SUMMARY
Development of a male hormonal contraceptive has been challenging ascribable to the failure to adequately suppress spermatogenesis in 5–10% of men. Methods to identify incomplete suppressors early in treatment might identify men most responsive to male hormonal contraceptives. We hypothesized that serum hormone and gonadotropin concentrations after 4 weeks of transdermal treatment with testosterone and Nestorone in a contraceptive trial would be associated with suppression of sperm concentrations to <1 million/mL after 24 weeks. Indeed, luteinizing hormone or follicle-stimulating hormone concentrations greater than 1 IU/L after 4 weeks of transdermal testosterone/nestorone treatment were 97% sensitive for predicting failure to suppress spermatogenesis after 24 weeks of treatment. Serum nestorone concentrations were significantly associated with suppression, but serum testosterone concentrations were not. Early suppression of gonadotropins is associated with, but does not ensure, adequate suppression of spermatogenesis. This information may allow for rapid identification of non-responders in male hormonal contraceptive trials.
Keywords: contraception, gonadotropins, nestorone, spermatogenesis, testosterone
INTRODUCTION
Worldwide, nearly half of all pregnancies are unintended, contributing to substantial negative health outcomes including maternal morbidity and mortality, complicated pregnancy outcomes and significant societal burdens (Sedgh et al., 2007). Despite a wide variety of reversible hormonal contraceptive methods available for women (Blumenthal & Edelman, 2008), no new reversible contraception options have been developed for men since the invention of condoms over 400 years ago. Many reversible male hormonal contraceptive regimens have been tested in clinical trials, using either testosterone alone or testosterone in combination with a progestin, to suppress gonadotropin secretion (Page et al., 2008). Reduced levels of circulating gonadotropins cause suppression of intratesticular testosterone concentrations and the combination of reduced intratesticular testosterone and reduced serum follicle-stimulating hormone (FSH) levels markedly suppresses spermatogenesis. Most trials evaluating male hormonal contraceptive regimens focus on suppression of spermatogenesis (assessed by seminal fluid sperm concentration) as the primary outcome for evaluating effectiveness of the regimen (Bebb et al., 1996; Kamischke et al., 2000, 2002; Gu et al., 2004; Page et al., 2006; Mommers et al., 2008). When effective at almost completely suppressing spermatogenesis (to < 1 million spermatozoa/mL seminal fluid), male hormonal contraceptive regimens are as efficacious as female hormonal contraceptive regimens at preventing pregnancy (World Health Organization, 1990, 1996; Gu et al., 2003, 2009; Turner et al., 2003; Nieschlag, 2007). One of the major challenges of male-focused regimens is the failure to sufficiently suppress spermatogenesis in all men to provide effective contraception (Liu et al., 2008).
Given the extended life cycle of developing spermatozoa (approximately 72 days in man), it takes about 3 months to determine whether a male hormonal contraceptive regimen effectively suppresses spermatogenesis in a given individual. Previous work has demonstrated that the addition of a progestin to androgens, the route of hormone administration, and ethnicity of the user are factors that significantly impact the rate and extent of suppression of spermatogenesis (Liu et al., 2008). However, these characteristics do not predict whether a particular regimen will be effective for a given individual. Thinking ahead to the eventual approved use of a male hormonal contraceptive regimen, an early means of predicting who will respond to the hormonal contraceptive method would help identify individuals that need dose adjustment or consideration of using a different contraceptive method. We hypothesized that early suppression of serum gonadotropin concentrations or increase in serum androgen or progestin concentrations would be associated with the ultimate clinical response (suppression of sperm concentration to <1 million/mL) to a male hormonal contraceptive regimen. In this study, to circumvent the known pulsatility of gonadotropin release from the pituitary in normal men (Baker et al., 1975; Matsumoto & Bremner, 1984; Plymate et al., 1989), we performed multiple measurements of gonadotropins at each time point to determine if multiple gonadotropin measurements in sequence (within a 60-min time period) would add to the model for identifying response, or failure to suppress sperm concentration to <1 million/mL. We analysed data from a recently completed male hormonal contraceptive trial using a novel transdermal combination regimen of testosterone gel and Nestorone gel to examine these relationships (Ilani et al., 2012).
MATERIALS AND METHODS
Study design
The study design has been previously reported (Ilani et al., 2012). In brief, healthy male volunteers between the ages of 18 and 50 years old, with normal andrological exam, normal baseline hormones and normal seminal fluid analysis, were enrolled at two academic medical centres as part of the Contraceptive Clinical Trial Network, the University of Washington Center for Research In Reproduction and Contraception, Seattle, WA, and the Center for Men's Health, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles, CA.
Of the 210 men screened, 99 met all criteria for enrolment and were randomized to one of three treatment groups: (i) Testosterone (T) gel 10 g + nestorone (NES) placebo gel (T+NES 0), (ii) T gel 10 g + 8 mg NES gel (T+NES 8), (iii) T gel 10 g + 12 mg NES gel (T+NES 12). Among the 111 men who did not enrol following screening, 42 withdrew consent or were lost to follow-up, 33 were excluded attributable to abnormal physical exam or routine laboratory measurement, 16 had possible substance abuse concerns, 15 had a sperm concentration below 15 million/mL on two consecutive semen analyses, and 5 had other reasons for not enrolling. T gel (Testogel) was manufactured by Besins Healthcare S.A. (Brussels, Belgium) and supplied by GOOGLIFE Healthcare (Den Haag, Netherlands). A quantity of 10 gm of 1% gel applied to the upper arms daily delivered approximately 10 mg T to the body per day. NES and placebo gel were produced by Antares Pharma (Basel, Switzerland) based on a formulation developed by the Population Council (New York, NY, USA). NES gel, when administered transdermally at doses of 8 and 12 mg daily, delivers approximately 800 and 1200 μg daily respectively (Sitruk-Ware, 1995). After randomization, gels were applied daily for 20–24 weeks and subjects returned for serum hormone concentrations after 2 weeks of treatment. After 4, 8, 12, 16, 20 and 24 weeks of treatment, and 2, 4 and 12 weeks after ending treatment, both serum hormones and seminal fluid analysis were obtained. All hormone measurements were obtained prior to the administration of the daily dose of medication, therefore approximately 24 h after the previous dose. In addition, at baseline and after 2, 4 and 24 weeks of treatment, serum gonadotropins were collected at three serial time points approximately 15 min apart and within a total of 60 min.
All subjects provided written consent prior to the initiation of screening and study procedures. This trial was registered at www.clinicaltrials.gov, National Clinical Trial no. 00891228 and 00229593.
Measurements
Seminal fluid analysis, including assessment of volume, sperm concentration, motility and morphology, were performed at each site according to the World Health Organization Laboratory Manual for the Examination of Human Semen (World Health Organization, 2010). Safety laboratory tests were analysed by the clinical laboratory at each site.
Serum NES concentrations were measured at the Population Council by radioimmunoassay as previously described (Ilani et al., 2012). Serum testosterone (T) and gonadotropins were measured at the Endocrine Metabolic and Research Laboratory at Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center. Serum T was measured by liquid chromatography tandem mass spectrometry (LC-MS/MS), and serum luteinizing hormone (LH) and FSH concentrations were measured by fluoroimmunometric assays as described previously (Ilani et al., 2012).
Statistical analysis
Gonadotropins were log-transformed prior to analysis attributable to a non-normal distribution. Gonadotropins at a given time point were compared using a one-way anova and Sheffé post hoc adjustment for multiple comparisons. Variables associated with significant suppression of spermatogenesis to either <1 million/mL or azoospermia were determined by univariate logistic regression. Sperm concentrations were used for outcomes given the goal threshold previously defined for a male hormonal contraceptive regimen of <1 million/mL as having similar efficacy as a female hormonal contraceptive (Nieschlag, 2007). Azoospermia was also studied as an outcome variable given that the ultimate goal of a male hormonal contraceptive would be to render most, if not all subjects, azoospermic, a state shown to be highly effective at preventing unintended pregnancy (World Health Organization, 1990).
A clinical prediction model for suppression of spermatogenesis was developed using backwards stepwise multivariate linear regression. For this analysis, we included the following variables: age, body mass index (BMI), race, treatment group, baseline serum LH and FSH concentrations, week 4 LH and FSH concentrations, baseline sperm concentration, week 4 serum T and NES concentrations, with week 24 sperm concentration as our continuous outcome variable. The regression analysis was then repeated using only the subjects who received active NES gel. All statistical analyses were completed using STATA 12.0 (College Park, TX, USA). For all analyses, an alpha of 0.05 was considered significant.
RESULTS
Subjects
Of the 99 subjects enrolled in the study, all 69 who completed 20 weeks or longer of treatment were included in the analysis. In this analysis, 65% of subjects were Caucasian, 14% Asian, 16% African American and 5% Native American. Subjects excluded from the efficacy analysis for non-adherence with nestorone application (detected by very low serum NES concentrations) (Ilani et al., 2012) were included in this analysis to address medication adherence as a factor in the regression model. Total subjects included in the analysis include 26 men in the T+NES 0 group, 21 men in the T+NES 8 group, and 22 men in the T+NES 12 group.
Serum gonadotropins
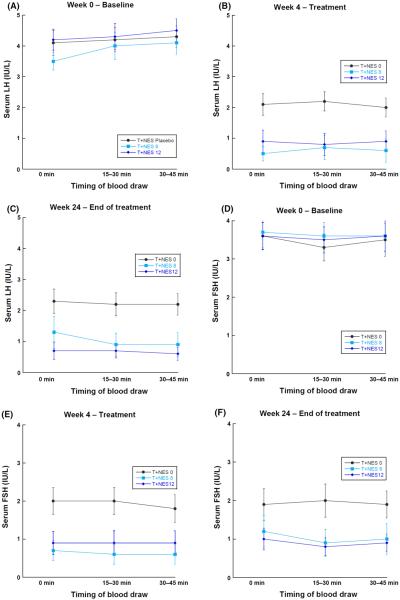
Serum gonadotropins were measured three times at approximately 15-min intervals at the baseline visit (week 0), and after 2, 4 and 24 weeks of treatment. Mean gonadotropin concentrations for each treatment group at each of the three collection points for a given treatment week are presented in Fig. 1. Average serum gonadotropins at 15 or 30 min following the initial blood draw did not differ from the initial serum gonadotropin concentration. Similarly, there were no significant differences among gonadotropin concentrations at each time point when analysing the change in gonadotropins as the absolute difference in gonadotropin concentrations. At week 0 (prior to the initiation of drug therapy), less than 10% of men exhibited variability of greater than 25% among the three serum gonadotropin samples drawn 15 min apart. Moreover, this variability was suppressed on treatment (data not shown), and did not affect the significance of the relationships between gonadotropin measurements and suppression of spermatogenesis.
Figure 1.
(A–F) Serum gonadotropins at baseline, 15–30 min, and 30–45 min at baseline, 4 and 20–24 weeks of treatment by treatment group, presented as mean ± standard error of the mean.
Predictors of suppression of spermatogenesis to <1 million sperm/mL
The association between subject characteristics, serum gonadotropins, and complete or near complete suppression of spermatogenesis (sperm concentration <1 million/mL) after 20–24 weeks of treatment are presented in Table 1. Neither age nor BMI helped identify subjects who did not adequately respond to this male hormonal contraceptive regimen, but race was significantly associated with the odds of failing to suppress spermatogenesis, with Caucasians having a significantly higher odds of failing to suppress compared to all other races. Subjects not receiving active NES (T+NES 0 group), had significantly higher odds of failing to suppress spermatogenesis to severe oligozoospermia than subjects receiving active NES. In addition, subjects with both serum gonadotropin concentrations greater than 1 U/L at any point during treatment had significantly greater odds of failing to suppress spermatogenesis.
Table 1.
Univariate gonadotropins and subject characteristics associated with sperm suppression to <1 million/mL or azoospermia
| Variable | <1 million/mL |
Azoospermia |
||
|---|---|---|---|---|
| OR (CI) | p-value | OR (CI) | p-value | |
| All treatment groups, n = 69 (T+NES 0, T+NES 8, T+NES 12) | ||||
| LH week 0 | 1.12 (0.36, 3.52) | 0.84 | 0.98 (0.31, 3.1) | 0.97 |
| LH week 2 | 2.81 (1.78, 4.46) | <0.001 | 2.4 (1.5, 3.6) | <0.001 |
| LH week 4 | 6.81 (2.98, 15.6) | <0.001 | 4.4 (2.2, 8.6) | <0.001 |
| LH week 24 | 5.0 (2.5, 9.95) | <0.001 | 7.6 (2.7, 21.1) | <0.001 |
| FSH week 0 | 0.82 (0.32, 2.11) | 0.69 | 0.78 (0.3, 2.0) | 0.61 |
| FSH week 2 | 2.03 (1.34, 3.07) | 0.001 | 1.9 (1.3, 2.9) | 0.001 |
| FSH week 4 | 3.37 (2.01, 5.66) | <0.001 | 3.1 (1.9, 5.2) | <0.001 |
| FSH week 24 | 3.47 (2.05, 5.87) | <0.001 | 3.3 (2.0, 5.6) | <0.001 |
| Age | 1.01 (0.97, 1.06) | 0.55 | 1.0 (0.97, 1.1) | 0.33 |
| BMI | 1.02 (0.88, 1.19) | 0.75 | 1.1 (.91, 1.2) | 0.46 |
| Race | 2.28 (1.21, 4.31) | 0.011 | 3.1 (142, 6.8) | 0.005 |
| Baseline sperm concentration | 1.0 (0.99, 1.01) | 0.92 | 1.0 (1.0, 1.0) | 0.82 |
| T+NES 0 vs. T + NES 8/12 | 8.7 (2.7, 27.9) | <0.001 | 5.8 (1.8, 18.4) | 0.003 |
| T+NES8 and T+NES 12 groups only, n = 43 | ||||
| LH week 0 | 1.10 (0.22, 5.63) | 0.91 | 0.98 (0.31, 3.08) | 0.97 |
| LH week 2 | 2.18 (1.24, 3.83) | 0.006 | 2.36 (1.54, 3.63) | <0.001 |
| LH week 4 | 27.2 (2.8, 258.4) | 0.004 | 4.36 (2.22, 8.58) | <0.001 |
| LH week 24 | 3.53 (1.67, 7.43) | 0.001 | 7.58 (2.71, 21.1) | <0.001 |
| FSH week 0 | 0.71 (0.18, 2.7) | 0.61 | 0.78 (0.30, 2.02) | 0.61 |
| FSH week 2 | 1.5 (0.93, 2.44) | 0.10 | 1.94 (1.29, 2.93) | 0.001 |
| FSH week 4 | 3.22 (1.65, 6.23) | 0.001 | 3.16 (1.88, 5.23) | <0.001 |
| FSH week 24 | 2.78 (1.51, 5.12) | 0.001 | 3.34 (1.99, 6.13) | <0.001 |
| Age | 1.04 (0.97, 1.10) | 0.25 | 1.02 (0.97, 1.07) | 0.33 |
| BMI | 1.02 (0.83, 1.25) | 0.84 | 1.06 (0.91, 1.23) | 0.46 |
| Race | 2.07 (101, 4.24) | 0.045 | 2.65 (1.18, 5.93) | 0.018 |
| Baseline sperm concentration | 1.00 (0.99, 1.01) | 0.70 | 1.0 (0.99, 1.01) | 0.82 |
| T+NES 8 vs. T+NES 12 | 0.45 (0.12, 1.68) | 0.23 | 0.5 (0.45, 1.7) | 0.27 |
The association between serum hormone concentrations and failure to suppress sperm concentrations to <1 million/mL or azoospermia after 20–24 weeks of treatment is presented in Table 2. Serum T concentrations were not associated with failure to suppress spermatogenesis at any time point. Similarly, the average serum T concentration over the entire course of treatment was not associated with suppression of spermatogenesis. In contrast, serum NES concentrations were significantly associated with suppression of spermatogenesis at all time points. Specifically, higher serum concentrations of NES increased the likelihood to adequately suppress spermatogenesis at weeks 20–24.
Table 2.
Univariate serum hormone concentrations associated with sperm suppression to <1 million/mL or azoospermia
| Serum testosterone concentration | <1 million/mL |
Azoospermia |
||
|---|---|---|---|---|
| OR (CI) | p-value | OR (CI) | p-value | |
| All treatment groups, n = 69 (T+NES 0, T+NES 8, T+NES 12) | ||||
| Baseline | 0.93 (0.22, 4.0) | 0.92 | 0.99 (0.23, 4.3) | 0.99 |
| Week 2 | 1.3 (0.56, 3.1) | 0.52 | 1.4 (0.60, 3.4) | 0.45 |
| Week 4 | 0.63 (0.29, 1.4) | 0.25 | 0.66 (0.30, 1.4) | 0.30 |
| Week 24 | 0.61 (0.23, 1.3) | 0.22 | 0.69 (0.32, 1.5) | 0.35 |
| Average week 4–24 | 0.78 (0.25, 2.5) | 0.67 | 0.81 (0.26, 2.6) | 0.72 |
| Serum hormone concentration | <1 million/mL |
Azoospermia |
||
|---|---|---|---|---|
|
|
OR (CI) |
p-value |
OR (CI) |
p-value |
| T+NES 8 and T+NES 12 groups only, n = 43 | ||||
| Baseline T | 0.78 (0.11, 5.5) | 0.80 | 0.87 (0.13, 5.7) | 0.89 |
| Week 2 T | 0.88 (0.31, 2.5) | 0.82 | 1.1 (0.40, 3.0) | 0.86 |
| Week 4 T | 0.44 (0.16, 1.2) | 0.10 | 0.56 (0.22, 1.4) | 0.22 |
| Week 24 T | 0.28 (0.08, 1.0) | 0.052 | 0.47 (0.16, 1.4) | 0.17 |
| Week 2 NES | 0.36 (0.17, 0.78) | 0.008 | 0.57 (0.31, 1.0) | 0.069 |
| Week 4 NES | 0.37 (0.18, 0.76) | 0.007 | 0.47 (0.24, 0.90) | 0.022 |
| Week 24 NES | 0.27 (0.12, 0.60) | 0.002 | 0.30 (0.13, 0.65) | 0.003 |
| Average T (week 4–24) | 0.26 (0.05, 1.4) | 0.12 | 0.45 (0.11, 1.9) | 0.28 |
| Average NES (week 4–24) | 0.78 (0.64, 0.94) | 0.008 | 0.82 (0.70, 0.96) | 0.016 |
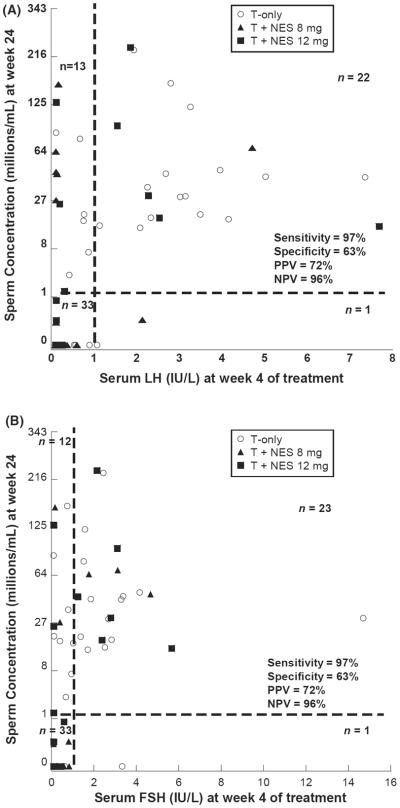
To determine if serum gonadotropins early in treatment (week 2 and week 4) were associated with suppression of spermatogenesis after 20–24 weeks of treatment, we compared serum LH and FSH with sperm concentration at the end of treatment (Fig. 2). In this analysis, higher serum gonadotropin concentrations after either 2 or 4 weeks of treatment were significantly associated with non-responsiveness. In particular, the serum gonadotropin concentrations at 4 weeks of treatment were highly sensitive (97% for both LH and FSH) for predicting the failure to respond to a male hormonal contraception regimen. Only one subject eventually suppressed spermatogenesis to <1 million/mL despite a week 4 LH greater than 1 IU/L (actual concentration 1.08 IU/L), whereas one different subject eventually suppressed spermatogenesis despite a week 4 FSH greater than 1 IU/L (actual concentration 3.02 IU/L). However, as a positive predictor of responsiveness, serum LH and FSH concentrations at week 4 have low specificity for suppression of spermatogenesis (63% for both gonadotropins), as roughly one-third of men with LH or FSH concentrations of less than 1 IU/L at 4 weeks of treatment failed to ultimately suppress spermatogenesis to less than 1 million sperm/mL of ejaculate after 20–24 weeks of treatment.
Figure 2.
(A, B) Relationship between sperm concentration at weeks 20–24 and serum LH (A) and serum FSH (B) at week 4 of treatment for subjects in all three treatment groups (T+NES 0, T+NES 8, T+NES 12). Note that a third root transformation was used for sperm concentration. NPV, negative predictive value; PPV, positive predictive value.
Because many factors contribute to the overall response to a male hormonal contraceptive regimen, we modelled the relative contribution of select variables that may be useful as early predictive markers of treatment failure using a backwards stepwise multivariate linear regression model (Table 3). In the analysis of all 69 subjects who completed the study, serum LH concentration at week 4 of treatment was noted to be the most significant factor (p < 0.002) associated with suppression of spermatogenesis at weeks 20–24. When analysing only the 43 subjects receiving active NES, the week 4 NES concentration was the most significant contributor to successful suppression of spermatogenesis at weeks 20–24.
Table 3.
Stepwise multivariate linear regression of factors associated with suppression of spermatogenesis at week 24
| Variable | Cumulative R2 | Δ R2 | p-value |
|---|---|---|---|
| All treatment groups (N = 69) | |||
| LH week 4 | 0.14 | – | 0.002 |
| Baseline sperm concentration | 0.24 | 0.10 | 0.001 |
| Baseline LH | 0.34 | 0.10 | 0.009 |
| Groups receiving nestorone (N = 43) | |||
| Nestorone concentration week 4 | 0.14 | – | 0.014 |
| Baseline sperm concentration | 0.23 | 0.09 | 0.037 |
For the analysis above, the total R2 value for all variables is reported as `cumulative R2'. The individual R2 value for any specific variable can be found under the column `Δ R2'.
When all treatment groups are considered, and the model includes all significant variables, 34% of the variability in sperm concentration is explained. Of the variables considered, serum LH concentration alone at 4 weeks is the most significant, accounting for 14% of the variation. In the 43 men receiving active NES together with T gel, the model explains 23% of the variability in sperm concentration, with serum NES concentration at 4 weeks explaining 14% of the variation (Table 3).
DISCUSSION
We used a recently completed clinical trial to evaluate specific variables in early treatment that are associated with overall response to a transdermal-only male hormonal contraceptive regimen. This model offers the opportunity to identify subjects early in the treatment regimen who are unlikely to respond to this contraceptive regimen and suggests variables that should be examined as predictors of treatment failure in future studies of this and other male hormonal contraceptives. In addition, this study suggests that, despite the known pulsatility of the pituitary hormones, a single serum gonadotropin measurement is as informative as rapid, serial gonadotropin sampling over 30 min. This could potentially simplify the design of future male hormonal contraceptive studies in predicting non-responders.
In addition, while earlier studies have suggested that multiple gonadotropin concentrations are necessary to more accurately characterize the baseline hormonal milieu for an individual (Baker et al., 1975), these studies were not performed in individuals receiving combined regimens of testosterone with a progestin, which significantly suppress serum gonadotropin concentrations and pulsatility. Moreover, the baseline gonadotropin pulsatility was less significant than seen in prior studies, possibly attributable to the serial measurements drawn within a short time period (15-min intervals completed within 60 min). Indeed, our analysis suggests that for men receiving male hormonal contraceptive regimens, three measurements within a 60-min window is not better than a single serum gonadotropin concentration to reflect the steady-state hormonal milieu suppressed by exogenous sex steroids. Therefore, while multiple measurements may characterize LH pulsatile secretion by the gonadotrophs, it does not contribute to understanding the efficacy of a male hormonal contraceptive regimen on spermatogenesis.
One important finding of this study is that the serum gonadotropin concentrations after 4 weeks of this transdermal male hormonal contraceptive regimen have a strong predictive value (96%) of suppression of spermatogenesis at 20–24 weeks of treatment. This suggests that men who do not have an adequate response to the regimen as reflected by a serum gonadotropin concentration below 1 IU/L after 4 weeks of treatment may be unlikely to adequately suppress spermatogenesis with more prolonged treatment using this regimen. Therefore, when considering potential approved use of a male hormonal contraceptive, men who fail to suppress serum gonadotropins to below 1 IU/L at 4 weeks could be counselled regarding the higher failure rate and instructed to use another method of contraception. Unfortunately, appropriate suppression of serum gonadotropins to below 1 IU/L at 4 weeks does not ensure suppression of spermatogenesis to concentrations below 1 million/mL at 20–24 weeks. As a result, only the `non-responders' with high gonadotropins can be identified early in the regimen by this approach. A separate study may allow us to determine if the subjects with suboptimal suppression of LH or FSH would benefit from a modified regimen (i.e. increased dose of either testosterone or progestin). The small number of non-responders with suppressed gonadotropins will not be identified in this way.
This study also revealed some additional important observations. Previous studies using other, non-transdermal, male hormonal contraceptive regimens have demonstrated that serum gonadotropins at the end of treatment are associated with sperm concentrations at the end of treatment and that serum gonadotropins prior to treatment have no significant association with the effectiveness of the contraceptive regimen as a univariate predictor (Handelsman et al., 1995; McLachlan et al., 2004). Our multivariate statistical models supported the importance of gonadotropin suppression. For example, when suppression of spermatogenesis was modelled in all subjects using multivariate linear regression, the week 4 serum LH concentrations were found to be the strongest predictor associated with suppression of spermatogenesis. Baseline LH was also significantly associated with suppression of spermatogenesis in this model as was the baseline sperm concentration. These findings suggest that reductions in LH (and presumably intratesticular testosterone) and baseline sperm production are key factors related to suppression of spermatogenesis in this study. However, the fact that these variables only explain 34% of the variation in suppression of spermatogenesis suggests that other unidentified variables, such as genetic differences between subjects, may explain much of the variation in sperm suppression. Our univariate model supported prior work showing race to be a significant factor in response to a male hormonal contraceptive regimen (Liu et al., 2008); however, the significance of race did not hold up as a major factor in the multivariate model.
In the multivariate model focusing on only the men receiving nestorone, serum NES concentrations, but not serum T concentrations, were significant predictors of response. This supports prior studies which have shown that serum T concentrations do not correlate with the effectiveness of suppression of spermatogenesis (Amory et al., 2001; McLachlan et al., 2004). The association between serum NES concentrations and spermatogenesis suppression indicates the importance of adding a progestin to the hormonal contraceptive regimen as shown by Ilani et al. (2012), and medication adherence as a predictor in overall success to the male hormonal contraceptive regimen. This finding is very similar to observations made in studies of female hormonal contraceptives, which have significantly worse outcomes under actual circumstances (typical use) as compared to ideal circumstances (perfect use) attributable to poor compliance (Rosenberg et al., 1995; Vaughan et al., 2008). Interestingly, when serum NES concentrations were added in this second model, the gonadotropin concentrations were no longer significantly associated with sperm suppression, likely ascribable to co-linearity between elevations in serum NES concentrations and suppression of serum LH concentrations.
In summary, we have analysed data from a transdermal male contraceptive study to determine characteristics associated with suppression of spermatogenesis. Significantly, we found that failure to suppress serum gonadotropins to low concentrations (<1 IU/L) after 4 weeks of drug administration is highly sensitive for failure to suppress spermatogenesis after 20–24 weeks of treatment. However, suppression of gonadotropins at this time point does not ensure the eventual suppression of spermatogenesis with continued treatment. While gonadotropin suppression and drug compliance are important elements of sperm suppression, our understanding of the factors associated with suppression of spermatogenesis is incomplete. Additional study of the genetic or intratesticular factors associated with spermatogenesis will be required before the more complete and uniform suppression of spermatogenesis required for these regimens to be introduced clinically can be achieved.
ACKNOWLEDGEMENTS
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development contracts HHSN 2752004033691 and HHSN 2751008060044U, and the Population Council. The University of Washington site was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54 HD-42454 as part of the Cooperative Contraceptive Research Centers Program and through K12 HD053984. The Los Angeles Center was supported by the Endocrinology, Metabolism and Nutrition Training Grant (T32 DK007571), and the UCLA Clinical and Translational Science Institute (UL1TR000124) at Harbor-UCLA Medical Center and Los Angeles Biomedical Research Institute. The authors acknowledge the gift of testosterone gel through Paul Piette, MD, Besins Health Care International; the help and assistance provided by Sharon Midler and her team for monitoring the study at Health Decisions; Elizabeth Ruiz, Xiao-Dan Han, Kathy Winter, Robert Bale Jr., Iris Nielsen, Connie Pete and Dorothy McGuinness for their help in coordinating the study; Christin Snyder, MD, for helping recruit and evaluate patients at the UW site; Andrew Leung, HTC, Sima Baravarian, PhD and technologists of the Hormone and Metabolic Research Laboratory at Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center for all the hormone analyses.
Footnotes
DISCLOSURE STATEMENT The authors have nothing to disclose.
REFERENCES
- Amory JK, Anawalt BD, Bremner WJ, Matsumoto AM. Daily testosterone and gonadotropin levels are similar in azoospermic and nonazoospermic normal men administered weekly testosterone: implications for male contraceptive development. J Androl. 2001;22:1053–1060. doi: 10.1002/j.1939-4640.2001.tb03445.x. [DOI] [PubMed] [Google Scholar]
- Baker HWG, Santen RJ, Burger HG, De Kretser DM, Hudson B, Pepperell RJ, et al. Rhythms in the secretion of gonadotropins and gonadal steroids. J Steroid Biochem. 1975;6:793–801. doi: 10.1016/0022-4731(75)90069-2. [DOI] [PubMed] [Google Scholar]
- Bebb RA, Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, Matsumoto AM. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab. 1996;81:757–762. doi: 10.1210/jcem.81.2.8636300. [DOI] [PubMed] [Google Scholar]
- Blumenthal PD, Edelman A. Hormonal contraception. Obstet Gynecol. 2008;112:670–684. doi: 10.1097/AOG.0b013e31818425b7. [DOI] [PubMed] [Google Scholar]
- Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK, et al. A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab. 2003;88:562–568. doi: 10.1210/jc.2002-020447. [DOI] [PubMed] [Google Scholar]
- Gu YQ, Tong JS, Ma DZ, Wang XH, Yuan D, Tang WH, et al. Male hormonal contraception: effects of injections of testosterone undecanoate and depot medroxyprogesterone acetate at eight-week intervals in Chinese men. J Clin Endocrinol Metab. 2004;89:2254–2262. doi: 10.1210/jc.2003-031307. [DOI] [PubMed] [Google Scholar]
- Gu Y, Liang X, Wu W, Liu M, Song S, Cheng L, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1910–1915. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Farley TM, Peregoudov A, Waites GM. Factors in nonuniform induction of azoospermia by testosterone enanthate in normal men. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Fertil Steril. 1995;63:125–133. [PubMed] [Google Scholar]
- Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, Page ST, et al. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab. 2012;97:3476–3486. doi: 10.1210/jc.2012-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamischke A, Ploger D, Venherm S, von Eckardstein S, von Eckardstein A, Nieschlag E. Intramuscular testosterone undecanoate with or without oral levonorgestrel: a randomized placebo-controlled feasibility study for male contraception. Clin Endocrinol (Oxf) 2000;53:43–52. doi: 10.1046/j.1365-2265.2000.01024.x. [DOI] [PubMed] [Google Scholar]
- Kamischke A, Heuermann T, Kruger K, von Eckardstein S, Schellschmidt I, Rubig A, et al. An effective hormonal male contraceptive using testosterone undecanoate with oral or injectable norethisterone preparations. J Clin Endocrinol Metab. 2002;87:530–539. doi: 10.1210/jcem.87.2.8218. [DOI] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, et al. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab. 2008;93:1774–1783. doi: 10.1210/jc.2007-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto AM, Bremner WJ. Modulation of pulsatile gonadotropin secretion by testosterone in man. J Clin Endocrinol Metab. 1984;58:609–614. doi: 10.1210/jcem-58-4-609. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, Robertson DM, Pruysers E, Ugoni A, Matsumoto AM, Anawalt BD, et al. Relationship between serum gonadotropins and spermatogenic suppression in men undergoing steroidal contraceptive treatment. J Clin Endocrinol Metab. 2004;89:142–149. doi: 10.1210/jc.2003-030616. [DOI] [PubMed] [Google Scholar]
- Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, Behre HM, et al. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2572–2580. doi: 10.1210/jc.2008-0265. [DOI] [PubMed] [Google Scholar]
- Nieschlag E. 10th Summit Meeting consensus: recommendations for regulatory approval for hormonal male contraception. October 22–23, 2006. Contraception. 2007;75:166–167. doi: 10.1016/j.contraception.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Anawalt BD, Irwig MS, Brockenbrough AT, Matsumoto AM, et al. Testosterone gel combined with depomedroxyprogesterone acetate is an effective male hormonal contraceptive regimen and is not enhanced by the addition of a GnRH antagonist. J Clin Endocrinol Metab. 2006;91:4374–4380. doi: 10.1210/jc.2006-1411. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocr Rev. 2008;29:465–493. doi: 10.1210/er.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989;10:366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355–360. [PubMed] [Google Scholar]
- Sedgh G, Henshaw S, Singh S, Ahman E, Shah IH. Induced abortion: estimated rates and trends worldwide. Lancet. 2007;370:1338–1345. doi: 10.1016/S0140-6736(07)61575-X. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. Transdermal application of steroid hormones for contraception. J Steroid Biochem Mol Biol. 1995;53:247–251. doi: 10.1016/0960-0760(95)00055-5. [DOI] [PubMed] [Google Scholar]
- Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, et al. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab. 2003;88:4659–4667. doi: 10.1210/jc.2003-030107. [DOI] [PubMed] [Google Scholar]
- Vaughan B, Trussell J, Kost K, Singh S, Jones R. Discontinuation and resumption of contraceptive use: results from the 2002 National Survey of Family Growth. Contraception. 2008;78:271–283. doi: 10.1016/j.contraception.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet. 1990;336:955–959. [PubMed] [Google Scholar]
- World Health Organization Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65:821–829. [PubMed] [Google Scholar]
- World Health Organization . World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. 5th edn World Health Organization; Geneva, Swtizerland: 2010. [Google Scholar]