Abstract
Summary
Melatonin, the neuro-hormone synthesized during the night, has recently seen an unexpected extension of its functional implications towards type 2 diabetes development, visual functions, sleep disturbances and depression. Transgenic mouse models were instrumental for the establishment of the link between melatonin and these major human diseases. Most of the actions of melatonin are mediated by two types of G protein-coupled receptors, named MT1 and MT2, which are expressed in many different organs and tissues. Understanding the pharmacology and function of mouse MT1 and MT2 receptors, including MT1/MT2 heteromers, will be of crucial importance to evaluate the relevance of these mouse models for future therapeutic developments. This review will critically discuss these aspects, and give some perspectives including the generation of new mouse models.
Keywords: melatonin, melatonin receptors, sleep, circadian rhythm, sleep, diabetes, retina, photoperiodism
Introduction
Melatonin is a neuro-hormone primarily synthesized by the pineal gland, but other cell types [e.g., retinal photoreceptors] are also capable of synthesizing it [1]. In the vast majority of organisms so far studied, melatonin synthesis occurs during the night, and duration of the synthesis is related to the length of the dark period [2].
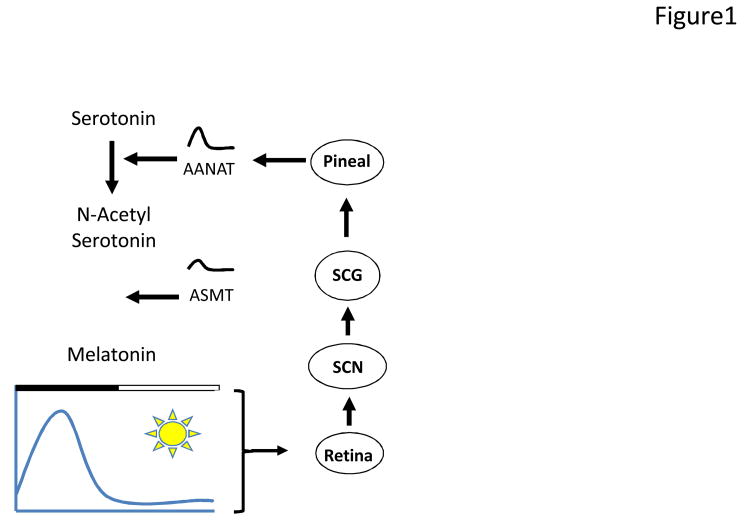
In mammals, pineal melatonin synthesis is under the control of the master circadian pacemaker, which is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN controls the timing of pineal melatonin synthesis via a sympathetic pathway that involves the activation of Arylalkylamine N-acetyltransferase (AANAT, the key regulatory enzyme in melatonin synthesis] via the cAMP-CREB pathway [1], Figure 1).
Figure 1.
Schematic drawing illustrating the regulation of pineal melatonin synthesis by the light/dark cycles via the SCN. During the daytime, the SCN inhibits melatonin synthesis, whereas at night the SCN sends a signal to the pineal to activate melatonin synthesis by increasing [over 100-fold] the transcription of the Aanat and then the activity of AANAT. AANAT converts serotonin to N-acetyl serotonin, and the Acetylserotonin N-Methyltransferase [ASMT] converts N-acetylserotonin to melatonin. ASMT transcription and activity also increases during the night, albeit to a lesser extent than AANAT.
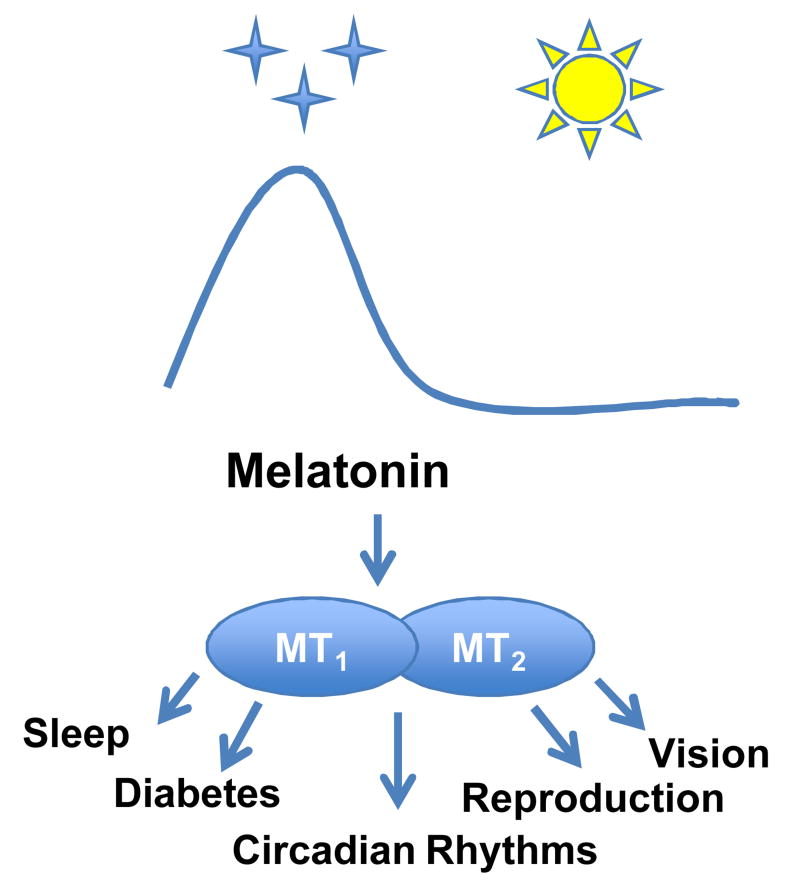
Two types of G protein-coupled receptors (GPCRs), named MT1 and MT2 [3-4], mediate the action of melatonin. These receptors are expressed in many different organs and tissues, and therefore melatonin modulates multiple aspects of human physiology. Consequently, dysfunction of the melatonergic system is often associated with sleep and circadian dysfunction [5], diabetes [6-8], retinal diseases [9-10], depression [11-12], autism spectrum disorders [13-14], and many neurodegenerative diseases, such as Alzheimer and Parkinson diseases [15-16].
In addition to the action mediated by MT1 and MT2 receptors, melatonin also can act as a free-radical scavenger, and thus as an antioxidant [8]. The action of melatonin as an antioxidant is believed to play an important role in protecting cells from aging and some neurodegenerative diseases [8].
Melatonin is currently used by millions of people around the world as a natural supplement for circadian and sleep disturbance. However, the mechanisms responsible for the beneficial effect of melatonin are still not fully understood. A significant advance in understanding the role of melatonin in the modulation of different physiological functions has been obtained by the development of transgenic mice lacking melatonin receptors. This review will focus on two aspects of melatonin receptors biology: 1) recent advances in MT1 and MT2 receptors pharmacology, including MT1/MT2 heteromers, and 2) the role that melatonin-receptor knockout (KO) mice has played over the last decade in improving our understanding of the numerous effects of melatonin on circadian rhythm regulation, sleep, vision, glucose homeostasis, and reproduction.
Melatonin receptor pharmacology is becoming more diverse
The availability of transgenic mouse models for melatonin receptors has concomitantly increased the interest in the properties of melatonin receptors from this species for fully understanding the differences and similarities to their human counterparts. The pharmacological properties of recombinant MT1 and MT2 receptors have been determined in several species including humans and mice [4, 17-23]. A recent study revealed strikingly similar overall profiles between recombinant human and mouse melatonin receptors of the same type [19]. This suggests that compounds with high affinity for mouse receptors have a high chance of being readily transposable into humans. However, these similarities might be somehow limited when considering ligands selective for MT1 or MT2, as detailed in the next section.
Towards ligands selective for mouse MT1 and MT2 melatonin receptors
Identification of selective ligands is an important step toward the development of drugs with improved side-effect profiles. Out of 39 compounds tested on recombinant murine melatonin receptors expressed in Chinese hamster ovary cells, only cis-4-Phenyl-2-propionamidotetralin (4P-PDOT) was selective, showing approximately 100-times higher affinity for MT2 [19]. Preferential binding of this compound to MT2 was confirmed in a second study on mouse MT1 and MT2 receptors expressed in HEK293 cells, although the affinity for MT2 was only 22-fold higher than for MT1 [24]. When compared with the human MT2 receptor, for which an approximately 300-fold higher affinity was reported [25], the selectivity of 4P-PDOT for mouse MT2 seems to be lower. More recently, IIK7 (N-Butanoyl 2-[9-methoxy-6H-iso¬indolo[2,1-a]indol-11-yl]¬ethan¬amine) was identified as a highly selective ligand for mouse MT2 receptors, having more than 1000-fold higher affinity for MT2 versus MT1 receptors [24]. For human MT2 receptors, IIK7 shows only a 90-fold higher affinity [26], indicating that despite a high overall conservation of pharmacological properties between species, there might exist some important differences between human and mouse receptors for MT1 and MT2 selective compounds. While these results on the IIK7 melatonin receptor agonist are encouraging, more systematic studies will be necessary to identify further selective melatonin receptor ligands and, in particular, MT1-selective agonists and MT1 and MT2-selective antagonists.
New insights from old and new melatonin receptor tracers
The overwhelming majority of ligand binding data on melatonin receptors have been obtained with the 2-[125I]-MLT radioactive tracer [21]. The tritiated [3H]melatonin tracer has been used less often because of its low specific activity. It is important to note that both radioligands are agonists, and thus are potentially able to sense the low affinity [G protein uncoupled] and high affinity [G protein-coupled] state. These two affinity states have indeed been detected in several studies [27-30]. Depending on the cellular background, the species and receptor type, only 15%–40% of the receptors were in the uncoupled state. These observations are fully compatible with previous observations in native tissues and transfected cells, demonstrating the formation of a stable complex between melatonin receptors and Gi proteins [31-33].
The recent study from Legros et al. [30] showed that affinities for ligands were similar for MT1 and MT2 receptors in the uncoupled state, the known MT1 and MT2 -specific differences [three- to tenfold] were only observed in the G protein-coupled state. This is an interesting point, as it suggests that the differences in binding affinities between receptor types are not due to intrinsic affinity differences between the binding sites of MT1 and MT2 receptors, but rather to the formation of different ternary [agonist-receptor-G protein] complexes, which bind melatonin ligands with different affinities. This conclusion is consistent with previous observations showing the formation of differential MT1 and MT2 receptor signaling complexes [34-35].
Recently, three further radiolabeled melatonin receptor agonists have been described [27]. The SD6 and S70254 compounds are based on the indole structure of melatonin carrying an iodoacetaminde side chain. The DIV880 compound has a different structure and corresponds to the iodinated analog of a bromo-compound identified in a screening project. Whereas SD6 has equally high affinity for human MT1 and MT2, [125I]-S70254 and [125I]-DIV880 bind only to MT2 receptors with high affinity. Interestingly, different radioligands detected different numbers of binding sites, suggesting that labeling of different receptor subpopulations depending on the radioligand. This supports the notion of the stabilization of ligand-dependent receptor conformations and formation of ligand-dependent signaling complexes.
In conclusion, melatonin receptor tracers with agonistic properties are able to detect different G protein-coupled and -uncoupled receptor complexes. Further studies will be needed to identify the first radiolabeled melatonin receptor antagonist, which will allow the detection of melatonin receptor binding sites independent of receptor activation.
Melatonin receptor oligomers are functionally relevant
Early studies suggested that melatonin receptors have the capacity to form dimers or higher-order oligomers [36-37]. A particularly interesting aspect of these studies was the possibility of MT1/MT2 heteromer formation. The potential physiological significance of these in vitro observations is supported by the co-expression of both receptor types in several tissues and the existence of a heteromer-specific pharmacological profile [38]. However, direct proof for formation of MT1/MT2 heteromers was lacking until recently. A new study supports the idea that MT1/MT2 heteromers do indeed exist in the mouse retina, where they are responsible for the melatonin-dependent increase in light sensitivity at night [24]. Co-expression of MT1 and MT2 in photoreceptor cells was shown at the mRNA level and heteromer formation at the protein level by co-immunoprecipitation and proximity ligation assay. By using MT1 and MT2 KO mice and transgenic mice overexpressing a dominant negative MT2 receptor inactive mutant, we could show that activation of both receptor types is mandatory to trigger the effect of melatonin on retinal light sensitivity. This effect was blocked by 4P-PDOT and luzindole, consistent with the idea that these two ligands are antagonist for MT1/MT2 heteromers. Injection of a low dose of the MT2-selective agonist IIK7 activating only the MT2 receptor protomer was unable to mimic the effect of melatonin. However, a higher dose of IIK7, activating MT2 and MT1 protomers, fully recapitulated the effect of melatonin. This study provides the first conclusive evidence for the existence and functional relevance of MT1/MT2 heteromers. These results can possibly be extended to humans, for which co-expression of both receptor types has been reported in photoreceptor cells [39-40]. Based on the large panel of tissues co-expressing MT1 and MT2 receptors, MT1/MT2 heteromers possibly exist in several other tissues and might be linked to other melatonin-related functions that have to be explored in future studies.
Signaling pathways: refining the perspective
MT1 and MT2 receptors have been shown to activate several signaling pathway [4, 35]. Both receptors are tightly coupled to the Gi/cAMP pathway, which appears to be also the case for MT1/MT2 heteromers [24] (Figure 2). Indeed, in vitro studies in transfected HEK293 cells suggest that heteromers inhibit forskolin-promoted cAMP production more potently and with higher efficiency with a more than tenfold difference in EC50 for melatonin as compared to homomers. The physiological meaning of this difference remains to be elucidated. Apart from coupling to the Gi/cAMP pathway, the MT1 receptor has been shown to couple to the Gq/PLC/Ca2+ pathway [32]. Studies on mouse MT1/MT2 heteromers indicate a significant amplification of the activation of this pathway in cells co-expressing both receptor types with improved amplitude and EC50 values as compared to cells expressing MT1 alone [>tenfold difference], shifting the melatonin dose-response curve more toward physiological melatonin concentrations. MT2 receptors were completely inactive in this pathway. Thus MT2 can be considered as a positive allosteric regulator of MT1 receptors in respect to the activation of the Gq/PLC pathway. Collectively, these data indicate that MT1/MT2 heteromers are coupled to Gi-and Gq-dependent signaling pathways, and that heteromers tend to be more potent and efficient in activating these pathways most likely due to positive allosteric interactions occurring at the receptor level [41].
Figure 2.
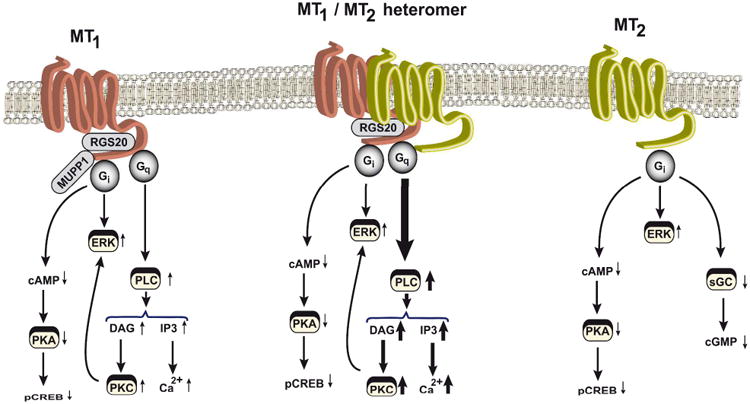
Principal melatonin-receptor signaling pathways. Depending on the type of melatonin-receptor complexes present in cells (MT1 homomers, MT2 homomers, or MT1/MT2 heteromers), the depicted signaling pathways are activated upon melatonin stimulation. Thickness of the arrows represents the potency and efficiency of the activation of the pathway. DAG, diacylglycerol; ERK, extracellular signal-regulated kinase; IP3, inositol triphosphate; MUPP1, multi-PDZ domain protein 1; pCREB, phospho-cAMP-response element-binding protein; PLC, phospholipase C; PKA, protein kinase A; PKC, protein kinase C; RGS20, regulator of G protein signaling 20; sGC, soluble guanylyl cyclase.
Melatonin has been show to activate other major signaling pathways such as the ERK1/2 and the PI3K/AKT pathways [7, 34, 42-43] (Figure 2). However, a full and detailed picture is still lacking. Information on the respective capacity of melatonin receptor homo- versus heteromers as well as the precise pathway used has still to be clarified. Currently available results suggest cell-type-dependent differences ranging from stimulation and activation of these pathways [44]. Furthermore, inhibition of the guanylyl cylcase pathway, which has been shown to be MT2-specific [45], also will be interesting to evaluate in the context of MT1/MT2 heteromers.
Taken together, research in recent years has revealed that melatonin receptor signaling is much more diverse than initially suspected. Depending on the cellular context, melatonin receptor homo- and heteromeric complexes can be formed. Recruitment of different G proteins and regulatory proteins into these complexes further diversifies receptors’ signaling capacity.
Melatonin-proficient versus melatonin-deficient mice
As noted, murine melatonin receptors appear to show a pharmacological profile that is similar to human receptors [19], but a clear understanding of melatonin signaling in mice is still lacking. This lack of data is due to the fact that there is some controversy about the capability of different strains of laboratory mice to produce melatonin. According to many authors, the vast majority of mouse strains are genetically incapable of synthesizing melatonin [46-49], and only CBA and C3H are considered melatonin-proficient strains, whereas most of the other laboratory strains [e.g., C57/BL6, Balb/C, SV129] are considered melatonin-deficient mice. However, it is worth noting that other authors have reported that C57/BL6 and many other strains [OF1 Swiss, BALB/c,] may also produce a small amount of melatonin for a brief period during the night [50-51]. Although the amounts of melatonin produced in these mice is small (10-30 pg/pineal vs. 200-300- pg/pineal of C3H or CBA mice [47, 50-51]), we cannot exclude the possibility that these levels of melatonin are sufficient to activate the MT1 and MT2 receptors in these so-called melatonin deficient-mice.
The recent identification of the Hiomt gene in the mouse genome [52] will facilitate the development of a “real” melatonin-deficient mouse [i.e., a mouse without the gene that codify for the enzyme responsible to convert N-acetylserotonin to melatonin]. The development of such a mouse will facilitate first the full understanding of the action of melatonin and then—by back-crossing these mice with melatonin-receptor KO mice—will also allow us to dissect the actions of melatonin as antioxidant from those mediated via the G protein-coupled receptors.
Melatonin receptor knockout mice as a key tool to understand melatonin action
Over the last 20 years, the use of transgenic mice in which specific genes have been ablated has provided important tools for understanding the function(s) of a specific gene. Melatonin-receptor KO mice were first developed by the Reppert laboratory in the late nineties [23, 53], and during the last decade many studies have used these transgenic mouse lines to investigate the effects that melatonin-receptor removal produces on the mouse physiology. These studies have provided clear experimental evidence on the importance of melatonin signaling in the regulation of many biological functions.
Circadian Rhythms
Several studies have shown that melatonin plays an important role in the entrainment of circadian rhythms, and remains the only pharmacological tool used to treat circadian dysfunction in humans [54]. Melatonin receptors are expressed in the SCN [3], MT1 being the most prevalent receptor. Administration of exogenous melatonin to SCN slices in vitro induces an acute inhibition of the neuron firing rate and phase-shifts the circadian rhythms of neuronal firing [55-57]. However, since most of the MT1 and MT2 receptor agonists and antagonists available lack the specificity to fully dissect the action of melatonin [24], this approach has not provided conclusive evidence about the role of the specific role of MT1 and MT2 receptors in the modulation of SCN function by melatonin.
The use of the melatonin receptors KOs has provided a clearer picture on the mechanisms by which melatonin can influence the circadian mammalian system. In the initial study using MT1 KO (MT1-/-), it was reported that the inhibitory effect of melatonin on SCN neuronal activity was no longer present, whereas the phase-shift response to melatonin appeared to be normal [23]. In addition, the phase-shifting response to melatonin in MT1-/- was blocked by pertussis toxin [23]; thus suggesting that MT2 receptors may be responsible for this phenomenon. Further studies have also shown that melatonin -- via MT1 receptors -- modulates cAMP responsive element (CREB) phosphorylation in the mouse SCN, since the induction of CREB phosphorylation induced by Pituitary adenylate cyclase-activating polypeptide (PACAP) was inhibited in MT1-/- mice [58]. This suggests that melatonin interacts with the circadian clock machinery via the cAMP-signaling pathway. However, it is important to note that at high melatonin concentration (100 nM and higher), melatonin can also affect the cAMP signaling pathways in mice lacking MT1 receptors, and co-application of 4P-PDOT abolished the inhibitory effects of melatonin on CREB phosporylation [58]. This suggests that MT2 may also be involved in the modulation of the cAMP pathway when melatonin is administered at pharmacological doses.
Indeed, a series of studies have indicated that MT2 receptors mediate the phase-shifting effect on melatonin in the SCN [59-60] via the PLC-PKC pathway [61]. To resolve this issue, Jin et al., [53] investigated the effect of melatonin administration on the SCN of MT2 KO mice [MT2-/-]. As expected in these mice, the inhibition of the SCN neural activity by melatonin was not affected, and the reduction in pCREB observed in MT1-/- at higher concentration is no longer present in MT1-/-MT2-/-, hence confirming that both receptors are involved in this response.
Finally additional studies have indicated that, although the activation of MT2 receptors is necessary for the phase-shift of the SCN firing in vitro, activation of MT1 receptors may be required for the melatonin-meditated phase shift of the circadian rhythms in locomotor activity [62] and the re-entrainment of the circadian rhythm of locomotor activity to six-hour phase advances is significantly slower in mice lacking MT2 receptors [63].
Sleep
In addition to being a well known player in the regulation of circadian dysfunctions, melatonin is also involved in the regulation of sleep. Although rodents are nocturnal animals (i.e., differently from humans they sleep during the day time when melatonin levels are low) these animal models may still represent an important tool to dissect the mechanisms by which melatonin can modulate sleep. Indeed studies in rats have shown that administration of melatonin can affect several sleep parameters such as rapid eye movement (REM) and non-rapid eye movement (NREM) sleep [64-65], whereas other studies have questioned the effectiveness of melatonin to affect sleep [66-67]. A recent study using a MT2 receptor agonist (IIK7) has suggested that the action of melatonin on sleep is mediated by MT2 receptors [68]. However, as noted, most of the melatonin agonists or antagonists available lack the specificity to conclusively demonstrate the type of melatonin receptors involved in the modulation of sleep. Therefore the use of a mouse lacking melatonin receptors is an essential tool for dissecting the contribution of melatonin to the regulation of sleep. Indeed, two recent studies have provided compelling experimental evidence on the involvement of melatonin receptors in the regulation of sleep in mice. In general it can be said that in mice melatonin can promote NREM sleep by acting on MT2 receptors located in the reticular thalamic nucleus [69]. This idea is supported by experimental data showing that infusion of a MT2 agonist in this nucleus or systemic administration increased the firing rate of the neurons in this area and such an effect can be blocked by the administration of a MT2 antagonist [69]. Consistently with these pharmacological data, mice lacking MT2 receptors showed a decrease in NREM sleep during the light phase [i.e., when mice usually sleep], whereas mice lacking MT1 increase the amount of NREM sleep during the dark phase. Furthermore, MT2 KOs increased the time of wakefulness during the light phase, and MT1 showed a decrease in wakefulness during the dark phase. Finally, in WT and MT2-/-, REM sleep lasted longer in the light phase than in the dark phase, and MT1-/- spend the same amount of time in REMS during the light or dark phase. Such a result would suggest that MT1 receptors may be involved in the regulation of the daily rhythm of REM [69]. Finally, an additional study, in which MT1/MT2 receptor KO mice were used, demonstrated that these mice showed an increase in wakefulness [probably due to the lack of MT2 receptors], and a reduction in REM sleep (as a consequence of the MT1 removal) [70]; this suggests that removal of melatonin receptors affects wakefulness rather than sleep. Thus the use of melatonin-receptor KO mice indicated that melatonin and its associated receptors are involved in the regulation of sleep and wake cycle, and MT1 and MT2 receptors differently affect the sleep and wake cycle.
Although these studies have laid the foundation for the dissection of melatonin receptors’ contribution to sleep, it must be noted that much work remains to be done. For example, the mice used in these studies were complete KO: therefore the use of inducible conditional melatonin-receptor KO mice in brain areas involved in the regulation of sleep and wakefulness may provide additional clues as to the role of melatonin in the regulation of sleep, and might be helpful in developing new tools to treat sleep disturbances.
Vision
As we have previously mentioned melatonin is also synthesized in the retina, where it plays an important role in the regulation of retinal physiology by acting on MT1 and MT2 receptors that are widely distributed in the retina and in other ocular structures [71-75]. Interestingly, melatonin receptors are abundantly expressed on the photoreceptor cells, thus suggesting that melatonin, in this case, may act as an autocrine signal to regulate its own synthesis.
Removal of these receptors has a profound effect of the retinal physiology, since it appears that melatonin via MT1 actually controls the daily rhythms of the scotopic and photopic electroretinogram (ERGs) and the scototopic threshold response [73-75]. Furthermore, the circadian regulation of the photopic ERG is also absent in MT1−/− mice; thus demonstrating that MT1 receptor signaling is required for the circadian regulation of the photic ERGs [75]. MT1 receptor removal also affects the viability of the photoreceptors and the retinal ganglion cells during aging [73].
Finally, a recently published study has shown that MT2 receptors have similar distribution of MT1 receptors within the retina, but MT2 mRNA seems to be absent in retinal ganglion cells [24]. Surprisingly, removal of MT2 receptors phenocopied the effects on the ERGs produced by the removal of the MT1 receptors [24], thus suggesting that in the mouse retina, and more precisely in the mouse photoreceptors, MT1 and MT2 receptors form heterodimers. Indeed, additional studies confirmed the presence of a functional MT1/MT2 heterodimer in the mouse photoreceptors [24].
These recent studies on the role played by melatonin and its associated receptors in the modulation of visual function have clearly demonstrated that melatonin is indeed a key player in retinal physiology. Removal of these receptors affects the viability of the photoreceptors and retinal ganglion cells, thus suggesting that melatonin can represent useful tools in the prevention of retinal cell loss that often occurs during the aging process and in some pathological conditions that are associated with aging (i.e., age related macular degeneration and glaucoma).
Diabetes
One of the new and most exciting news of the recent years in the melatonin field has been the discovery that polymorphisms in the genes encoding human melatonin receptors (MTNR1A and MTNR1B) may be in involved in the pathogenesis of type-2 diabetes (T2D) [6-8, 76]. These studies have confirmed earlier studies in rats that established a possible link between melatonin and glucose metabolism [77-78, Figure 3]. In more recent years, data supporting a specific role for melatonin in the modulation of insulin secretion has been documented. Indeed, both melatonin receptors are present within pancreatic islets, although there are some discrepancies in their relative distribution. While both receptors were found to be present within β-cells of human and rodent islets [5], a study utilizing islets derived from melatonin-receptor KO mice reported expression of MT2 solely in β-cells and exclusive expression of MT1 within pancreatic α-cells [79]. However, regardless of discrepant observations in localization, several studies have arrived at the conclusion that melatonin receptors exert a predominantly inhibitory effect, at least in rodent β-cells on insulin secretion via receptor-mediated attenuation of adenylate cyclase and guanlylate cyclase [80-83]. In contrast to the insulin secretory response, glucagon is secreted from pancreatic α-cells in response to low blood glucose levels and stimulates hepatic glucose output. In vitro studies utilizing a glucagon producing mouse pancreatic α-cell line, αTC1.9, established that melatonin administration produces a direct stimulatory effect on glucagon secretion via a PLC dependent mechanism [84]. This secretory response was subsequently blocked in the presence of luzindole and 4P-PDOT; thus demonstrating that melatonin receptors within pancreatic islets are coupled to signaling pathways involved in the modulation of both insulin and glucagon secretion.
Figure 3.
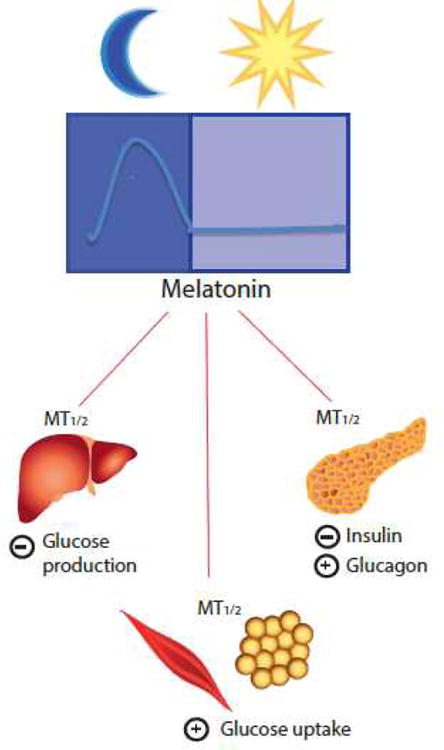
Melatonin receptors are involved in the regulation of glucose metabolism. Evidence from in vitro and in vivo studies has demonstrated that signaling through melatonin receptors interacts with many facets of glucose metabolism. Within pancreatic islets, melatonin receptors are coupled to signaling pathways, which exert an inhibitory effect on insulin secretion from β-cells and a stimulatory effect on glucagon secretion from α-cells. In peripheral tissues, melatonin receptors appear to positively regulate glucose uptake within skeletal muscle and adipose tissue, and negatively regulate nocturnal glucose production by the liver.
In addition to studies examining the role of melatonin on the secretion of gluco-regulatory hormones, it has been postulated that signaling through melatonin receptors enhances systemic glucose tolerance via a direct effect on glucose uptake. In support of this notion, in vitro studies have demonstrated that melatonin administration, independent of insulin, is capable of stimulating glucose uptake in both skeletal muscle and adipose tissue [42, 85], and, in mouse muscle cells, the effects of melatonin on glucose uptake were found to be mediated via an IRS-1/PI-3-kinase-dependent pathway [41]. Along similar lines, intra-cerebroventricular infusion of melatonin in rats proved capable of stimulating tyrosine phosphorylation of the insulin receptor, Akt, and IRS-1 [86]. These results support the intriguing possibility of intracellular cross talk between the melatonin and insulin signaling system.
In line with the data obtained in rodents, recent genome-wide association studies also have demonstrated that polymorphisms in both MT1 and MT2 are associated with altered glucose metabolism. Variants in MT2 have been linked to impairments in both insulin secretion and increased fasting glucose levels [6-8], and variants in MT1 have also been shown to be associated with an increased risk of developing polycystic ovarian syndrome, an endocrine disorder marked by insulin resistance and T2D onset [87]. By re-sequencing the coding region of the MTNR1B gene coding for the MT2 receptor, 40 variants have been identified and functionally characterized. Corresponding mutants with impaired receptor signaling did strongly associate with the T2D risk, indicating that loss of melatonin receptor function is positively associated with disease risk [7].
To date, only a few studies have characterized mechanisms underlying glucose homeostasis in melatonin receptor KO mice [83, 88-89]. The first study examining the effect of melatonin on rhythms in glucose metabolism demonstrated that mice lacking MT1 exhibited higher mean blood glucose levels than WT, MT2-/-, and MT1-/-/MT2-/- mice [87], and a subsequent investigation further established the MT1 KO mice are glucose intolerant, and insulin resistant, with respect to WT and MT2-/- animals [89].
Additional support for an inhibitory role of melatonin signaling in the modulation of insulin secretion also can be found in a study in which islets derived from melatonin receptor KO mice were found to have enhanced insulin secretion in the presence of melatonin [1uM] compared to WT islets. However, it is important to note that these results could not be elicited using physiological concentrations of melatonin [10 nM], and in WT islets attenuation of insulin secretion in the presence of melatonin could not be demonstrated [88]. In contrast to the studies examining insulin secretion, basal glucagon secretion was affected by the presence of melatonin receptors since basal glucagon secretion was significantly reduced in pancreatic islets of MT2-/- and MT1/MT2-/- mice in comparison to WT and MT1-/- mice [83].
Melatonin, photoperiod, and reproduction
Melatonin is synthesized at night, and the timing of its production correlates with the duration of the period of darkness. Thus melatonin signaling is believed to be the internal signal by which the organisms may perceive the seasonal changes in the photoperiod and thus regulate the reproductive cycles. In some vertebrate species, the length of photoperiod regulates the reproductive season (e.g., hamsters, sheep, and many others), and the hypophyseal pars tuberalis (PT) transduces the seasonal changes in the melatonin rhythmic profile into a pattern of prolactin secretion [90]. Although the reproductive system of the mouse is not sensitive to photoperiod, the development of the melatonin-receptor KOs have provided an important tool for dissecting the mechanisms by which melatonin regulates reproduction in photoperiodic species. Recent studies have shown that many of the genes and proteins that are responsible for the generation of the circadian oscillation are not only present in the SCN, but are also expressed in many peripheral tissues [91]. These genes are also found in the PT, and it is believed that melatonin signaling can affect the pattern of expression of these clock genes/proteins. Consistently with this hypothesis, it has been demonstrated that rhythmic expression of the clock gene Period 1 in the pituitary gland depends on the heterologous sensitization of the adenosine A2b receptors via the activation MT1signaling during the night [92].
Additional studies have reported that the rhythmic expression of several other clock genes (Per1, Per 2, Bmal1, and Cry 1) in the mouse PT depend on MT1 signaling as well [93], and MT1 and MT2 receptors are also involved in the control of the activity of lactotroph cells in the pars dystalis [94]. Finally, a recent paper has investigated the effect of melatonin, signaling removal on two genes responsible for photoperiod gonadal reproduction (e.g., type 2 and 3 deidodinase). These genes are present in the ependymal cell layer [EC] where they are transcriptionally regulated by the hormone thyrotropin secreted by the PT. In C3H mice with intact melatonin receptors, Deiodinase 2 (Dio2) mRNA levels are low, and no significant changes are observed with a different photoperiod; whereas Deiodinase 3 [Dio3] mRNA levels are significantly increased in a light-dark cycle with a short day. In mice lacking MT1 and MT1/MT2 receptors, photoperiodic changes in Dio3 mRNA were no longer present, whereas MT2−/− mice retained the response [95]. Additional experiments using C57/BL indicated that administration of exogenous melatonin down-regulated Dio2 mRNA and induced Dio3 mRNA under a long day. These effects were not observed in C57/BL6 mice in which the MT1 receptors had been genetically removed [95]. Such a result further indicates that MT1 signaling is crucial for the photoperiodic response of gene expression in the EC and thus for the photoperiodic regulation of gonadal activity.
Conclusions and Outlook
The development of melatonin-receptor KO mice has significantly improved our understanding of melatonin in the regulation of mouse physiology; furthermore, it has helped to elucidate the underlying mechanisms and started to pave the way towards a potential role of melatonin in disease development. Several exciting aspects remain to be elucidated in the future. For example, the mechanism by which this hormone regulates circadian rhythms via MT1 and MT2 receptors is still not fully understood, and may differ between different tissues. The same questions remain to be solved for the action of melatonin in the modulation of sleep and glucose homeostasis and reproduction. Furthermore, the full impact of MT1/MT2 heteromers remains to be elucidated in the numerous tissues co-expressing both receptors. Finally, emerging experimental evidence indicates that, in human post-mortem samples, melatonin-receptor expression seems to be affected by aging [15], depression [11-12], Alzheimer's disease [15], and Parkinson's disease [16]. Therefore, it would be beneficial to explore whether mice lacking melatonin receptors might represent a good model for investigating the pathogenesis of these diseases.
Acknowledgments
Supported by grants from the National Institutes of Health Grants NS43459, EY028821, EY022216 and by 5U54NS060659, S21MD000101, G12-RR03034, U54RR026137 and -T32 HL103104 (G.T.) and INSERM, CNRS, the “Who am I?” laboratory of excellence no. ANR-11-LABX-0071 funded by the French government through its “Investments for the Future” program operated by the French National Research Agency (ANR) under grant no. ANR-11-IDEX-0005-01, [Grants ANR RPIB 2012 “MED-HET-REC-2”, “Fondation Recherche Médicale” [Grant FRM DEQ20130326503] and a doctoral fellowship from the CODDIM 2009 (Région Ile-de-France) to R.J.
References
- 1.Klein DC, Coon SL, Roseboom PH, Weller JL, et al. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–57. [PubMed] [Google Scholar]
- 2.Korf HW, von Gall C. Mice, melatonin and the circadian system. Mol Cell Endocrinol. 2006;252:57–68. doi: 10.1016/j.mce.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Reppert SM. Melatonin receptors: molecular biology of a new family of G-protein-coupled receptors. J Biol Rhythms. 1997;12:528–531. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- 4.Dubocovich ML, Delagrange P, Krause DN, Sugden D, et al. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009;35:183–92. doi: 10.1002/biof.23. [DOI] [PubMed] [Google Scholar]
- 6.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, Sparsø T, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 8.Bonnefond A, Clement N, Fawcett K, Yengo L, et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44:297–301. doi: 10.1038/ng.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi C, Pan X, Yan H, Guo M, et al. Effects of melatonin in age-related macular degeneration. Ann N Y Acad Sci. 2005;1057:384–392. doi: 10.1196/annals.1356.029. [DOI] [PubMed] [Google Scholar]
- 10.Rosen R, Hu DN, Perez V, Tai K, et al. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis. 2009;15:1673–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YH, Ursinus J, Zhou JN, Scheer FA, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013;148:357–67. doi: 10.1016/j.jad.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Etain B, Dumaine A, Bellivier F, Pagan C, et al. Genetic and functional abnormalities of the melatonin biosynthesis pathway in patients with bipolar disorder. Hum Mol Genet. 2012;21:4030–4037. doi: 10.1093/hmg/dds227. [DOI] [PubMed] [Google Scholar]
- 13.Melke J, Goubran Botros H, Chaste P, Betancur C, et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol Psychiatry. 2008;13:90–98. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Li J, Ruan Y, Lu T, et al. Sequencing ASMT identifies rare mutations in Chinese Han patients with autism. PLoS One. 2013;8:e53727. doi: 10.1371/journal.pone.0053727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu YH, Zhou JN, Van Heerikhuize J, Jockers R, et al. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer's disease. Neurobiol Aging. 2007;28:1239–1247. doi: 10.1016/j.neurobiolaging.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Adi N, Mash DC, Ali Y, Singer C, et al. Melatonin MT1 and MT2 receptor expression in Parkinson's disease. Med Sci Monit. 2010;16:BR61–67. [PubMed] [Google Scholar]
- 17.Audinot V, Bonnaud A, Grandcolas L, Rodriguez M, et al. Molecular cloning and pharmacological characterization of rat melatonin MT1 and MT2 receptors. Biochem Pharmacol. 2008;75:2007–2019. doi: 10.1016/j.bcp.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Coge F, Guenin SP, Fery I, Migaud M, et al. The end of a myth: cloning and characterization of the ovine melatonin MT[2] receptor. Br J Pharmacol. 2009;158:1248–1262. doi: 10.1111/j.1476-5381.2009.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devavry S, Legros C, Brasseur C, Cohen W, et al. Molecular pharmacology of the mouse melatonin receptors MT[1] and MT[2] Eur J Pharmacol. 2012;677:15–21. doi: 10.1016/j.ejphar.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Mailliet F, Audinot V, Malpaux B, Bonnaud A, et al. Molecular pharmacology of the ovine melatonin receptor: comparison with recombinant human MT1 and MT2 receptors. Biochem Pharmacol. 2004;67:667–677. doi: 10.1016/j.bcp.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Zlotos DP, Jockers R, Cecon E, Rivara S, et al. MT1 and MT2 Melatonin Receptors: Ligands, Models, Oligomers, and Therapeutic Potential. J Med Chem. 2013 doi: 10.1021/jm401343c. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Weaver DR, Jin X, Shearman LP, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;49:1248–1253. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 23.Roca AL, Godson C, Weaver DR, Reppert SM. Structure, characterization, and expression of the gene encoding the mouse Mel1a melatonin receptor. Endocrinology. 1996;137:3469–3477. doi: 10.1210/endo.137.8.8754776. [DOI] [PubMed] [Google Scholar]
- 24.Baba K, Benleulmi-Chaachoua A, Journe AS, Kamal M, et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. 2013;6:ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:365–375. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- 26.Sugden D, Yeh LK, Teh MT. Design of subtype selective melatonin receptor agonists and antagonists. Reprod Nutr Dev. 1990;39:335–44. doi: 10.1051/rnd:19990306. [DOI] [PubMed] [Google Scholar]
- 27.Legros C, Matthey U, Grelak T, Pedragona-Moreau S, et al. New Radioligands for Describing the Molecular Pharmacology of MT1 and MT2 Melatonin Receptors. Int J Mol Sci. 2013;14:8948–8962. doi: 10.3390/ijms14058948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niles LP. GTP modulates [125I]iodomelatonin binding to a picomolar-affinity site in the Syrian hamster hypothalamus. Eur J Pharmacol. 1990;189:95–98. doi: 10.1016/0922-4106(90)90234-o. [DOI] [PubMed] [Google Scholar]
- 29.Witt-Enderby PA, Dubocovich ML. Characterization and regulation of the human ML1A melatonin receptor stably expressed in Chinese hamster ovary cells. Mol Pharmacol. 1996;50:166–174. [PubMed] [Google Scholar]
- 30.Legros C, Devavry S, Caignard S, Tessier C, et al. Melatonin MT1 and MT2 receptors display different molecular pharmacologies only in the G-protein coupled state. Br J Pharmacol. 2014;171:186–201. doi: 10.1111/bph.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett P, MacLean A, Morgan PJ. Evidence for multiple forms of melatonin receptor-G-protein complexes by solubilization and gel electrophoresis. J Neuroendocrinol. 1994;6:509–515. doi: 10.1111/j.1365-2826.1994.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 32.Brydon L, Roka F, Petit L, de Coppet P, et al. Dual signaling of human Mel1a melatonin receptors via G[I2], G[I3], and G[Q/11] proteins. Mol Endocrinol. 1999;13:2025–2038. doi: 10.1210/mend.13.12.0390. [DOI] [PubMed] [Google Scholar]
- 33.Roka F, Brydon L, Waldhoer M, Strosberg AD, et al. Tight association of the human Mel[1a]-melatonin receptor and G[i]: precoupling and constitutive activity. Mol Pharmacol. 1999;56:1014–1024. doi: 10.1124/mol.56.5.1014. [DOI] [PubMed] [Google Scholar]
- 34.Guillaume JL, Daulat AM, Maurice P, Levoye A, et al. The PDZ protein mupp1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J Biol Chem. 2008;283:16762–16771. doi: 10.1074/jbc.M802069200. [DOI] [PubMed] [Google Scholar]
- 35.Maurice P, Daulat AM, Turecek R, Ivankova-Susankova K, et al. Molecular organization and dynamics of the melatonin MT receptor/RGS20/G[i] protein complex reveal asymmetry of receptor dimers for RGS and G[i] coupling. EMBO J. 2010;29:3646–3659. doi: 10.1038/emboj.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, et al. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:21522–21527. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- 37.Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66:312–321. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- 38.Levoye A, Jockers R, Ayoub MA, Delagrange P, et al. Are G protein-coupled receptor heterodimers of physiological relevance?--Focus on melatonin receptors. Chronobiol Int. 2006;23:419–426. doi: 10.1080/07420520500521863. [DOI] [PubMed] [Google Scholar]
- 39.Savaskan E, Wirz-Justice A, Olivieri G, Pache M, et al. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem. 2002;50:519–526. doi: 10.1177/002215540205000408. [DOI] [PubMed] [Google Scholar]
- 40.Savaskan E, Jockers R, Ayoub M, Angeloni D, et al. The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer's disease. Curr Alzheimer Res. 2007;4:47–51. doi: 10.2174/156720507779939823. [DOI] [PubMed] [Google Scholar]
- 41.Jockers R, Maurice P, Boutin JA, Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new. Br J Pharmacol. 2008;154:1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faria JA, Kinote A, Ignacio-Souza LM, de Araujo TM, et al. Melatonin acts through MT1/MT2 receptors to activate hypothalamic Akt and suppress hepatic gluconeogenesis in rats. Am J Physiol Endocrinol Metab. 2013;305:E230–242. doi: 10.1152/ajpendo.00094.2013. [DOI] [PubMed] [Google Scholar]
- 43.Ha E, Yim SV, Chung JH, Yoon KS, et al. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J Pineal Res. 2006;41:67–72. doi: 10.1111/j.1600-079X.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 44.Mao L, Dauchy RT, Blask DE, Slakey LM, et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3beta. Mol Endocrinol. 2012;26:1808–1820. doi: 10.1210/me.2012-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petit L, Lacroix I, de Coppet P, Strosberg AD, et al. Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3 ‘-5 ‘-monophosphate pathway. Biochem Pharmacol. 1999;58:633–639. doi: 10.1016/s0006-2952(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 46.Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- 47.Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 48.Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 49.Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;163:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 50.Conti A, Maestroni GJ. HPLC validation of a circadian melatonin rhythm in the pineal gland of inbred mice. J Pineal Res. 1996;20:138–144. doi: 10.1111/j.1600-079x.1996.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 51.Vivien-Roels B, Malan A, Rettori MC, et al. Daily variations in pineal melatonin concentrations in inbred and outbred mice. J Biol Rhythms. 1998;13:403–409. doi: 10.1177/074873098129000228. [DOI] [PubMed] [Google Scholar]
- 52.Kasahara T, Abe K, Mekada K, Yoshiki A, et al. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci USA. 2010;107:6412–6417. doi: 10.1073/pnas.0914399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin X, von Gall C, Pieschl RL, Gribkoff VK, et al. Targeted disruption of the mouse Mel[1b] melatonin receptor. Mol Cell Biol. 2003;23:1054–1060. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 55.Shibata S, Cassone VM, Moore RY. Effects of melatonin on neuronal activity in the rat suprachiasmatic nucleus in vitro. Neurosci Lett. 1989;97:140–144. doi: 10.1016/0304-3940(89)90153-5. [DOI] [PubMed] [Google Scholar]
- 56.Stehle J, Vanecek J, Vollrath L. Effects of melatonin on spontaneous electrical activity of neurons in rat suprachiasmatic nuclei: an in vitro iontophoretic study. J Neural Transm. 1989;78:173–177. doi: 10.1007/BF01252503. [DOI] [PubMed] [Google Scholar]
- 57.Benloucif S, Dubocovich ML. Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms. 1996;11:113–125. doi: 10.1177/074873049601100204. [DOI] [PubMed] [Google Scholar]
- 58.von Gall C, Weaver DR, Kock M, Korf HW, et al. Melatonin limits transcriptional impact of phosphoCREB in the mouse SCN via the Mel1a receptor. Neuroreport. 2000;11:1803–1807. doi: 10.1097/00001756-200006260-00002. [DOI] [PubMed] [Google Scholar]
- 59.Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, et al. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–1220. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- 60.Hunt AE, Al-Ghoul WM, Gillette MU, Dubocovich ML. Activation of MT[2] melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280:C110–118. doi: 10.1152/ajpcell.2001.280.1.C110. [DOI] [PubMed] [Google Scholar]
- 61.McArthur AJ, Hunt AE, Gillette MU. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology. 1997;138:627–634. doi: 10.1210/endo.138.2.4925. [DOI] [PubMed] [Google Scholar]
- 62.Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, et al. Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res. 2005;39:113–120. doi: 10.1111/j.1600-079X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 63.Pfeffer M, Rauch A, Korf HW, von Gall C. The endogenous melatonin [MT] signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol Int. 2012;29:415–429. doi: 10.3109/07420528.2012.667859. [DOI] [PubMed] [Google Scholar]
- 64.Holmes SW, Sugden D. Effects of melatonin on sleep and neurochemistry in the rat. Br J Pharmacol. 1982;76:95–101. doi: 10.1111/j.1476-5381.1982.tb09194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mirmiran M, Pévet P. Effects of melatonin and 5-methoxytryptamine on sleep-wake patterns in the male rat. J Pineal Res. 1986;3:135–141. doi: 10.1111/j.1600-079x.1986.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 66.Mailliet F, Galloux P, Poisson D. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology [Berl] 2001;156:417–26. doi: 10.1007/s002130100769. [DOI] [PubMed] [Google Scholar]
- 67.Wang F, Li J, Wu C, Yang J, Xu F, et al. The GABA[A] receptor mediates the hypnotic activity of melatonin in rats. Pharmacol Biochem Behav. 2003;74:573–578. doi: 10.1016/s0091-3057(02)01045-6. [DOI] [PubMed] [Google Scholar]
- 68.Fisher SP, Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci Lett. 2009;457:93–96. doi: 10.1016/j.neulet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, et al. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci. 2011;31:18439–18452. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Comai S, Ochoa-Sanchez R, Gobbi G. Sleep-wake characterization of double MT1/MT2receptor knockout mice and comparison with MT1and MT2receptor knockout mice. Behav Brain Res. 2013;243:231–238. doi: 10.1016/j.bbr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 71.Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiechmann AF, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alcantara-Contreras S, Baba K, Tosini G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neuroscience Letters. 2011;494:61–64. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sengupta A, Baba K, Mazzoni F, Pozdeyev NV, et al. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS One. 2011;6:e24483. doi: 10.1371/journal.pone.0024483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karamitri A, Renault N, Clement N, Guillaume JL, et al. Minireview: Toward the establishment of a link between melatonin and glucose homeostasis: association of melatonin MT2 receptor variants with type 2 diabetes. Mol Endocrinol. 2013;27:1217–1233. doi: 10.1210/me.2013-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milcu S. The effect of pinealectomy on plasma insulin in rats. In: GEW Wolstenholme, Knight J., editors. The pineal gland. Edinburgh/London: Churchill Livingstone; 1971. pp. 345–357. 1971. [Google Scholar]
- 78.Diaz B, Blazquez E. Effect of pinealectomy on plasma glucose, insulin and glucagon levels in the rat. Horm Metab Res. 1986;18:225–229. doi: 10.1055/s-2007-1012279. [DOI] [PubMed] [Google Scholar]
- 79.Nagorny CL, Sathanoori R, Voss U, Mulder H, et al. Distribution of melatonin receptors in murine pancreatic islets. J Pineal Res. 2011;50:412–417. doi: 10.1111/j.1600-079X.2011.00859.x. [DOI] [PubMed] [Google Scholar]
- 80.Kemp DM, Ubeda M, Habener JF. Identification and functional characterization of melatonin Mel 1a receptors in pancreatic beta cells: potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol Cell Endocrinol. 2002;191:157–166. doi: 10.1016/s0303-7207(02)00064-3. [DOI] [PubMed] [Google Scholar]
- 81.Peschke E, Muhlbauer E, Musshoff U, Csernus VJ, et al. Receptor MT1 mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J Pineal Res. 2002;33:63–71. doi: 10.1034/j.1600-079x.2002.02919.x. [DOI] [PubMed] [Google Scholar]
- 82.Picinato MC, Haber EP, Cipolla-Neto J, Curi R, et al. Melatonin inhibits insulin secretion and decreases PKA levels without interfering with glucose metabolism in rat pancreatic islets. J Pineal Res. 2002;33:156–160. doi: 10.1034/j.1600-079x.2002.02903.x. [DOI] [PubMed] [Google Scholar]
- 83.Stumpf I, Muhlbauer E, Peschke E. Involvement of the cGMP pathway in mediating the insulin-inhibitory effect of melatonin in pancreatic beta-cells. J Pineal Res. 2008;45:318–327. doi: 10.1111/j.1600-079X.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 84.Bahr I, Muhlbauer E, Albrecht E, Peschke E. Evidence of the receptor-mediated influence of melatonin on pancreatic glucagon secretion via the Galphaq protein-coupled and PI3K signaling pathways. J Pineal Res. 2012;53:390–398. doi: 10.1111/j.1600-079X.2012.01009.x. [DOI] [PubMed] [Google Scholar]
- 85.Lima FB, Matsushita DH, Hell NS, Dolnikoff MS, et al. The regulation of insulin action in isolated adipocytes. Role of the periodicity of food intake, time of day and melatonin. Braz J Med Biol Res. 1994;27:995–1000. [PubMed] [Google Scholar]
- 86.Anhe GF, Caperuto LC, Pereira-Da-Silva M, Souza LC, et al. In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J Neurochem. 2004;90:559–566. doi: 10.1111/j.1471-4159.2004.02514.x. [DOI] [PubMed] [Google Scholar]
- 87.Li C, Shi Y, You L, Wang L, et al. Melatonin receptor 1A gene polymorphism associated with polycystic ovary syndrome. Gynecol Obstet Invest. 2011;72:130–134. doi: 10.1159/000323542. [DOI] [PubMed] [Google Scholar]
- 88.Muhlbauer E, Gross E, Labucay K, Wolgast S, et al. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606:61–71. doi: 10.1016/j.ejphar.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 89.Contreras-Alcantara S, Baba K, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity [Silver Spring] 2010;18:1861–1863. doi: 10.1038/oby.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lincoln G. Melatonin modulation of prolactin and gonadotrophin secretion. Systems ancient and modern. Adv Exp Med Biol. 1999;460:137–153. doi: 10.1007/0-306-46814-x_16. [DOI] [PubMed] [Google Scholar]
- 91.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.von Gall C, Garabette ML, Kell CA, Frenzel S, et al. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002;5:234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- 93.Jilg A, Moek J, Weaver DR, Korf HW, et al. Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signalling. Eur J Neurosci. 2005;22:2845–2854. doi: 10.1111/j.1460-9568.2005.04485.x. [DOI] [PubMed] [Google Scholar]
- 94.Sheynzon P, Korf HW. Targeted deletions of Mel1a and Mel1b melatonin receptors affect pCREB levels in lactotroph and pars intermedia cells of mice. Neurosci Lett. 2006;407:48–52. doi: 10.1016/j.neulet.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 95.Yasuo S, Yoshimura T, Ebihara S, Korf HW. Melatonin transmits photoperiodic signals through the MT1 melatonin receptor. J Neurosci. 2009;29:2885–2889. doi: 10.1523/JNEUROSCI.0145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]