,14 Levine and colleagues first evaluated the use of a 12-core biopsy, using 2 consecutive sets of sextant biopsy in a single sitting. They demonstrated an increase in cancer detection to 31% overall, with only 21% being detected on the first sextant alone.14 Other researchers have demonstrated that prostate CDRs on repeat biopsy vary as a function of the extent of the initial biopsy.15 If a prior negative biopsy utilized a sextant scheme, the CDR was 39% with a repeat extended biopsy, whereas if a prior negative biopsy utilized an extended scheme, the CDR of the repeat biopsy decreased to 21–28%. Use of repeat saturation (20 to 24 cores) biopsy after initial saturation biopsy has been shown to have a CDR of 24%, similar to the CDR of 29% for biopsies following an initial sextant biopsy (p=0.08).15 The authors from this study concluded that the false-negative rate for repeat prostate biopsies after an initial saturation biopsy is equivalent to that following traditional biopsy and they recommended against saturation prostate biopsy for primary biopsy.
Although it has been demonstrated that T1c cancers are present more frequently in the transition zone than T2 cancers, the yield of routine transition zone biopsy remains low.9,16,17 Because relatively few cancers are found uniquely in the transition zone, it is unlikely that repeat biopsies would be avoided by routine transition-zone sampling. No difference has been seen in the number of men requiring a repeat biopsy when evaluating the role of transition-zone sampling on initial and repeat biopsy.17 Sampling the anterior apical peripheral zone on repeat biopsy identified 36.0% with cancer exclusively in the anterior apical peripheral zone cores. The CDR from the anterior apical peripheral zone sites was significantly higher in the repeat biopsies than in the initial biopsies (p<0.01), suggesting a predominance of missed cancers in this location.18 Apical cores and extreme anterior apical cores have been shown to increase unique cancer detection and minimize the potential for misdiagnosis and need for repeat biopsy.8 Few studies have evaluated the NPV of far-lateral sampling of the prostate. However, lateral sampling appears to improve clinical NPV because several cancers are identified only in the lateral sample.
d. Pathology concordance between biopsy and radical prostatectomy
Several studies have demonstrated that extended biopsy schemes improve biopsy concordance with prostatectomy specimens. Concordance rates of prostate cancer grade, when an extended biopsy scheme is used, are as high as 85%, compared to 50% with a sextant biopsy.19–21 Upgrading of the Gleason score has been shown to be significantly less likely with the extended scheme (17% vs. 41% for the sextant scheme, p<0.001).20 Similarly, 14% of the prostate cancers detected using extended biopsy schemes have been shown to be under-graded compared to 25% of cancers detected using sextant schemes (p=0.01).21 The results of biopsy schemes involving saturation biopsies (more than 12 cores) appear to have a higher concordance rate with results from prostatectomy (59%) than a scheme involving fewer than 12 cores (47%, p=0.05).22
Apical and laterally directed sampling improves the ability to predict pathological features on prostatectomy, while the concordance of transition-zone biopsies with radical prostatectomy pathology is poor. In a study evaluating individually labeled, preoperative apical core biopsies and corresponding prostatectomy specimens, Rogatsch et al. determined the positive predictive value for identifying the tumor location correctly was 71.1%, while the lack of cancer in the apical biopsy had an negative predictive value of 75.5%.23 Cancer concordance of transition-zone biopsies and prostatectomy specimens range from approximately 20–40%.24 The role of lateral sampling of the prostate was evaluated by Singh et al. who showed that laterally directed cores were independent predictors of pathological features at prostatectomy.25
2. INFLUENCE OF BIOPSY TECHNIQUE
a. Transrectal
i. End-fire vs Side-fire cancer detection rates
Currently, two different approaches are used for transrectal prostate sampling including an end-fire or side-fire configuration of the biopsy probe (Figure 1). Evidence from retrospective studies has initially suggested that an end-fire configuration results in a greater prostate cancer detection rate than a side-fire configuration. In a study of 2674 patients, Ching et al26 evaluated 2,674 patients who underwent prostate biopsy and showed a prostate CDR for end-fire versus side-fire probes of 45.8% versus 38.5%, respectively. Similar results were shown by Paul et al27 in a study of 2625 subjects (31.3% vs 21.5%). Ching et al also found that the use of an end-fire probe on repeat biopsy significantly increased prostate cancer detection (odds ratio [OR] 1.59, 95% confidence interval [CI]: 1.03–2.46).28 It has been hypothesized that improved cancer detection with the end-fire approach may be due, in part, to a better ability to sample the apex, lateral regions, and the anterior gland due to its needle angle.
Fig. 1.
End-fire (A) and side-fire (B) configurations of the transrectal ultrasound biopsy probe.
Although both of the aforementioned studies included large numbers of patients, they were retrospective, and the number of cores and biopsy schemes were not standardized. To address this concern, Rom and colleagues performed a prospective randomized multicenter study comparing prostate cancer detection rates of end-fire and side-fire transrectal ultrasound probe configurations. The prostate cancer detection rate did not differ between the end-fire and side-fire probe (34.3% vs 34.4%, p=0.972).29 Recently, Raber et al confirmed these findings in a study comparing end-fire and side-fire configurations in 1705 patients undergoing first biopsy and re-biopsy.30 No significant difference was found between the two probes in the first biopsy and re-biopsy sets (38% vs 36.5%, P= 0.55; 10.8% vs 9.3%, p=0.7). The side-fire transrectal probe has been associated with a better patient tolerance profile.30,31
ii. Computerized templates for prostate biopsy
Computerized templates offer a biopsy strategy with reliable sampling of the same locations in the prostate each time. Available platforms typically convert 2-dimensional ultrasound data to a 3D model or image of the prostate. This technique takes into account the variability in prostate volume and shape, and allows reproducible sampling through accurate needle placement, in a known and recorded location. Furthermore, in theory, such a biopsy should allow better negative accuracy through reproducible spatial sampling of all essential areas of the gland. If an initial biopsy is negative for cancer, subsequent biopsies can be arrayed in such a way as to sample different regions of the prostate, which could potentially reduce sampling error. While such schemes do not increase cancer detection, they allow more reproducible sampling, which increases positive predictive value with regard to cancer location. This has potential value in monitoring or treatment planning.
Currently computerized biopsy systems have been studied for purposes of systematic biopsy: TargetScan® and Artemis. 3D transrectal prostate mapping with TargetScan® performed with an endorectal ultrasound probe has been studied in 2 series. In a retrospective multicenter review, a comparative analysis of 140 TargetScan® biopsies and 23 associated prostatectomy specimens demonstrated pathologic concordance in 52%.32 In a single institution study, a simulation on 20 radical prostatectomy specimens showed that TargetScan® biopsy correctly identified cancer in 16 (80%) of the glands. This technique was reproducible between different operators as demonstrated by an 85% biopsy core concordance.33
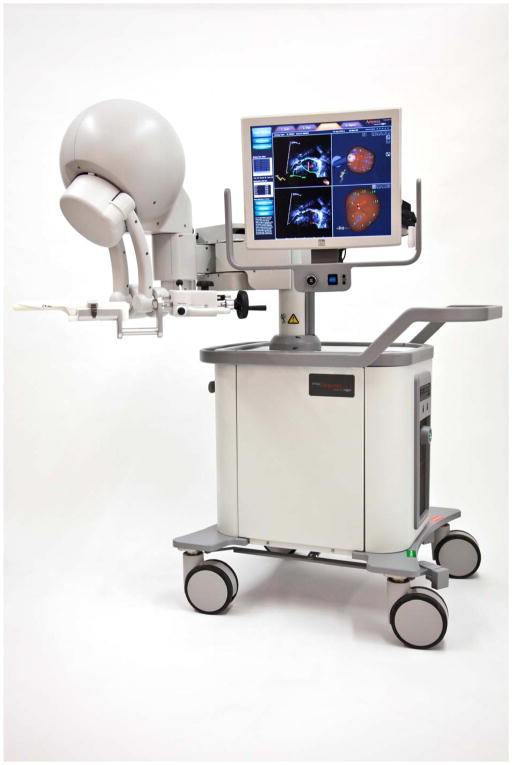
Artemis® is a 3D imaging and navigation system that converts 2D monochromatic ultrasound images to an enhanced 3D color image allowing manipulation, planning, and management of the prostate biopsy process (Figure 2). While capable of performing a computer-directed template biopsy, as in the case of TargetScan®, Artemis® is distinct in that spatial-tracking of the arm provides exact recording of the location of the biopsy core. This allows the user to return to the previous biopsy site at a later setting if considering surveillance or re-biopsy. Natarajan, et al, completed Artemis® biopsy in 180/218 men. In the tracking study, they were able to return to the same needle position with a recorded error of 1.2 mm +/− 1.1 mm.34
Fig. 2.
Artemis 3D imaging and navigation system. Courtesy of Eigen, Grass Valley, CA; with permission.
b. MRI-guided prostate biopsy
While conventional biopsy has relied upon improving sampling through increasing numbers of cores, an alternative approach is to reduce sampling error through localization. Recent improvements in multiparametric magnetic resonance imaging (MRI) have allowed accurate localization of prostate cancer.35,36 A number of investigators have evaluated the impact of pre-biopsy MRI followed by targeted biopsy on cancer detection. The use of MR-targeted biopsy has been studied in the setting of previous negative biopsy,37 men with no history of previous biopsy,38 and, most recently, those on active surveillance.39,40
Among men undergoing repeat biopsy, 54% of men were found to have cancer only identified on the MR-targeted cores. Cancers in this setting are most often found in the anterior prostate or apex.7 Among men presenting with no previous history of cancer, pre-biopsy MRI appears to have the potential to stratify the risk of prostate cancer through the application of a suspicion score.41,42 In a study of 555 men, Haffner, et al demonstrated that MR-targeted biopsy identified fewer cancers overall when compared to systematic biopsy (236/302 vs 290/ 302, respectively), but detected a comparable number of clinically significant cancers (236/249 vs 237/249).43 All cancers detected by MR-targeted approach were deemed significant. In addition, more cancer was identified per core, suggesting the potential for more accurate risk stratification. A number of subsequent studies have shown similar results44 suggesting a potential to utilize MRI not only to improve cancer detection, but also to reduce over-detection of indolent disease. The ability to improve risk stratification through better sampling of cancer has also been suggested by several studies evaluating the impact of MR-targeted biopsy in active surveillance patients.45–47
3. REPEAT PROSTATE BIOPSY
a. Indications
i. HGPIN, ASAP, rising PSA
There is no consensus regarding the need for repeat biopsy in men with previous negative sampling. Potential indications include abnormal histology, rising PSA, or persistence of an elevated PSA. Historically, men diagnosed with isolated high grade intraepithelial neoplasia (HGPIN) were recommended to undergo immediate repeat biopsy given the high likelihood of concordant occult cancer, but, upon the implementation of extended core biopsy in clinical practice it was noted that the likelihood of cancer detection upon immediate repeat biopsy was small.48 The recent EAU, AUA and NCCN guidelines reported that the presence of HGPIN diagnosis no longer represents an indication for immediate repeat biopsy.49 Epstein and Herawi have asserted that the prostate cancer risk at repeat prostate biopsy after HGPIN diagnosis (22%) is similar to the risk of cancer detection after an initial benign biopsy.50 Additionally, prospective trials have failed to demonstrate an association between the presence of HGPIN at initial prostate biopsy and subsequent prostate cancer at repeat prostate biopsy.51,52 However, studies by Benecchi et al.53, and Netto and Epstein54 have identified the presence of HGPIN as a risk factor in their analyses and included HGPIN in their repeat prostate biopsy nomograms.
The number of HGPIN foci appears to be an important prognosticator and influences the suggested management protocols. For example, Godoy et al55 and Merrimen et al56 have found that isolated HGPIN does not warrant any further prostate biopsy. Similarly, data from the Cleveland Clinic has shown that upon comparison of men with multifocal and isolated HGPIN on initial saturation biopsy, an 80% and 0% likelihood of prostate cancer on repeat prostate biopsy was observed, respectively.57 These findings, taken together, demonstrate that a single focus may have limited clinical significance, with minimally increase risk of prostate cancer development. Multifocal HGPIN, however, more than doubles the risk of de novo prostate cancer development. The NCCN guidelines recommend that for patients with multifocal HGPIN (≥2 cores) on an extended pattern biopsy, repeat biopsy be performed within the first year.
Another critical predictor of cancer risk in men with isolated HGPIN is the interval to biopsy. Studies of serial delayed interval biopsy suggest that repeat biopsy can be performed at longer intervals than 1 year.48,55 Our group has advocated that serial prostate biopsy every 3 years based upon our early observation of prostate cancer in 26% of men biopsied 3 years after initial diagnosis, and our subsequent demonstration of a similar persistent risk on 6 year biopsy.48 Among a large cohort of men with isolated HGPIN followed for 3 years as part of the placebo arm of a chemoprevention trial, cancer was demonstrated in 34.7% of men with serial biopsy performed each year.58
The natural history of atypical small acinar proliferation (ASAP) is less well defined than that of HGPIN, but, if ASAP is present in the initial biopsy specimen, the risk of diagnosing prostate cancer on subsequent biopsy is significantly increased. Unlike HGPIN, ASAP represents uncertainty regarding the diagnosis of cancer. Studies of repeat biopsy have shown a detection rate for prostatic adenocarcinoma as high as 55% after an initial diagnosis of ASAP59 and up to 58% when found in combination with HGPIN on initial biopsy.60 Regardless of PSA values, current recommendations are to rebiopsy all patients with ASAP in their initial biopsy specimen within 3 to 6 months. The typical technique of biopsy is focal saturation to the region of observed atypia.
A rising prostate-specific antigen after a negative prostate biopsy may indicate undiagnosed cancer, while a persistently elevated PSA may draw concern of a missed occult cancer. In the presence of a rising PSA after a negative biopsy a low threshold for repeat biopsy should be entertained. An important consideration is adequacy of the initial prostate biopsy, taking into account the number of cores taken and anatomical sites sampled, areas of under sampling, length of each core, and quality of the tissues sampled. Most studies of repeat prostate biopsy following extended initial prostate indicate that up to 30% of patients have cancers that were not previously identified.61,62 A repeat biopsy strategy may include focal saturation, extended 21-core, saturation biopsy, or image guidance to improve the detection rate.
b. Technique
i. Focal saturation, 12 core, saturation
When performing repeat biopsies, it is important to recognize that the region of the prostate potentially undersampled in a 12-core biopsy scheme is the anterior apex.7 The entire apex of the prostate is comprised of peripheral zone and, although extended schemes do sample the apex and lateral apex, additional cores should be taken from the anterior apex on repeat biopsy. Similarly, repeat biopsy should include the transition zone as supported by the European Association of Urology guidelines.63
The precise labeling of the initial prostate locations is important to direct rebiopsy in a more concentrated fashion into the region of the initial ASAP.50,64 Allen et al64 have demonstrated earlier that the chance of detecting prostate cancer greatly increases by performing a rebiopsy not only of the atypical site but also of adjacent contralateral and adjacent ipsilateral areas. However, Scattoni et al has previously reported that a precise spatial concordance between ASAP and prostate cancer was present in only 33% of the cases, similar to the likelihood of finding prostate cancer in an adjacent or a nonadjacent site.65
Contemporary recommendations for the technique of repeat prostate biopsy suggests that a repeated 10- to 12-core extended biopsy scheme remains the most frequently used technique, with additional cores from suspected areas by modern imaging or the anterior and transition zone. As compared to standard extended techniques (10–14 cores), repeat saturation biopsies (20–24 cores) increase the CDR (24.9 % vs. 32.7 %, p = 0.0075).62
a. Transperineal saturation biopsy
According to AUA and NCCN guidelines, a saturation prostate biopsy may be considered in men with a prior negative biopsy and persistent suspicion of prostate cancer. The transperineal biopsy technique allows for improved sampling of the apex and anterior zones which are common sites of cancer detection on repeat biopsy. In a series of 92 consecutive men with at least two negative prior transrectal biopsies, most of the tumors detected on transperineal saturation biopsy were found in the anterior zone (83.3%).66 Transrectal and transperineal prostate saturation repeat biopsies have a similar cancer detection rate.67
i. MRI-guided repeat prostate biopsy
MRI-guided repeat biopsy has the potential to reduce the sampling error of the initial biopsy through localization of disease (Figure 3). In a recent meta-regression comparing cancer detection on repeat prostate biopsy, Nelson et al compared transperineal, ultrasound guided transrectal saturation, and MRI-guided biopsy where cancer detection rates were 30.0%, 36.8%, and 37.6%, respectively.68 Meta-regression analysis showed that MRI-guided biopsy had significantly higher cancer detection than transperineal biopsy. There were no significant differences however between median Gleason scores among the three biopsy strategies. The authors concluded that in the re-biopsy setting, it is unclear which strategy offers the highest CDR. However, MRI-guided biopsies may potentially detect more prostate cancers than other modalities and can achieve this with fewer biopsy cores.
Fig. 3.
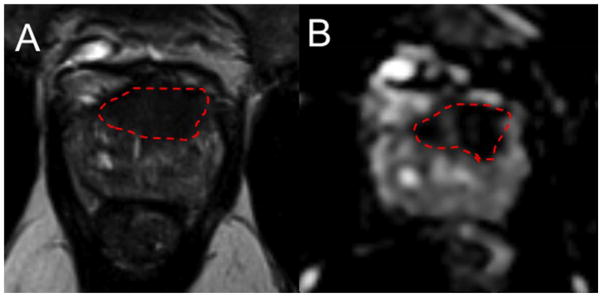
MRI-guided repeat prostate biopsy after a negative 12-core template biopsy. MRI demonstrated a left anterior mid-to-apex transition zone lesion that appeared to intimately involve anterior fibromuscular stroma (suspicion score 4/5) on T2 weighed imaging (A) and apparent diffusion coefficient map (B). Targeted biopsy revealed Gleason score 3+4=7 prostate cancer in 2 of 2 cores, 20–70% of each core.
4. PAIN CONTROL
a. Technique of anesthesia
Improvements in anesthesia techniques have allowed urologists to sample a greater number of cores and from different locations in the gland including the ability to perform a saturation biopsy procedure in an office setting.62 Both rectal and prostatic anesthesia may limit pain during the procedure. Intrarectal local anesthesia has been used both as lubrication in order to reduce friction and protect the mucosa during instrumentation as well as ease the discomfort with introduction of the ultrasound probe. A variety of anesthetic agents have been employed including lidocaine, prilocaine, nifidipine, and dimethyl sulphoxide in various combinations with varied results. A periprostatic nerve block is commonly used in TRUS-guided biopsy where the optimal injection site seems to be localized in the angle between the prostate and the seminal vesicles, which can be easily identified as a hypoechoic area on TRUS. A concentration of 1% lidocaine, 5 ml per side, is sufficient to provide pain relief. Periprostatic nerve block is associated with significantly less pain during biopsy than lidocaine gel or placebo69 and is superior to intrarectal instillation of anesthetic cream70 Extensive biopsy protocols may be comfortably performed in office setting using local anesthesia with 22 ml 1% lidocaine injection71 Despite lack of a standardized dose or optimal technique, periprostatic anesthetic infiltration should be considered the gold standard.72 Intraprostatic anesthesia has been provided in combination with periprostatic nerve block resulting in improved pain control,73 but further studies are needed to delineate location, technique and dosages. Historically, the transperineal approach to prostate biopsy has been performed under general anesthesia, but recent studies have demonstrated the combination of pudendal and periprostatic nerve block is well tolerated and improves pain reduction without the need for general anesthesia.74
5. COMPLICATIONS
a. Incidence of prostate biopsy complications
According to the AUA clinical guidelines on the incidence, prevention, and complications related to prostate needle biopsy, the most common urological side effects of a prostate needle biopsy include hematuria, rectal bleeding, hematospermia, urinary tract infection, acute urinary retention.75,76 Erectile dysfunction and vasovagal response have also been noted to occur in patients undergoing prostate biopsy (Table 2).
Table 2.
Incidence of prostate biopsy complications
| Complication | Incidence |
|---|---|
| Hematuria | 23–84% |
| Rectal bleeding | 17–45% |
| Hematospermia | 12–93% |
| Urinary tract infection | 2–6% |
| Bacteremia | 0.1–2.2% |
| Hospitalization | 0.6–4.1% |
| Erectile dysfunction | 2.2% |
| Urinary retention | 1–7% |
| Vasovagal response | 1.4–5.3% |
b. Bleeding complications
Episodes of significant bleeding after prostate biopsy may occur in 1–4% of patients.77 Recent data suggest that hematuria is noted in 23–84%, rectal bleeding in 17–45%, and hematospermia in 12–93% of men post prostate needle biopsy. However relatively fewer men who underwent a biopsy perceived hematuria (6%), rectal bleeding (3%), and hematospermia as a major/moderate problem (27%).5,78–80
i. Prevention of prostate biopsy complications
Current considerations for the prevention of bleeding complications after prostate biopsy include holding anticoagulation, including warfarin, aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), herbal supplements, and clopidogrel, for 7–10 days prior to the biopsy when it is possible to do so. For those patients with underlying coagulopathy or who are on warfarin, prostatic biopsy should not be performed until the international normalized ratio has been corrected below 1.5. Several studies have evaluated the safety of maintaining anticoagulation during biopsy. From the data it appears that stopping aspirin may be unnecessary, as it does not increase the incidence or severity of bleeding complications.81–83 Aspirin may, however, prolong the duration of self-limiting hematuria and rectal bleeding.81,83 Similar trends demonstrating no increased risk of bleeding have been noted in evaluating the safety of continuing warfarin during biopsy.84,85 Taken together, it appears as though aspirin may be continued during the procedure if there is any concern about the safety of withholding it. Data on stopping warfarin and clopidogrel are limited and the risks between cardiovascular or thromboembolic events when stopping anticoagulation must be weighed against the risk for bleeding and associated complications with continuation.76
ii. Influence of technique
In a meta-analysis and review of prostate biopsy results, Shen et al determined there was no significant differences in the incidence of major or minor complications between the tranperineal and transrectal technique.86 In prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy, Hara et al found no differences in rectal bleeding, hematuria, or hematopsermia between the two techniques.87 A major consideration is the potential to reduce infection by utilizing a transrectal approach, particularly in view of the increasing rate of infection following biopsy noted in recent years.88
iii. Management
Severe rectal bleeding may be managed initially with bed rest, volume resuscitation, and transfusion. If the patient’s condition does not improve while under observation, options for management include digital compression, rectal tamponade with a tampon,89 inflated condom, or inflated foley catheter balloon.90 Colonoscopy with injection of epinephrine and polidocanol or use of sclerotherapeutic agents, angiography with embolization,91 transrectal exploration, and suturing are alternative means of stopping rectal bleeding.92,93 Hematuria may be managed similarly with bed rest, volume resuscitation and transfusion. Cystoscopy or anoscopy with coagulation of bleeding points may be used in more severe cases.
c. Infectious complications
Most infectious complications after prostate biopsy are limited to symptomatic urinary tract infection and low-grade febrile illness, which can be readily treated with oral or intravenous antibiotics; however, post-biopsy sepsis has emerged as a risk of this procedure. The incidence of infectious complications following prostate biopsy in large multi-institutional studies ranges from 0.1%–7%, depending upon the antimicrobial prophylactic regimen used,88,94,95 with approximately 30%–50% of these patients having accompanying bacteremia.96,97 The risk of hospitalization for infectious complications in contemporary studies ranges from 0.6–4.1%.95 The reported incidence of UTI after prostate biopsy typically ranges between 2% and 6%.98 Bacteremia is frequently accompanied by severe sepsis, which has an overall incidence of 0.1%–2.2% following prostate biopsy.96 One recent study reported that among post–TRUS biopsy patients hospitalized with E. coli bacteremia, 25% had severe sepsis requiring intensive care unit admission.99 In terms of repeat prostate biopsy, Loeb et al demonstrated a repeat biopsy session was not associated with a greater risk of infectious (OR 0.81, p = 0.39) or serious noninfectious urological complications (OR 0.94, p = 0.82) compared to the initial biopsy.100
i. Prevention
According to the AUA Best Practice Statement, TRUS-guided prostate biopsy, performed through a grossly contaminated field, requires important preventative considerations. There is wide variation in the approach to the preparation of the rectum. Some studies found no benefit to either preprocedural povidine-iodine96 or sodium biphosphate enemas.101 However, another study found that a bisacodyl suppository rectal preparation the night before or morning of the procedure decreased infectious complications.102
The AUA Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis recommends a Fluoroquinolone or 1st/2nd/3rd generation cephalosporin prior to biopsy.103 Currently, no conclusive data have been found to support either the use of long-course (3 days) over short (1 day) fluoroquinolone regimens, or multiple vs single dose schedules.104 Although antibiotic prophylaxis is largely effective in preventing infection, leading to a low incidence of sepsis, recently there have been increasing quinolone-resistant infection resulting from more frequent use of quinolones in the population overall, including at the time of transrectal prostate biopsy.105 Prebiopsy screening with rectal swabs may allow identification of those men harboring antibiotic resistant organisms in their endogenous gastrointestinal flora prebiopsy, and for whom fluoroquinolone prophylaxis may not be appropriate.94 This strategy has revealed a prevalence of about 22% of men harboring fluoroquinolone resistant bacteria.94,106 Taylor and colleagues targeted specific antimicrobial prophylaxis based on rectal swab results.107 These authors were able to show a non-significant reduction in post-prostate biopsy infections from 2.6% to 0% (p=0.12) and a potential cost savings per infectious complication averted. However, these methods have not been broadly used and the determination of true benefit requires further prospective investigation.
The need for routine urine culture prior to prostate biopsy is unclear; urine culture appears only to be useful in the decision to refrain from prostate biopsy when bacterial growth is evident.108 The use of urinalysis or urine dipstick prior to prostate biopsy is widespread; however, there are no published studies to document its benefit.
ii. Technique
In a prospective randomized study comparing transperineal and transrectal systematic 12-core prostate biopsy, Hara et al found no differences in the rates sepsis or post-biopsy fevers.87 Similarly, Miller et al found similar rates of sepsis when comparing the 2 biopsy techniques.109 Shen et al determined there was no significant differences in the incidence of major or minor complications between the transperineal and transrectal technique in a large meta-analysis.86
iii. Management
At present, there are no published guidelines for the management of post–prostate biopsy infections. However, in addition to patient-specific prophylactic regimens, consideration should be given to empiric followed by culture driven antimicrobial therapy if a patient presents with post-biopsy sepsis. Previous studies have demonstrated that inappropriate empiric therapy of E. coli. bloodstream infections is associated with an increased risk of mortality.110 Broader spectrum empiric antimicrobial coverage should be considered for post–prostate biopsy sepsis compared to that given for other causes of community-onset urosepsis as prostate biopsy was actually a risk factor for bacteremia with multidrug-resistant E. coli.99 Moreover, other individual risk factors that should be considered when choosing appropriate empiric therapy including prior exposure to fluoroquinolones. Initial therapy must cover E. coli, the most common pathogen, as well as numerous other organisms. Prior to treatment, a urine culture, and blood cultures if the patient is febrile, should be obtained.
a. Quality of life
ii. Erectile dysfunction
Recent data has suggested an associated between prostate biopsy, lower urinary tract symptoms, and erectile dysfunction. In their randomized trial of 145 men, Klein et al. authors found that prostate biopsy may cause urinary symptoms and erectile dysfunction, regardless of anesthesia or number of cores sampled as shown by a decreased in IIEF-5 and an increase in IPSS scores in their study.111 Erectile dysfunction was noted in 2.2% of men in a study be Akyol and Adayener possibly due to nerve injury caused by the biopsy needle.112 However, in a study by Helfand et al, cancer diagnosis appears to have an adverse effect on the erectile function of men undergoing prostate biopsy but no effect on lower urinary tract symptoms.113 Similarly, serial prostate biopsies appear to have an adverse effect on erectile function in men with prostate cancer on active surveillance but do not affect lower urinary tract symptoms.114 Several other studies, however, have suggested the effects of the prostate needle biopsy are transient with no significant differences in men with and without prostate cancer.115,116
i. Urinary retention
Urinary retention requiring temporary catheterization develops in up to 1% of men undergoing transrectal prostate biopsy.80,117,118 Men with enlarged glands and higher International Prostate Symptom Scores are more prone to develop post biopsy retention.118 Data suggest that starting higher-risk patients on an alpha blocker prior to prostate biopsy may prevent episodes of urinary retention.119 Higher rates of acute urinary retention have been noted in men undergoing transperineal prostate biopsy.120
ii. Other
Excessive anxiety and discomfort from the endorectal probe may produce a moderate or severe vasovagal response in 1.4% to 5.3% of patients121,122 and may require termination of the procedure. Placing the patient in the Trendelenburg position and use of intravenous hydration usually resolve these symptoms, with further intervention as clinically indicated
CONCLUSIONS
A 12-core systematic biopsy that incorporates apical and far-lateral cores in the template distribution allows maximal cancer detection, avoidance of a repeat biopsy, while minimizing the detection of insignificant prostate cancers. MRI guided prostate biopsy has an evolving role in both initial and repeat prostate biopsy strategies, potentially improving sampling efficiency, increasing detection of clinically significant cancers, and reducing detection of insignificant cancers. Hematuria, hematospermia, and rectal bleeding are common complications of prostate needle biopsy, but are generally self-limiting and well tolerated. All men should receive antimicrobial prophylaxis prior to biopsy. Fluoroquinolones or cephalosporins remain the recommended prophylactic antibiotics, although the frequency of quinolone-resistant infections is increasing.
KEY POINTS.
A 12-core systematic biopsy that incorporates apical and far-lateral cores in the template distribution allows maximal cancer detection, avoidance of a repeat biopsy, while minimizing the detection of insignificant prostate cancers.
Endfire and sidefire ultrasound probes, along with transrectal and transperineal approaches to prostate biopsy, have similar cancer detection rates and complications.
MRI guided prostate biopsy has an evolving role in both initial and repeat prostate biopsy strategies, potentially improving sampling efficiency, increasing detection of clinically significant cancers, and reducing detection of insignificant cancers.
Hematuria, hematospermia, and rectal bleeding are common complications of prostate needle biopsy, but they are generally self-limiting and well tolerated.
Fluoroquinolones or cephalosporins remain the recommended prophylactic antibiotics, although the frequency of quinolone-resistant infections is increasing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taneja SSBM, Carter HB, Schellhammer P, Cookson MS, Gomella LG, Troyer D, Wheeler TM, Schlossberg S, Penson DF. White Paper: AUA/Optimal Techniques of Prostate Biopsy and Specimen Handling. 2013 doi: 10.1016/j.juro.2013.02.072. http://www.auanet.org/common/pdf/education/clinicalguidance/Prostate-Biopsy-WhitePaper.pdf. [DOI] [PMC free article] [PubMed]
- 2.Bjurlin MA, Carter HB, Schellhammer P, Cookson MS, Gomella LG, Troyer D, Wheeler TM, Schlossberg S, Penson DF, Taneja SS. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 2013;189:2039–46. doi: 10.1016/j.juro.2013.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, Evans R, Johnston D, Chen M. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163:152–7. [PubMed] [Google Scholar]
- 4.Guichard G, Larre S, Gallina A, Lazar A, Faucon H, Chemama S, Allory Y, Patard JJ, Vordos D, Hoznek A, et al. Extended 21-sample needle biopsy protocol for diagnosis of prostate cancer in 1000 consecutive patients. Eur Urol. 2007;52:430–5. doi: 10.1016/j.eururo.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 5.de la Taille A, Antiphon P, Salomon L, Cherfan M, Porcher R, Hoznek A, Saint F, Vordos D, Cicco A, Yiou R, et al. Prospective evaluation of a 21-sample needle biopsy procedure designed to improve the prostate cancer detection rate. Urology. 2003;61:1181–6. doi: 10.1016/s0090-4295(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 6.Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605–12. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
- 7.Meng MV, Franks JH, Presti JC, Jr, Shinohara K. The utility of apical anterior horn biopsies in prostate cancer detection. Urologic oncology. 2003;21:361–5. doi: 10.1016/s1078-1439(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 8.Moussa AS, Meshref A, Schoenfield L, Masoud A, Abdel-Rahman S, Li J, Flazoura S, Magi-Galluzzi C, Fergany A, Fareed K, et al. Importance of additional “extreme” anterior apical needle biopsies in the initial detection of prostate cancer. Urology. 2010;75:1034–9. doi: 10.1016/j.urology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Bazinet M, Karakiewicz PI, Aprikian AG, Trudel C, Aronson S, Nachabe M, Peloquin F, Dessureault J, Goyal M, Zheng W, et al. Value of systematic transition zone biopsies in the early detection of prostate cancer. J Urol. 1996;155:605–6. [PubMed] [Google Scholar]
- 10.Singh H, Canto EI, Shariat SF, Kadmon D, Miles BJ, Wheeler TM, Slawin KM. Improved detection of clinically significant, curable prostate cancer with systematic 12-core biopsy. J Urol. 2004;171:1089–92. doi: 10.1097/01.ju.0000112763.74119.d4. [DOI] [PubMed] [Google Scholar]
- 11.Meng MV, Elkin EP, DuChane J, Carroll PR. Impact of increased number of biopsies on the nature of prostate cancer identified. J Urol. 2006;176:63–8. doi: 10.1016/S0022-5347(06)00493-9. [DOI] [PubMed] [Google Scholar]
- 12.Haas GP, Delongchamps NB, Jones RF, Chandan V, Serio AM, Vickers AJ, Jumbelic M, Threatte G, Korets R, Lilja H, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99:1484–9. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 13.Chen ME, Troncoso P, Johnston DA, Tang K, Babaian RJ. Optimization of prostate biopsy strategy using computer based analysis. J Urol. 1997;158:2168–75. doi: 10.1016/s0022-5347(01)68188-6. [DOI] [PubMed] [Google Scholar]
- 14.Levine MA, Ittman M, Melamed J, Lepor H. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol. 1998;159:471–5. doi: 10.1016/s0022-5347(01)63951-x. [DOI] [PubMed] [Google Scholar]
- 15.Lane BR, Zippe CD, Abouassaly R, Schoenfield L, Magi-Galluzzi C, Jones JS. Saturation technique does not decrease cancer detection during followup after initial prostate biopsy. J Urol. 2008;179:1746–50. doi: 10.1016/j.juro.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 16.Fleshner NE, Fair WR. Indications for transition zone biopsy in the detection of prostatic carcinoma. J Urol. 1997;157:556–8. [PubMed] [Google Scholar]
- 17.Terris MK, Pham TQ, Issa MM, Kabalin JN. Routine transition zone and seminal vesicle biopsies in all patients undergoing transrectal ultrasound guided prostate biopsies are not indicated. J Urol. 1997;157:204–6. [PubMed] [Google Scholar]
- 18.Orikasa K, Ito A, Ishidoya S, Saito S, Endo M, Arai Y. Anterior apical biopsy: is it useful for prostate cancer detection? Int J Urol. 2008;15:900–4. doi: 10.1111/j.1442-2042.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 19.Elabbady AA, Khedr MM. Extended 12-core prostate biopsy increases both the detection of prostate cancer and the accuracy of Gleason score. Eur Urol. 2006;49:49–53. doi: 10.1016/j.eururo.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Mian BM, Lehr DJ, Moore CK, Fisher HA, Kaufman RP, Jr, Ross JS, Jennings TA, Nazeer T. Role of prostate biopsy schemes in accurate prediction of Gleason scores. Urology. 2006;67:379–83. doi: 10.1016/j.urology.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 21.San Francisco IF, DeWolf WC, Rosen S, Upton M, Olumi AF. Extended prostate needle biopsy improves concordance of Gleason grading between prostate needle biopsy and radical prostatectomy. J Urol. 2003;169:136–40. doi: 10.1016/S0022-5347(05)64053-0. [DOI] [PubMed] [Google Scholar]
- 22.Kahl P, Wolf S, Adam A, Heukamp LC, Ellinger J, Vorreuther R, Solleder G, Buettner R. Saturation biopsy improves preoperative Gleason scoring of prostate cancer. Pathol Res Pract. 2009;205:259–64. doi: 10.1016/j.prp.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Rogatsch H, Horninger W, Volgger H, Bartsch G, Mikuz G, Mairinger T. Radical prostatectomy: the value of preoperative, individually labeled apical biopsies. J Urol. 2000;164:754–7. doi: 10.1097/00005392-200009010-00031. [DOI] [PubMed] [Google Scholar]
- 24.Haarer CF, Gopalan A, Tickoo SK, Scardino PT, Eastham JA, Reuter VE, Fine SW. Prostatic transition zone directed needle biopsies uncommonly sample clinically relevant transition zone tumors. J Urol. 2009;182:1337–41. doi: 10.1016/j.juro.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 25.Singh H, Canto EI, Shariat SF, Kadmon D, Miles BJ, Wheeler TM, Slawin KM. Six additional systematic lateral cores enhance sextant biopsy prediction of pathological features at radical prostatectomy. J Urol. 2004;171:204–9. doi: 10.1097/01.ju.0000100220.46419.8b. [DOI] [PubMed] [Google Scholar]
- 26.Ching CB, Moussa AS, Li J, Lane BR, Zippe C, Jones JS. Does transrectal ultrasound probe configuration really matter? End fire versus side fire probe prostate cancer detection rates. J Urol. 2009;181:2077–82. doi: 10.1016/j.juro.2009.01.035. discussion 2082–3. [DOI] [PubMed] [Google Scholar]
- 27.Paul R, Korzinek C, Necknig U, Niesel T, Alschibaja M, Leyh H, Hartung R. Influence of transrectal ultrasound probe on prostate cancer detection in transrectal ultrasound-guided sextant biopsy of prostate. Urology. 2004;64:532–6. doi: 10.1016/j.urology.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Ching CB, Zaytoun O, Moussa AS, Li J, Avallone A, Jones JS. Type of transrectal ultrasonography probe influences prostate cancer detection rates on repeat prostate biopsy. BJU Int. 2012;110:E46–9. doi: 10.1111/j.1464-410X.2011.10689.x. [DOI] [PubMed] [Google Scholar]
- 29.Rom M, Pycha A, Wiunig C, Reissigl A, Waldert M, Klatte T, Remzi M, Seitz C. Prospective randomized multicenter study comparing prostate cancer detection rates of end-fire and side-fire transrectal ultrasound probe configuration. Urology. 2012;80:15–8. doi: 10.1016/j.urology.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 30.Raber M, Scattoni V, Gallina A, Freschi M, De Almeyda EP, Girolamo VD, Montorsi F, Rigatti P. Does the transrectal ultrasound probe influence prostate cancer detection in patients undergoing an extended prostate biopsy scheme? Results of a large retrospective study. BJU Int. 2012;109:672–7. doi: 10.1111/j.1464-410X.2011.10522.x. [DOI] [PubMed] [Google Scholar]
- 31.Moussa AS, El-Shafei A, Diaz E, Gao T, Zaytoun OM, Fareed K, Ulchaker JC, Jones JS. Identification of the variables associated with pain during transrectal ultrasonography-guided prostate biopsy in the era of periprostatic nerve block: the role of transrectal probe configuration. BJU Int. 2013 doi: 10.1111/j.1464-410X.2012.11689.x. [DOI] [PubMed] [Google Scholar]
- 32.Megwalu II, Ferguson GG, Wei JT, Mouraviev V, Polascik TJ, Taneja S, Black L, Andriole GL, Kibel AS. Evaluation of a novel precision template-guided biopsy system for detecting prostate cancer. BJU Int. 2008;102:546–50. doi: 10.1111/j.1464-410X.2008.07832.x. [DOI] [PubMed] [Google Scholar]
- 33.Andriole GL, Bullock TL, Belani JS, Traxel E, Yan Y, Bostwick DG, Humphrey PA. Is there a better way to biopsy the prostate? Prospects for a novel transrectal systematic biopsy approach. Urology. 2007;70:22–6. doi: 10.1016/j.urology.2007.06.1128. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan S, Marks LS, Margolis DJ, Huang J, Macairan ML, Lieu P, Fenster A. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334–42. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isebaert S, Van den Bergh L, Haustermans K, Joniau S, Lerut E, De Wever L, De Keyzer F, Budiharto T, Slagmolen P, Van Poppel H, et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J Magn Reson Imaging. 2013;37:1392–401. doi: 10.1002/jmri.23938. [DOI] [PubMed] [Google Scholar]
- 36.Delongchamps NB, Rouanne M, Flam T, Beuvon F, Liberatore M, Zerbib M, Cornud F. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 2011;107:1411–8. doi: 10.1111/j.1464-410X.2010.09808.x. [DOI] [PubMed] [Google Scholar]
- 37.Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, Huang J, Dorey FJ, Reiter RE, Marks LS. Value of Targeted Prostate Biopsy Using Magnetic Resonance-Ultrasound Fusion in Men with Prior Negative Biopsy and Elevated Prostate-specific Antigen. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park BK, Park JW, Park SY, Kim CK, Lee HM, Jeon SS, Seo SI, Jeong BC, Choi HY. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol. 2011;197:W876–81. doi: 10.2214/AJR.11.6829. [DOI] [PubMed] [Google Scholar]
- 39.Vargas HA, Akin O, Afaq A, Goldman D, Zheng J, Moskowitz CS, Shukla-Dave A, Eastham J, Scardino P, Hricak H. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188:1732–8. doi: 10.1016/j.juro.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margel D, Yap SA, Lawrentschuk N, Klotz L, Haider M, Hersey K, Finelli A, Zlotta A, Trachtenberg J, Fleshner N. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol. 2012;187:1247–52. doi: 10.1016/j.juro.2011.11.112. [DOI] [PubMed] [Google Scholar]
- 41.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Futterer JJ. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–57. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenkrantz AB, Kim S, Lim RP, Hindman N, Deng FM, Babb JS, Taneja SS. Prostate Cancer Localization Using Multiparametric MR Imaging: Comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert Scales. Radiology. 2013 doi: 10.1148/radiol.13122233. [DOI] [PubMed] [Google Scholar]
- 43.Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, Jones JS, Villers A. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171–8. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 44.Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23:43–50. doi: 10.1097/MOU.0b013e32835ad3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park BH, Jeon HG, Choo SH, Jeong BC, Seo SI, Jeon SS, Choi HY, Lee HM. Role of multiparametric 3.0 tesla magnetic resonance imaging in prostate cancer patients eligible for active surveillance. BJU Int. 2013 doi: 10.1111/bju.12423. [DOI] [PubMed] [Google Scholar]
- 46.Stamatakis L, Siddiqui MM, Nix JW, Logan J, Rais-Bahrami S, Walton-Diaz A, Hoang AN, Vourganti S, Truong H, Shuch B, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119:3359–66. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mullins JK, Bonekamp D, Landis P, Begum H, Partin AW, Epstein JI, Carter HB, Macura KJ. Multiparametric magnetic resonance imaging findings in men with low-risk prostate cancer followed using active surveillance. BJU Int. 2013;111:1037–45. doi: 10.1111/j.1464-410X.2012.11641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefkowitz GK, Taneja SS, Brown J, Melamed J, Lepor H. Followup interval prostate biopsy 3 years after diagnosis of high grade prostatic intraepithelial neoplasia is associated with high likelihood of prostate cancer, independent of change in prostate specific antigen levels. J Urol. 2002;168:1415–8. doi: 10.1016/S0022-5347(05)64463-1. [DOI] [PubMed] [Google Scholar]
- 49.Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, Zattoni F. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006;175:820–34. doi: 10.1016/S0022-5347(05)00337-X. [DOI] [PubMed] [Google Scholar]
- 51.Gallo F, Chiono L, Gastaldi E, Venturino E, Giberti C. Prognostic significance of high-grade prostatic intraepithelial neoplasia (HGPIN): risk of prostatic cancer on repeat biopsies. Urology. 2008;72:628–32. doi: 10.1016/j.urology.2007.11.115. [DOI] [PubMed] [Google Scholar]
- 52.Gokden N, Roehl KA, Catalona WJ, Humphrey PA. High-grade prostatic intraepithelial neoplasia in needle biopsy as risk factor for detection of adenocarcinoma: current level of risk in screening population. Urology. 2005;65:538–42. doi: 10.1016/j.urology.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Benecchi L, Pieri AM, Melissari M, Potenzoni M, Pastizzaro CD. A novel nomogram to predict the probability of prostate cancer on repeat biopsy. J Urol. 2008;180:146–9. doi: 10.1016/j.juro.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 54.Netto GJ, Epstein JI. Widespread high-grade prostatic intraepithelial neoplasia on prostatic needle biopsy: a significant likelihood of subsequently diagnosed adenocarcinoma. Am J Surg Pathol. 2006;30:1184–8. doi: 10.1097/01.pas.0000213324.97294.54. [DOI] [PubMed] [Google Scholar]
- 55.Godoy G, Huang GJ, Patel T, Taneja SS. Long-term follow-up of men with isolated high-grade prostatic intra-epithelial neoplasia followed by serial delayed interval biopsy. Urology. 2011;77:669–74. doi: 10.1016/j.urology.2010.07.519. [DOI] [PubMed] [Google Scholar]
- 56.Merrimen JL, Jones G, Srigley JR. Is high grade prostatic intraepithelial neoplasia still a risk factor for adenocarcinoma in the era of extended biopsy sampling? Pathology. 2010;42:325–9. doi: 10.3109/00313021003767306. [DOI] [PubMed] [Google Scholar]
- 57.Lee MC, Moussa AS, Yu C, Kattan MW, Magi-Galluzzi C, Jones JS. Multifocal high grade prostatic intraepithelial neoplasia is a risk factor for subsequent prostate cancer. J Urol. 2010;184:1958–62. doi: 10.1016/j.juro.2010.06.137. [DOI] [PubMed] [Google Scholar]
- 58.Taneja SS, Morton R, Barnette G, Sieber P, Hancock ML, Steiner M. Prostate cancer diagnosis among men with isolated high-grade intraepithelial neoplasia enrolled onto a 3-year prospective phase III clinical trial of oral toremifene. J Clin Oncol. 2013;31:523–9. doi: 10.1200/JCO.2012.41.7634. [DOI] [PubMed] [Google Scholar]
- 59.Mancuso PA, Chabert C, Chin P, Kovac P, Skyring T, Watt WH, Napaki S. Prostate cancer detection in men with an initial diagnosis of atypical small acinar proliferation. BJU Int. 2007;99:49–52. doi: 10.1111/j.1464-410X.2007.06544.x. [DOI] [PubMed] [Google Scholar]
- 60.Scattoni V, Roscigno M, Freschi M, Briganti A, Fantini GV, Bertini R, Salonia A, Montorsi F, Rigatti P. Predictors of prostate cancer after initial diagnosis of atypical small acinar proliferation at 10 to 12 core biopsies. Urology. 2005;66:1043–7. doi: 10.1016/j.urology.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Campos-Fernandes JL, Bastien L, Nicolaiew N, Robert G, Terry S, Vacherot F, Salomon L, Allory Y, Vordos D, Hoznek A, et al. Prostate cancer detection rate in patients with repeated extended 21- sample needle biopsy. Eur Urol. 2009;55:600–6. doi: 10.1016/j.eururo.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 62.Zaytoun OM, Moussa AS, Gao T, Fareed K, Jones JS. Office based transrectal saturation biopsy improves prostate cancer detection compared to extended biopsy in the repeat biopsy population. J Urol. 2011;186:850–4. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 63.Chun FK, Epstein JI, Ficarra V, Freedland SJ, Montironi R, Montorsi F, Shariat SF, Schroder FH, Scattoni V. Optimizing performance and interpretation of prostate biopsy: a critical analysis of the literature. Eur Urol. 2010;58:851–64. doi: 10.1016/j.eururo.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 64.Allen EA, Kahane H, Epstein JI. Repeat biopsy strategies for men with atypical diagnoses on initial prostate needle biopsy. Urology. 1998;52:803–7. doi: 10.1016/s0090-4295(98)00291-x. [DOI] [PubMed] [Google Scholar]
- 65.Scattoni V, Raber M, Abdollah F, Roscigno M, Deho F, Angiolilli D, Maccagnano C, Gallina A, Capitanio U, Freschi M, et al. Biopsy schemes with the fewest cores for detecting 95% of the prostate cancers detected by a 24-core biopsy. Eur Urol. 2010;57:1–8. doi: 10.1016/j.eururo.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Mabjeesh NJ, Lidawi G, Chen J, German L, Matzkin H. High detection rate of significant prostate tumours in anterior zones using transperineal ultrasound-guided template saturation biopsy. BJU Int. 2012;110:993–7. doi: 10.1111/j.1464-410X.2012.10972.x. [DOI] [PubMed] [Google Scholar]
- 67.Abdollah F, Novara G, Briganti A, Scattoni V, Raber M, Roscigno M, Suardi N, Gallina A, Artibani W, Ficarra V, et al. Trans-rectal versus trans-perineal saturation rebiopsy of the prostate: is there a difference in cancer detection rate? Urology. 2011;77:921–5. doi: 10.1016/j.urology.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 68.Nelson AW, Harvey RC, Parker RA, Kastner C, Doble A, Gnanapragasam VJ. Repeat prostate biopsy strategies after initial negative biopsy: meta-regression comparing cancer detection of transperineal, transrectal saturation and MRI guided biopsy. PLoS One. 2013;8:e57480. doi: 10.1371/journal.pone.0057480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lynn NN, Collins GN, Brown SC, O’Reilly PH. Periprostatic nerve block gives better analgesia for prostatic biopsy. BJU Int. 2002;90:424–6. doi: 10.1046/j.1464-410x.2002.02902.x. [DOI] [PubMed] [Google Scholar]
- 70.Adamakis I, Mitropoulos D, Haritopoulos K, Alamanis C, Stravodimos K, Giannopoulos A. Pain during transrectal ultrasonography guided prostate biopsy: a randomized prospective trial comparing periprostatic infiltration with lidocaine with the intrarectal instillation of lidocaine-prilocain cream. World J Urol. 2004;22:281–4. doi: 10.1007/s00345-003-0386-4. [DOI] [PubMed] [Google Scholar]
- 71.Matlaga BR, Lovato JF, Hall MC. Randomized prospective trial of a novel local anesthetic technique for extensive prostate biopsy. Urology. 2003;61:972–6. doi: 10.1016/s0090-4295(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 72.Autorino R, De Sio M, Di Lorenzo G, Damiano R, Perdona S, Cindolo L, D’Armiento M. How to decrease pain during transrectal ultrasound guided prostate biopsy: a look at the literature. J Urol. 2005;174:2091–7. doi: 10.1097/01.ju.0000181212.51025.06. [DOI] [PubMed] [Google Scholar]
- 73.Cam K, Sener M, Kayikci A, Akman Y, Erol A. Combined periprostatic and intraprostatic local anesthesia for prostate biopsy: a double-blind, placebo controlled, randomized trial. J Urol. 2008;180:141–4. doi: 10.1016/j.juro.2008.03.052. discussion 144–5. [DOI] [PubMed] [Google Scholar]
- 74.Iremashvili VV, Chepurov AK, Kobaladze KM, Gamidov SI. Periprostatic local anesthesia with pudendal block for transperineal ultrasound-guided prostate biopsy: a randomized trial. Urology. 2010;75:1023–7. doi: 10.1016/j.urology.2009.09.083. [DOI] [PubMed] [Google Scholar]
- 75.Association AU. AUA/SUNA White Paper on the Incidence, Prevention and Treatment of Complications Related to Prostate Needle Biopsy. Avaliable at: http://www.auanet.org/common/pdf/education/clinical-guidance/AUA-SUNA-PNB-White-Paper.pdf.
- 76.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, Rosario DJ, Scattoni V, Lotan Y. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–92. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 77.American Urological Association. Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis. 2008 Available at: http://www.auanet.org/content/media/antimicroprop08.pdf.
- 78.Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, Davis M, Catto JW, Avery K, Neal DE, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ. 2012;344:d7894. doi: 10.1136/bmj.d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghani KR, Dundas D, Patel U. Bleeding after transrectal ultrasonography-guided prostate biopsy: a study of 7-day morbidity after a six-, eight- and 12-core biopsy protocol. BJU Int. 2004;94:1014–20. doi: 10.1111/j.1464-410X.2004.05096.x. [DOI] [PubMed] [Google Scholar]
- 80.Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826–30. doi: 10.1016/s0090-4295(02)01958-1. [DOI] [PubMed] [Google Scholar]
- 81.Giannarini G, Mogorovich A, Valent F, Morelli G, De Maria M, Manassero F, Barbone F, Selli C. Continuing or discontinuing low-dose aspirin before transrectal prostate biopsy: results of a prospective randomized trial. Urology. 2007;70:501–5. doi: 10.1016/j.urology.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 82.Maan Z, Cutting CW, Patel U, Kerry S, Pietrzak P, Perry MJ, Kirby RS. Morbidity of transrectal ultrasonography-guided prostate biopsies in patients after the continued use of low-dose aspirin. BJU Int. 2003;91:798–800. doi: 10.1046/j.1464-410x.2003.04238.x. [DOI] [PubMed] [Google Scholar]
- 83.Halliwell OT, Yadegafar G, Lane C, Dewbury KC. Transrectal ultrasound-guided biopsy of the prostate: aspirin increases the incidence of minor bleeding complications. Clin Radiol. 2008;63:557–61. doi: 10.1016/j.crad.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 84.Chowdhury R, Abbas A, Idriz S, Hoy A, Rutherford EE, Smart JM. Should warfarin or aspirin be stopped prior to prostate biopsy? An analysis of bleeding complications related to increasing sample number regimes. Clin Radiol. 2012;67:e64–70. doi: 10.1016/j.crad.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Ihezue CU, Smart J, Dewbury KC, Mehta R, Burgess L. Biopsy of the prostate guided by transrectal ultrasound: relation between warfarin use and incidence of bleeding complications. Clin Radiol. 2005;60:459–63. doi: 10.1016/j.crad.2004.10.014. discussion 457–8. [DOI] [PubMed] [Google Scholar]
- 86.Shen PF, Zhu YC, Wei WR, Li YZ, Yang J, Li YT, Li DM, Wang J, Zeng H. The results of transperineal versus transrectal prostate biopsy: a systematic review and meta-analysis. Asian J Androl. 2012;14:310–5. doi: 10.1038/aja.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hara R, Jo Y, Fujii T, Kondo N, Yokoyoma T, Miyaji Y, Nagai A. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008;71:191–5. doi: 10.1016/j.urology.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 88.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–4. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maatman TJ, Bigham D, Stirling B. Simplified management of post-prostate biopsy rectal bleeding. Urology. 2002;60:508. doi: 10.1016/s0090-4295(02)01772-7. [DOI] [PubMed] [Google Scholar]
- 90.Dunn IBU, MJ, Kirk D. Profuse rectal bleeding after prostatic biopsy: a life-threatening complication dealt with simply. BJU International. 2000;86:910. [Google Scholar]
- 91.Smith JC, Jr, Kerr WS, Athanasoulis CA, Waltman AC, Ring EJ, Baum S. Angiographic management of bleeding secondary to genitourinary tract surgery. J Urol. 1975;113:89–92. doi: 10.1016/s0022-5347(17)59415-x. [DOI] [PubMed] [Google Scholar]
- 92.Gonen M, Resim S. Simplified treatment of massive rectal bleeding following prostate needle biopsy. Int J Urol. 2004;11:570–2. doi: 10.1111/j.1442-2042.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 93.Pacios E, Esteban JM, Breton ML, Alonso MA, Sicilia-Urban JJ, Fidalgo MP. Endoscopic treatment of massive rectal bleeding following transrectal ultrasound-guided prostate biopsy. Scand J Urol Nephrol. 2007;41:561–2. doi: 10.1080/00365590601116832. [DOI] [PubMed] [Google Scholar]
- 94.Liss MA, Chang A, Santos R, Nakama-Peeples A, Peterson EM, Osann K, Billimek J, Szabo RJ, Dash A. Prevalence and significance of fluoroquinolone resistant Escherichia coli in patients undergoing transrectal ultrasound guided prostate needle biopsy. J Urol. 2011;185:1283–8. doi: 10.1016/j.juro.2010.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, Loblaw DA, Trachtenberg J, Stanimirovic A, Simor AE, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183:963–8. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 96.Otrock ZK, Oghlakian GO, Salamoun MM, Haddad M, Bizri AR. Incidence of urinary tract infection following transrectal ultrasound guided prostate biopsy at a tertiary-care medical center in Lebanon. Infect Control Hosp Epidemiol. 2004;25:873–7. doi: 10.1086/502312. [DOI] [PubMed] [Google Scholar]
- 97.Zaytoun OM, Vargo EH, Rajan R, Berglund R, Gordon S, Jones JS. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology. 2011;77:1035–41. doi: 10.1016/j.urology.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 98.Williamson DA, Barrett LK, Rogers BA, Freeman JT, Hadway P, Paterson DL. Infectious complications following transrectal-ultrasound (TRUS) guided prostate biopsy: new challenges in the era of multi-drug resistant Escherichia coli. Clin Infect Dis. 2013 doi: 10.1093/cid/cit193. [DOI] [PubMed] [Google Scholar]
- 99.Williamson DA, Roberts SA, Paterson DL, Sidjabat H, Silvey A, Masters J, Rice M, Freeman JT. Escherichia coli bloodstream infection after transrectal ultrasound-guided prostate biopsy: implications of fluoroquinolone-resistant sequence type 131 as a major causative pathogen. Clin Infect Dis. 2012;54:1406–12. doi: 10.1093/cid/cis194. [DOI] [PubMed] [Google Scholar]
- 100.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Is repeat prostate biopsy associated with a greater risk of hospitalization? Data from SEER-Medicare. J Urol. 2013;189:867–70. doi: 10.1016/j.juro.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carey JM, Korman HJ. Transrectal ultrasound guided biopsy of the prostate. Do enemas decrease clinically significant complications? J Urol. 2001;166:82–5. [PubMed] [Google Scholar]
- 102.Jeon SS, Woo SH, Hyun JH, Choi HY, Chai SE. Bisacodyl rectal preparation can decrease infectious complications of transrectal ultrasound-guided prostate biopsy. Urology. 2003;62:461–6. doi: 10.1016/s0090-4295(03)00470-9. [DOI] [PubMed] [Google Scholar]
- 103.American Urological Association. Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis. 2012 Available at: http://www.auanet.org/content/media/antimicroprop08.pdf.
- 104.Zani EL, Clark OA, Rodrigues Netto N., Jr Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev. 2011:CD006576. doi: 10.1002/14651858.CD006576.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pepin J. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol. 2012;62:453–9. doi: 10.1016/j.eururo.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 106.Steensels D, Slabbaert K, De Wever L, Vermeersch P, Van Poppel H, Verhaegen J. Fluoroquinolone-resistant E. coli in intestinal flora of patients undergoing transrectal ultrasound-guided prostate biopsy--should we reassess our practices for antibiotic prophylaxis? Clin Microbiol Infect. 2012;18:575–81. doi: 10.1111/j.1469-0691.2011.03638.x. [DOI] [PubMed] [Google Scholar]
- 107.Taylor AK, Zembower TR, Nadler RB, Scheetz MH, Cashy JP, Bowen D, Murphy AB, Dielubanza E, Schaeffer AJ. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187:1275–9. doi: 10.1016/j.juro.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 108.Horcajada JP, Busto M, Grau S, Sorli L, Terradas R, Salvado M, Lorente JA, Gonzalez A, Knobel H. High prevalence of extended-spectrum beta-lactamase-producing enterobacteriaceae in bacteremia after transrectal ultrasound-guided prostate biopsy: a need for changing preventive protocol. Urology. 2009;74:1195–9. doi: 10.1016/j.urology.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 109.Miller J, Perumalla C, Heap G. Complications of transrectal versus transperineal prostate biopsy. ANZ J Surg. 2005;75:48–50. doi: 10.1111/j.1445-2197.2005.03284.x. [DOI] [PubMed] [Google Scholar]
- 110.Tumbarello M, Trecarichi EM, Bassetti M, De Rosa FG, Spanu T, Di Meco E, Losito AR, Parisini A, Pagani N, Cauda R. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother. 2011;55:3485–90. doi: 10.1128/AAC.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klein T, Palisaar RJ, Holz A, Brock M, Noldus J, Hinkel A. The impact of prostate biopsy and periprostatic nerve block on erectile and voiding function: a prospective study. J Urol. 2010;184:1447–52. doi: 10.1016/j.juro.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 112.Akyol I, Adayener C. Transient impotence after transrectal ultrasound-guided prostate biopsy. J Clin Ultrasound. 2008;36:33–4. doi: 10.1002/jcu.20417. [DOI] [PubMed] [Google Scholar]
- 113.Helfand BT, Glaser AP, Rimar K, Zargaroff S, Hedges J, McGuire BB, Catalona WJ, McVary KT. Prostate cancer diagnosis is associated with an increased risk of erectile dysfunction after prostate biopsy. BJU Int. 2013;111:38–43. doi: 10.1111/j.1464-410X.2012.11268.x. [DOI] [PubMed] [Google Scholar]
- 114.Fujita K, Landis P, McNeil BK, Pavlovich CP. Serial prostate biopsies are associated with an increased risk of erectile dysfunction in men with prostate cancer on active surveillance. J Urol. 2009;182:2664–9. doi: 10.1016/j.juro.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 115.Akbal C, Turker P, Tavukcu HH, Simsek F, Turkeri L. Erectile function in prostate cancer-free patients who underwent prostate saturation biopsy. Eur Urol. 2008;53:540–4. doi: 10.1016/j.eururo.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 116.Chrisofos M, Papatsoris AG, Dellis A, Varkarakis IM, Skolarikos A, Deliveliotis C. Can prostate biopsies affect erectile function? Andrologia. 2006;38:79–83. doi: 10.1111/j.1439-0272.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- 117.Berger AP, Gozzi C, Steiner H, Frauscher F, Varkarakis J, Rogatsch H, Bartsch G, Horninger W. Complication rate of transrectal ultrasound guided prostate biopsy: a comparison among 3 protocols with 6, 10 and 15 cores. J Urol. 2004;171:1478–80. doi: 10.1097/01.ju.0000116449.01186.f7. discussion 1480–1. [DOI] [PubMed] [Google Scholar]
- 118.Zaytoun OM, Anil T, Moussa AS, Jianbo L, Fareed K, Jones JS. Morbidity of prostate biopsy after simplified versus complex preparation protocols: assessment of risk factors. Urology. 2011;77:910–4. doi: 10.1016/j.urology.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 119.Challacombe B, Dasgupta P, Patel U, Amoroso P, Kirby R. Recognizing and managing the complications of prostate biopsy. BJU Int. 2011;108:1233–4. doi: 10.1111/j.1464-410X.2011.10621.x. [DOI] [PubMed] [Google Scholar]
- 120.Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology. 2013;81:1142–6. doi: 10.1016/j.urology.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 121.Djavan B, Waldert M, Zlotta A, Dobronski P, Seitz C, Remzi M, Borkowski A, Schulman C, Marberger M. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol. 2001;166:856–60. [PubMed] [Google Scholar]
- 122.Rodriguez LV, Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: a prospective study and review of the literature. J Urol. 1998;160:2115–20. doi: 10.1097/00005392-199812010-00045. [DOI] [PubMed] [Google Scholar]
- 123.Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997;157:199–202. discussion 202–3. [PubMed] [Google Scholar]
- 124.Naughton CK, Miller DC, Mager DE, Ornstein DK, Catalona WJ. A prospective randomized trial comparing 6 versus 12 prostate biopsy cores: impact on cancer detection. J Urol. 2000;164:388–92. [PubMed] [Google Scholar]
- 125.Presti JC, Jr, Chang JJ, Bhargava V, Shinohara K. The optimal systematic prostate biopsy scheme should include 8 rather than 6 biopsies: results of a prospective clinical trial. J Urol. 2000;163:163–6. [PubMed] [Google Scholar]
- 126.Gore JL, Shariat SF, Miles BJ, Kadmon D, Jiang N, Wheeler TM, Slawin KM. Optimal combinations of systematic sextant and laterally directed biopsies for the detection of prostate cancer. J Urol. 2001;165:1554–9. [PubMed] [Google Scholar]
- 127.Philip J, Ragavan N, Desouza J, Foster CS, Javle P. Effect of peripheral biopsies in maximising early prostate cancer detection in 8-, 10- or 12-core biopsy regimens. BJU Int. 2004;93:1218–20. doi: 10.1111/j.1464-410X.2004.04857.x. [DOI] [PubMed] [Google Scholar]
- 128.Shim HB, Park HK, Lee SE, Ku JH. Optimal site and number of biopsy cores according to prostate volume prostate cancer detection in Korea. Urology. 2007;69:902–6. doi: 10.1016/j.urology.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 129.Scattoni V, Roscigno M, Raber M, Deho F, Maga T, Zanoni M, Riva M, Sangalli M, Nava L, Mazzoccoli B, et al. Initial extended transrectal prostate biopsy--are more prostate cancers detected with 18 cores than with 12 cores? J Urol. 2008;179:1327–31. doi: 10.1016/j.juro.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 130.Pepe P, Aragona F. Saturation prostate needle biopsy and prostate cancer detection at initial and repeat evaluation. Urology. 2007;70:1131–5. doi: 10.1016/j.urology.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 131.Jones JS, Patel A, Schoenfield L, Rabets JC, Zippe CD, Magi-Galluzzi C. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol. 2006;175:485–8. doi: 10.1016/S0022-5347(05)00211-9. [DOI] [PubMed] [Google Scholar]
- 132.Ploussard G, Nicolaiew N, Marchand C, Terry S, Vacherot F, Vordos D, Allory Y, Abbou CC, Salomon L, de la Taille A. Prospective evaluation of an extended 21-core biopsy scheme as initial prostate cancer diagnostic strategy. Eur Urol. 2012 doi: 10.1016/j.eururo.2012.05.049. [DOI] [PubMed] [Google Scholar]