Abstract
Background
Critically ill patients are medically complex and may benefit from a multidisciplinary approach to care.
Methods
We conducted a population-based retrospective cohort study of medical patients admitted to Pennsylvania acute hospitals (N=169) from July 1, 2004 to June 30, 2006, linking a statewide hospital organizational survey to hospital discharge data. Multivariate logistic regression was used to determine the independent relationship between daily multidisciplinary rounds and 30-day mortality.
Results
112 hospitals and 107,324 patients were included in the final analysis. Overall 30-day mortality was 18.3%. After adjusting for patient and hospital characteristics, multidisciplinary care was associated with significant reductions in the odds of death (OR=0.84, 95% CI: 0.76–0.93, p=0.001). When stratifying by intensivist physician staffing, the lowest odds of death were in ICUs with high-intensity physician staffing and multidisciplinary care teams (OR=0.78, 95% CI: 0.68–0.89, p<0.0001), followed by ICUs with low intensity physician staffing and multidisciplinary care teams (OR=0.88, 95%CI: 0.79–0.97, p=0.014), compared to hospitals with low intensity physician staffing but without multidisciplinary care teams. The effects of multidisciplinary care were consistent across key subgroups including patients with sepsis, patients requiring invasive mechanical ventilation, and patients in the highest quartile of severity of illness
Conclusions
Daily rounds by a multidisciplinary team are associated with lower mortality among medical ICU patients. The survival benefit of intensivist physician staffing is in part explained by the presence of multidisciplinary teams in high-intensity staffed ICUs.
INTRODUCTION
Over four million intensive care unit (ICU) admissions occur annually in the United States each year.1 These patients are often at high risk of death—mortality for critical illness syndromes such as acute lung injury and sepsis ranges from 25 to 50% and 20% of Americans die with intensive care services.2–5 One approach to lowering ICU mortality is to optimize the organization of ICU services.6 For example, a large body of literature indicates that the presence of trained intensivist physicians is associated with improved survival,7 leading many policy makers to call for expansion of the intensivist-led model of critical care.8 Unfortunately there are not enough trained intensivists to meet either current or future demand, and only a minority of ICUs are currently staffed in this manner.9, 10
A potential complement to intensivist staffing is a multidisciplinary care model in which physicians, nurses, respiratory therapists, clinical pharmacists, and other staff members provide critical care as a team. A multidisciplinary approach acknowledges the complexities of modern critical care and the important role of communication between providers in delivering comprehensive care. Such a model is endorsed by the Society of Critical Care Medicine and the American Association of Critical Care Nurses11, 12. Yet, unlike intensivist physician staffing, little research has systematically evaluated the relationship between multidisciplinary care and outcomes, and there are few data to justify widespread adoption of this approach. Existing studies are generally single center in nature with limited ability to adjust for variations in case-mix or temporal trends between time periods.13–15
The objective of our study was to determine the independent effect of multidisciplinary care teams on the mortality of critically ill patients, using a multi-center hospital-level organizational survey and patient-level outcomes data. We also sought to determine the interaction between multidisciplinary care teams and intensivist physician staffing to see if part of the benefit of intensivist staffing could be explained by multidisciplinary care. We hypothesized that multidisciplinary care teams would be associated with improved critical care survival, particularly in settings without high-intensity physician staffing.
METHODS
Study design and patients
We conducted a retrospective cohort study using state discharge data from the Pennsylvania Health Care Cost Containment Council (PHC4). PHC4 collects clinical and administrative data on all patients discharged from non-federal hospitals within the Commonwealth of Pennsylvania. All discharges between July 1, 2004 and June 30, 2006 were eligible for the analysis. We excluded pediatric hospitals, rehabilitation hospitals, long-term acute care hospitals, and specialty surgery hospitals. Patient level data were linked to the Pennsylvania Department of Health’s death records to obtain each patient’s vital status at 30 days after admission. Hospital characteristics were obtained from the hospital-level data file maintained by PHC4 and the 2005 American Hospital Association Annual Survey.
Data on ICU care models were obtained from a cross-sectional, mixed-mode organizational survey of Pennsylvania hospitals.16 The survey was conducted between June 1, 2005 and May 31, 2006 and completed by each hospital’s chief nursing officer. A total of 118 hospitals completed the survey (69.8%). Four hospitals completed the survey but did not respond to questions about ICU care models, resulting in 114 hospitals with complete responses. Responding and non-responding hospitals were similar in bed size, community size, teaching status and other key characteristics.17 All questions about ICU structure and organization were specific to the hospital’s single ICU that treats the majority of adult, non-cardiac, non-surgical patients. Consequently this analysis is restricted to medical patients and the single ICU in each hospital that primarily serves those patients, typically either a medical or mixed medical-surgical ICU.
We identified patients admitted to an ICU using revenue codes specific to intensive care. We excluded patients less than 18 years of age at the time of admission and patients in hospitals that did not fully respond to the survey. Additionally, because we only had organizational data on the single, primary non-cardiac non-surgical ICU at each hospital, we excluded patients with a primary cardiac, surgical or neurological diagnosis as defined by International Classification of Diseases, 9th Revision—Clinical Modification (ICD-9-CM) discharge codes and discharge diagnosis related groups. Because the discharge data do not specify the actual admission ICU, for hospitals with more than one ICU (27.7% of total) it is possible that some excluded patients were actually admitted to the ICU of interest. To evaluate for possible selection bias we compared the mortality of excluded patients in hospitals with the different types of multidisciplinary care models described below.
Variables and risk adjustment
The primary exposure of interest was each hospital’s response to the question: “Does the intensive care unit have daily multidisciplinary ICU rounds consisting of the physician, nurse, and other health care professionals (e.g. social worker, respiratory therapist, pharmacist)?” This response was coded as either yes or no. Each study ICU operated under a single care model. We did not have more detailed information on the exact components of the multidisciplinary team or the training of the physician that directed rounds. The secondary exposure of interest was each ICU’s physician staffing model. Physician staffing models were reported in the survey as no intensivist, optional intensivist consult, mandatory intensivist consult and primary intensivist management. These groups were further categorized into high-intensity (mandatory consult or primary intensivist management) and low-intensity (optional intensivist consult or no intensivist) physician staffing, according to prior reports.7
We also sought to evaluate the relationship between multidisciplinary care and intensivist physician staffing with respect to their effect on mortality. We created four groups of hospitals based on their combination of multidisciplinary care and physician staffing models: (1) low-intensity staffing without multidisciplinary care teams; (2) low intensity staffing with multi-disciplinary care teams; (3) high-intensity staffing with multidisciplinary care teams; and (4) high-intensity staffing without multidisciplinary care teams. We excluded hospitals in the last group (high-intensity staffing without multi-disciplinary care teams) as there were not enough hospitals to accurately estimate outcomes in that category.
The primary outcome variable was mortality within 30 days of hospital admission. We controlled for potential confounding variables that could be related to the multidisciplinary care teams, physician staffing model, and mortality. Risk adjustment variables were selected a priori and included age, gender, admission source (emergency room, inter-hospital transfer, or direct), chronic conditions as defined by Elixhauser comorbidities modeled as indicator covariates, ventilation status on admission defined by ICD-9-CM procedure codes, primary diagnosis as defined by ICD-9-CM diagnosis codes, hospital teaching status determined by each hospital’s resident-to-bed ratio (non-teaching: ratio=0; small teaching: ratio between 0 and 0.2; large teaching: ratio >0.2), ICU type (medical, mixed medical-surgical, or mixed medical-coronary), region of Pennsylvania defined by PHC4, and each hospital’s average annual admission volume.18–21 We further controlled for severity of illness using the MediQual Atlas probability of in-hospital death, a validated risk adjustment tool for hospitalized patients using key clinical and demographic variables measured on admission.22 Reported areas under the curve for MediQual Atlas mortality prediction in medical patients range from 0.837 to 0.874, which are comparable to other common ICU risk-adjustment systems.23, 24 The MediQual Atlas score is automatically calculated by PHC4 on patients admitted to Pennsylvania hospitals but may be absent due to missing clinical data.
Analysis
We compared descriptive statistics for hospitals and patients by care model group using the chi-square test or the t-test, as appropriate. To determine the independent effect of multidisciplinary care and high-intensity staffing on 30-day mortality, we created patient-level multivariate logistic regression models controlling for potential confounders described above. We modeled categorical variables using indicator covariates and continuous variables using quadratic splines. We created three separate models: a model with multidisciplinary care teams alone (model 1), a model with physician staffing alone (model 2), and a model with the grouped multidisciplinary care team/physician staffing variable (model 3). The last model was designed to evaluate the interaction between high-intensity staffing and multidisciplinary care, given that we could not control for both in a single multivariate model. In all models we used generalized estimating equations with robust Huber-White confidence intervals to account for potential clustering within hospitals.25 We assessed model discrimination using the C-statistic. We also performed three prospectively-defined subgroup analyses to examine the effects of staffing models on high risk patients. Subgroups of interest were patients in the top quartile of the MediQual Atlas score, patients receiving invasive mechanical ventilation as defined by ICD-9-CM procedure code, and patients with severe sepsis defined using previously validated criteria.2, 26
To account for missing MedQual scores we performed multiple imputation using Markov Chain Monte Carlo simulation, creating 10 imputed datasets and combing regression results according to previously described method.27, 28 We also performed a sensitivity analysis dropping patients with missing MedQual scores under the assumption of missing completely at random (i.e., purely for administrative rather than clinical reasons).29 For the subgroup of patients in the highest quartile we MediQual Atlas score we analyzed only the complete cases.
The multiple imputation was performed with SAS 9.2 (Cary, NC). Other statistical analyses were performed with Stata 10.0 (College Station, TX). All tests are two-tailed, and a p value less than or equal to 0.05 was considered significant. This research was approved by the Institutional Review Boards of the University of Pennsylvania and the University of Pittsburgh.
RESULTS
A total of 471,112 patients were admitted to Pennsylvania hospital ICUs during the study period (Figure). We excluded 55 hospitals with survey non-response or incomplete response, and two hospitals with high-intensity staffing and no multi-disciplinary care teams. Further excluding patients with non-medical diagnoses resulted in 112 hospitals and 107,324 patients in the final analysis. The care model in the ICU of interest was low-intensity/no multidisciplinary care in 54 hospitals (48.2%); low-intensity/ multidisciplinary care in 36 hospitals (32.1%); and high-intensity/multidisciplinary care in 22 hospitals (19.6%). Among excluded patients, mortality was similar between the different staffing groups (low-intensity/no multidisciplinary care: 8.0%; low-intensity/multidisciplinary care: 9.3%; high-intensity/multidisciplinary care: 8.7%).
Hospital characteristics are shown in Table 1. High-intensity physician staffing and multi-disciplinary care teams were more common in teaching hospitals and hospitals with critical care fellowships. High-intensity physician staffing and multi-disciplinary care teams were also more common in large hospitals and hospitals with a high volume of annual admissions. In hospitals with high-intensity staffing, the ICU of interest tended to be a medical specialty ICU, compared to hospitals with low-intensity staffing, where the ICU of interest tended to be a mixed medical-surgical or mixed medical-cardiac ICU. High-intensity physician staffed ICUs also tended to be larger than low-intensity staffed ICUs.
Table 1.
Hospital and intensive care unit characteristics
| Low intensity/no multidisciplinary care (n=54) |
Low intensity/multidisciplinary care (n=36) |
High intensity/multidisciplinary care (n=22) |
p-value | |
|---|---|---|---|---|
| Hospital characteristics | ||||
| Teaching status, No. (%) | ||||
| Non-teaching | 42 (78) | 22 (61) | 7 (32) | 0.001 |
| Small teaching | 11 (20) | 9 (25) | 8 (36) | |
| Large teaching | 1 (2) | 5 (14) | 7 (32) | |
| Critical Care Fellowship, No. (%) | 1 (2) | 3 (8) | 4 (18) | 0.041 |
| Number of beds, median [IQR] | 128 [77–208] | 198 [84–311] | 286 [144–645] | <0.001 |
| ICUs in hospital | ||||
| Median [IQR] | 1 [1–1] | 1 [1–2] | 1 [1–3] | 0.002 |
| Range | 1–5 | 1–5 | 1–5 | |
| Ownership, No. (%) | ||||
| Non-profit | 52 (96) | 36 (100) | 19 (86) | 0.060 |
| For profit | 2 (4) | 0 (0) | 3 (14) | |
| Annual non-surgical ICU admissions, median [IQR] | 272 [147–402] | 380 [196–705] | 588 [304–1103] | 0.036 |
| ICU characteristics | ||||
| ICU type, No. (%) | ||||
| Medical | 2 (4) | 4 (11) | 9 (41) | 0.001 |
| Combined medical-coronary | 19 (35) | 9 (25) | 2 (9) | |
| Combined medical-surgical | 33 (61) | 23 (64) | 11 (50) | |
| Number of beds, median [IQR] | 11 [6–16] | 15 [9–29] | 21 [16–48] | <0.001 |
ICU = intensive care unit; IQR = interquartile range
Patient demographics were generally similar between groups (Table 2). Patients in ICUs with high-intensity physician staffing and multidisciplinary care were more likely to require mechanical ventilation, were more likely to carry a diagnosis of sepsis, and had a higher probability of in-hospital death. Accordingly, unadjusted in-hospital mortality was higher in ICUs with high-intensity staffing and multidisciplinary care teams (16.4%) compared to hospitals with low-intensity staffing and multidisciplinary care teams (13.9%) and low-intensity staffing without multidisciplinary care teams (11.2%).
Table 2.
Patient Characteristics
| Variable | Low intensity/no multidisciplinary care (n=39, 549) |
Low intensity/ multidisciplinary care (n=34, 348) |
High intensity/ multidisciplinary care (n=33, 427) |
p-value |
|---|---|---|---|---|
| Age, mean (SD) | 65.4 (17.9) | 64.3 (18.6) | 62.0 (17.8) | <0.001 |
| Female (%) | 50.7 | 50.7 | 48.9 | <0.001 |
| Race (%) | ||||
| White | 90.6 | 82.8 | 72.8 | <0.001 |
| Black | 5.8 | 12.8 | 18.2 | |
| Other | 3.6 | 4.4 | 9.1 | |
| Admission source (%) | ||||
| Emergency department | 76.1 | 85.1 | 66.3 | <0.001 |
| Direct admit | 18.8 | 10.2 | 20.0 | |
| Hospital transfer | 1.0 | 2.8 | 6.5 | |
| SNF transfer | 2.6 | 1.2 | 1.6 | |
| Other facility | 1.5 | 0.7 | 5.6 | |
| Charlson Comorbidity Index (%) | ||||
| 0 | 21.4 | 22.5 | 20.1 | <0.001 |
| 1–2 | 45.0 | 43.8 | 43.9 | |
| 3–4 | 21.0 | 20.6 | 21.3 | |
| 5+ | 12.6 | 13.1 | 14.7 | |
| Primary Diagnosis (%) | ||||
| COPD/ Asthma | 5.8 | 4.9 | 3.6 | <0.001 |
| Gastrointestinal-other | 6.1 | 4.9 | 4.5 | |
| Gastrointestinal bleed | 4.9 | 4.9 | 4.2 | |
| Human immunodeficiency virus | 0.2 | 0.3 | 0.5 | |
| Hypertension | 3.1 | 3.0 | 2.5 | |
| Complication | 4.4 | 4.1 | 6.8 | |
| General | 20.1 | 17.7 | 16.0 | |
| Infection | 4.1 | 4.0 | 3.3 | |
| Liver disease | 2.1 | 2.3 | 3.7 | |
| Neurological infection | 0.3 | 0.4 | 0.7 | |
| Oncology | 3.7 | 4.0 | 6.7 | |
| Overdose | 6.6 | 8.7 | 6.3 | |
| Pneumonia | 9.6 | 8.8 | 6.3 | |
| Psychiatric | 1.6 | 2.2 | 1.4 | |
| Pulmonary-other | 5.2 | 5.6 | 5.2 | |
| Respiratory failure | 10.9 | 12.9 | 14.8 | |
| Sepsis | 8.2 | 9.0 | 10.6 | |
| Vascular | 2.5 | 2.5 | 3.0 | |
| Requiring mechanical ventilation (%) | 17.0 | 26.2 | 31.3 | <0.001 |
| Sepsis (%) | 7.4 | 10.8 | 14.0 | <0.001 |
| ICU length of stay, median [IQR] | 2 [1–4] | 2 [1–5] | 2 [1–5] | <0.001 |
| Hospital length of stay, median [IQR] | 5 [3–9] | 5 [3–10] | 6 [3–11] | <0.001 |
| Unadjusted in-hospital mortality (%) | 11.2 | 13.9 | 16.4 | <0.001 |
| MediQual predicted death probability (%)* | ||||
| 0.00–0.05 | 5 | 3.7 | 2.6 | <0.001 |
| 0.05–0.10 | 29.4 | 26.7 | 25.0 | |
| 0.10–0.15 | 17.1 | 16.4 | 16.3 | |
| 0.15–0.25 | 19.3 | 19.5 | 19.3 | |
| 0.25–1.0 | 29.2 | 33.9 | 36.8 | |
| Discharge location for survivors (%) | ||||
| Home | 62.1 | 55.8 | 61.6 | <0.001 |
| Other hospital | 10.0 | 10.1 | 5.6 | |
| Skilled nursing facility | 18.5 | 22.0 | 19.0 | |
| LTAC | 1.5 | 2.5 | 3.7 | |
| Hospice | 1.9 | 3.0 | 2.7 | |
| Other | 6.0 | 6.6 | 7.4 |
Percentages are reflective of patients with non-missing data (n = 86,286)
SNF = skilled nursing facility; COPD = chronic obstructive pulmonary disease; IQR = interquartile range
In the primary analysis all multivariate models had C-statistics ≥ 0.85. Controlling for hospital and patient characteristics and accounting for clustering by center, but not accounting for intensivist staffing, multidisciplinary care teams were associated with a 16% reduction in the odds of death (OR=0.84, 95% CI: 0.76 – 0.93, p = 0.001) (Table 3). High-intensity physician staffing alone was associated with a similar reduction in the odds of death (OR=0.84, 95% CI: 0.75 – 09.4, p=0.002). When we simultaneously evaluated multi-disciplinary care teams and high-intensity staffing in a stratified model, the lowest odds of death were in ICUs with both high-intensity physician staffing and multidisciplinary care teams (OR=0.78, 95% CI: 0.68–0.89, p<0.0001), followed by ICUs with multidisciplinary care teams and low-intensity physician staffing (OR=0.88, 95%CI: 0.79–0.97, p=0.014), compared to hospitals without either multidisciplinary care teams or low intensity staffing.
Table 3.
Association between intensivist physician staffing and 30-day mortality for all patients*
| Model 1: Multidisciplinary care staffing alone | Model 2: Intensivist physician staffing alone | Model 3: Interaction between intensivist physician staffing and multidisciplinary care teams | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) |
P value | Variable | Odds Ratio (95% CI) |
P value | Variable | Odds Ratio (95% CI) |
P value |
| No multidisciplinary care | 1.00 | — | Low intensity | 1.00 | — | Low intensity/no multidisciplinary care | 1.00 | — |
| Multidisciplinary care | 0.84 (0.76–0.93) |
0.001 | High intensity | 0.84 (0.75–0.94) |
0.002 | Low intensity/ multidisciplinary care | 0.88 (0.79–0.97) |
0.014 |
| High intensity/ multidisciplinary care | 0.78 (0.68–0.89) |
<0.0001 | ||||||
Estimates are adjusted for age, gender, admission source, Elixhauser comorbidities, mechanical ventilation status, MediQual severity score, primary diagnosis, teaching status, ICU type, region and annual volume. Total n = 107,301.
In the subgroup analyses C statistics ranged from 0.71 to 0.78. Results were similar in the three planned subgroup analyses, with significant mortality reductions observed with multi-disciplinary care teams and high intensity physician staffing in patients with sepsis, patients requiring mechanical ventilation, and patients in the highest quartile of severity of illness (Table 4). Odds ratios from the complete case analyses excluding the 21,038 patients with missing MediQual scores (19.6% of total) scores were all within 0.01 of the odds ratios from the multiple imputation analyses, with similar confidence intervals.
Table 4.
Planned subgroup analyses for association between intensivist physician staffing and 30-day mortality*
| Subgroup | Model 1: Multidisciplinary care staffing alone | Model 2: Intensivist physician staffing alone | Model 3: Interaction between intensivist physician staffing and multidisciplinary care | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) |
P value | Variable | Odds Ratio (95% CI) |
P value | Variable | Odds Ratio (95% CI) |
P value | |
| Ventilated patients | No multidisciplinary care | 1.00 | — | Low intensity | 1.00 | — | Low intensity/no multidisciplinary care | 1.00 | — |
| (n=26,177) | Multidisciplinary care | 0.81 (0.71–0.92) |
0.001 | High intensity | 0.83 (0.71–0.96) |
0.015 | Low intensity/ multidisciplinary care | 0.85 (0.74–0.96) |
0.010 |
| High intensity/ multidisciplinary care | 0.74 (0.63–0.88) |
0.001 | |||||||
| Top quartile of MedQual | No multidisciplinary care | 1.00 | — | Low intensity | 1.00 | — | Low intensity/no multidisciplinary care | 1.00 | — |
| (n=21,591) | Multidisciplinary care | 0.83 (0.74–0.93) |
0.001 | High intensity | 0.82 (0.73–0.91) |
<0.001 | Low intensity/ multidisciplinary care | 0.88 (0.78–0.99) |
0.028 |
| High intensity/ multidisciplinary care | 0.76 (0.67–0.85) |
<0.001 | |||||||
| Sepsis patients | No multidisciplinary care | 1.00 | — | Low intensity | 1.00 | — | Low intensity/no multidisciplinary care | 1.00 | — |
| (n=11,321) | Multidisciplinary care | 0.80 (0.70–0.92) |
0.002 | High intensity | 0.81 (0.70–0.94) |
0.005 | Low intensity/ multidisciplinary care | 0.85 (0.73–0.98) |
0.028 |
| High intensity/ multidisciplinary care | 0.74 (0.63–0.87) |
<0.001 | |||||||
Estimates are adjusted for age, gender, admission source, Elixhauser comorbidities, mechanical ventilation status, MediQual severity score, primary diagnosis, teaching status, ICU type, region and annual volume.
DISCUSSION
In a large population-based sample of hospitals, daily rounds by a multidisciplinary care team were independently associated with lower mortality in ICU patients. In a stratified model that included intensivist physician staffing, multidisciplinary care was associated with a significant mortality reduction in ICUs with low-intensity physician staffing, conveying a decreased risk of death that approached, but did not equal, ICUs with high-intensity physician staffing. These results suggest that in hospitals without high-intensity physician staffing multi-disciplinary rounds are likely to improve patient outcomes.
Several mechanisms may explain these findings. Multidisciplinary rounds may facilitate implementation of best clinical practices such as evidence-based treatments for acute lung injury, sepsis, and prevention of ICU complications.30–32 Pharmacist participation on rounds is associated with fewer adverse-drug events33 and alone may be associated with lower mortality among ICU patients.34 Multidisciplinary rounds may also improve communication between care providers.35 Communication may facilitate implementation of respiratory-therapy and nurse-driven protocols for weaning and sedation, which can reduce duration of mechanical ventilation and shorten ICU length of stay.36, 37
Our findings have important implications for the organization of critical care services. First, this study provides empiric evidence to support a multidisciplinary model of critical care. Based on these results and expert opinion voiced in consensus guidelines, it is reasonable for hospitals to implement routine multidisciplinary rounds when staffing capabilities allow.11 Additionally, our results provide insight into ways to improve mortality in ICUs without intensivist physician staffing. Workforce analyses suggest that there are not enough intensivists to meet demand, and as a consequence only a minority of ICUs in the United States are staffed by trained intensivists.9, 10 ICU directors report that lack of enough trained of intensivists is a key barrier to implementing an intensivist model of care.38 Our study shows that hospitals without the ability to implement high-intensity physician staffing can still achieve significant mortality reductions by implementing a multidisciplinary, team-based approach.
Our results also confirm prior studies showing that high-intensity physician staffing lowers mortality in the ICU. Several cohort studies indicate that intensivist-led critical care is associated with improved outcomes in the ICU.7, 39, 40 The benefit of intensivists was recently called into question by a study suggesting higher mortality in ICUs staffed by intensivists.41 Although the etiology of this discrepant finding is unknown, possibilities include the self-selected nature of hospitals in the cohort, selective referral of high-risk patients to intensivists, use of in-hospital rather than 30-day mortality which can lead to discharge bias.42–44 We demonstrate a benefit from intensivist-staffing in a population-based cohort of hospitals consistent with prior reports. Additionally, our study expands the literature on intensivist staffing by demonstrating a potential mechanism for the effect. Despite the wealth of literature on intensivist physician staffing, few studies are directed at understanding how intensivists achieve superior outcomes.45 Our results show that the benefit of intensivists is due, at least in part, to the multidisciplinary care models typically found in intensivist-led ICUs.
We did not have detailed information about the characteristics of the multidisciplinary team, such as team size, the training and experience of the physician leading the team, or the exact ancillary staff members comprising the team. Given the wording of our questionnaire, we expect that at a minimum the multidisciplinary teams included the primary physician, the bedside nurse, and at least one other care provider. Still, although our study provides empirical support for a multidisciplinary approach to care, we are unable to identify either specific attributes of the multidisciplinary team or an optimal team size that may be associated with improved outcomes. Questions remain about how medical teams function, and even what defines a medical team in practice. These topics are important areas for future research. We also do not know the ideal role of the physician in multidisciplinary rounds. In some ICUs a single intensivist-trained physician may direct rounds on all ICU patients, while in others multiple physicians of varying backgrounds may lead rounds on their patients at different times. Each of these organizational styles would meet our definition of multidisciplinary rounds. Future work should empirically examine the benefits and limitations of these different care models.
Our analysis has several limitations. First, we only had data on care models at a single ICU at each hospital—the ICU primarily providing care to non-surgical, non-cardiac patients. We excluded patients unlikely to have received care in that ICU. These results do not necessarily generalize to surgical, cardiac, and neurological patients, or specialty ICUs serving those populations. Because the PHC4 discharge data do not specify exactly in which ICU the patient received care, in hospitals with more than one ICU it is possible that we excluded some patients that received care in the ICU of interest, and included some patients that did not. The finding that mortality was similar among excluded patients in each group suggests that any misclassification bias would be minimal. Second, we did not have organizational data on 55 hospitals that not fully complete our survey. Although survey respondents were similar to non-respondents, response bias cannot be ruled out. Finally, we were unable to observe the effects of high intensity staffing models without multidisciplinary care teams due to a low number of hospitals in this category. We cannot comment on whether multidisciplinary care teams improve outcomes within high-intensity staffed ICUs.
With the aging of the population, demand for critical care is certain to rise in the coming years. Evidence-based strategies on how to best organize and manage ICUs are needed.46 We demonstrate that daily rounds by a multidisciplinary care team are associated with lower mortality in the ICU. Clinicians, hospital administrators and policy makers can use these results to help optimally organize critical care services and potentially improve outcomes for critically ill patients in hospitals where intensivist staffing is not available.
Figure.
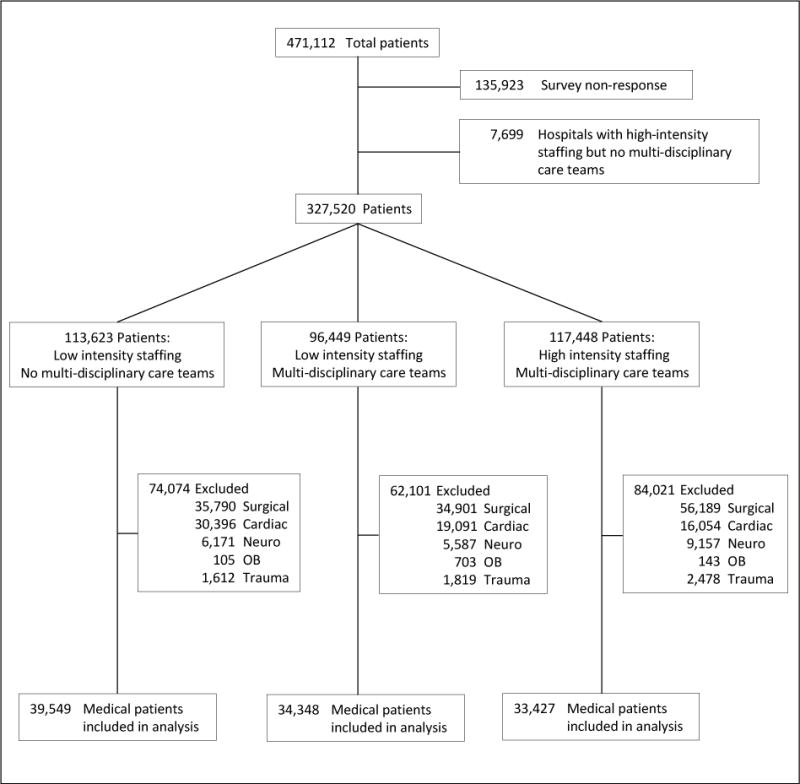
Flow diagram of patients into the study. OB = obstetric; Neuro = neurological.
Acknowledgments
Pennsylvania Health Care Cost Containment Council disclaimer: The following statement is provided and required by the Pennsylvania Health Care Cost Containment Council (PHC4): PHC4 has provided this data in an effort to further PHC4’s mission of educating the public and containing health care costs in Pennsylvania. PHC4, its agents and staff, have made no representation, guarantee, or warranty, expressed or implied, that the data—financial, patient, payor and physician specific information—are error-free, or that the use of the data will avoid differences of opinion or interpretation, or disputes with those who use published reports or purchased data. PHC4, its agents and staff, will bear no responsibility or liability for the results of the analysis, or consequences of its use.
Role of the sponsors: This work was funded by grants from the National Institutes of Health’s National Institute on Aging (K08AG21921, Barnato) and National Heart Lung and Blood Institute (K23HL082650, Kahn); and a grant from the Leonard Davis Institute of Health Economics (Kahn). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript
Footnotes
This work was presented in abstract form on July 14, 2009 at the 7th World Congress on Health Economics in Beijing, China.
There are no financial conflicts of interest to disclose.
Data access and responsibility: Dr. Kahn had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985–2000: an analysis of bed numbers, use, and costs. Crit Care Med. 2004;32:1254–9. doi: 10.1097/01.ccm.0000128577.31689.4c. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–43. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–9. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmel S, Rowan K. Variation in intensive care unit outcomes: a search for the evidence on organziational factors. Curr Opin Crit Care. 2001;7:284–296. doi: 10.1097/00075198-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–62. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 8.Milstein A, Galvin RS, Delbanco SF, Salber P, Buck CR., Jr Improving the safety of health care: the leapfrog initiative. Eff Clin Pract. 2000;3:313–6. [PubMed] [Google Scholar]
- 9.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J., Jr Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762–70. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 10.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA. Critical care delivery in the United States: Distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016–1024. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 11.Brilli RJ, Spevetz A, Branson RD, et al. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29:2007–19. doi: 10.1097/00003246-200110000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Durbin CG., Jr Team model: advocating for the optimal method of care delivery in the intensive care unit. Crit Care Med. 2006;34:S12–7. doi: 10.1097/01.CCM.0000199985.72497.D1. [DOI] [PubMed] [Google Scholar]
- 13.Burns SM, Earven S, Fisher C, et al. Implementation of an institutional program to improve clinical and financial outcomes of mechanically ventilated patients: one-year outcomes and lessons learned. Crit Care Med. 2003;31:2752–63. doi: 10.1097/01.CCM.0000094217.07170.75. [DOI] [PubMed] [Google Scholar]
- 14.Young MP, Gooder VJ, Oltermann MH, Bohman CB, French TK, James BC. The impact of a multidisciplinary approach on caring for ventilator-dependent patients. Int J Qual Health Care. 1998;10:15–26. doi: 10.1093/intqhc/10.1.15. [DOI] [PubMed] [Google Scholar]
- 15.Sebat F, Johnson D, Musthafa AA, et al. A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest. 2005;127:1729–43. doi: 10.1378/chest.127.5.1729. [DOI] [PubMed] [Google Scholar]
- 16.Lin CY, Farrell MH, Lave JR, Angus DC, Barnato AE. Organizational determinants of hospital end-of-life treatment intensity. Med Care. 2009;47:524–30. doi: 10.1097/MLR.0b013e31819261bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C, Farrell MH, Lave JR, Angus DC, Barnato AE. Organizational determinants of hospital end-of-life treatment intensity. Medical Care. 2009 doi: 10.1097/MLR.0b013e31819261bd. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 19.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–9. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 22.Iezzoni LI, Moskowitz MA. A clinical assessment of MedisGroups. JAMA. 1988;260:3159–63. doi: 10.1001/jama.260.21.3159. [DOI] [PubMed] [Google Scholar]
- 23.Iezzoni LI. The risks of risk adjustment. JAMA. 1997;278:1600–7. doi: 10.1001/jama.278.19.1600. [DOI] [PubMed] [Google Scholar]
- 24.Kuzniewicz MW, Vasilevskis EE, Lane R, et al. Variation in ICU risk-adjusted mortality: impact of methods of assessment and potential confounders. Chest. 2008;133:1319–27. doi: 10.1378/chest.07-3061. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 26.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177:279–84. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambler G, Omar RZ, Royston P. A comparison of imputation techniques for handling missing predictor values in a risk model with a binary outcome. Stat Methods Med Res. 2007;16:277–98. doi: 10.1177/0962280206074466. [DOI] [PubMed] [Google Scholar]
- 28.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–98. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 29.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–64. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 30.The Acute Respiratory Distress Syndrome Network. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 31.Ferrer R, Artigas A, Levy MM, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 32.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 33.Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282:267–70. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 34.MacLaren R, Bond CA, Martin SJ, Fike D. Clinical and economic outcomes of involving pharmacists in the direct care of critically ill patients with infections. Crit Care Med. 2008;36:3184–9. doi: 10.1097/CCM.0b013e31818f2269. [DOI] [PubMed] [Google Scholar]
- 35.Shortell SM, Zimmerman JE, Rousseau DM, et al. The performance of intensive care units: does good management make a difference? Med Care. 1994;32:508–25. doi: 10.1097/00005650-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–9. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 37.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 38.Kahn JM, Matthews FA, Angus DC, Barnato AE, Rubenfeld GD. Barriers to implementing the Leapfrog Group recommendations for intensivist physician staffing: a survey of intensive care unit directors. J Crit Care. 2007;22:97–103. doi: 10.1016/j.jcrc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Treggiari MM, Martin DP, Yanez ND, Caldwell E, Hudson LD, Rubenfeld GD. Effect of intensive care unit organizational model and structure on outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2007;176:685–90. doi: 10.1164/rccm.200701-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathens AB, Rivara FP, Mackenzie EJ, et al. The impact of an intensivist-model ICU on trauma-related mortality. Ann Surg. 2006;244:545–554. doi: 10.1097/01.sla.0000239005.26353.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med. 2008;148:801–9. doi: 10.7326/0003-4819-148-11-200806030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasilevskis EE, Kuzniewicz MW, Dean ML, et al. Relationship between discharge practices and intensive care unit in-hospital mortality performance: evidence of a discharge bias. Med Care. 2009;47:803–12. doi: 10.1097/MLR.0b013e3181a39454. [DOI] [PubMed] [Google Scholar]
- 43.Kahn JM, Kramer AA, Rubenfeld GD. Transferring critically ill patients out of hospital improves the standardized mortality ratio: a simulation study. Chest. 2007;131:68–75. doi: 10.1378/chest.06-0741. [DOI] [PubMed] [Google Scholar]
- 44.Rubenfeld GD, Angus DC. Are intensivists safe? Ann Intern Med. 2008;148:877–9. doi: 10.7326/0003-4819-148-11-200806030-00010. [DOI] [PubMed] [Google Scholar]
- 45.Kahn JM, Brake H, Steinberg KP. Intensivist physician staffing and the process of care in academic medical centres. Qual Saf Health Care. 2007;16:329–33. doi: 10.1136/qshc.2007.022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnato AE, Kahn JM, Rubenfeld GD, et al. Prioritizing the organization and management of intensive care services in the United States: the PrOMIS Conference. Crit Care Med. 2007;35:1003–11. doi: 10.1097/01.CCM.0000259535.06205.B4. [DOI] [PubMed] [Google Scholar]


