Abstract
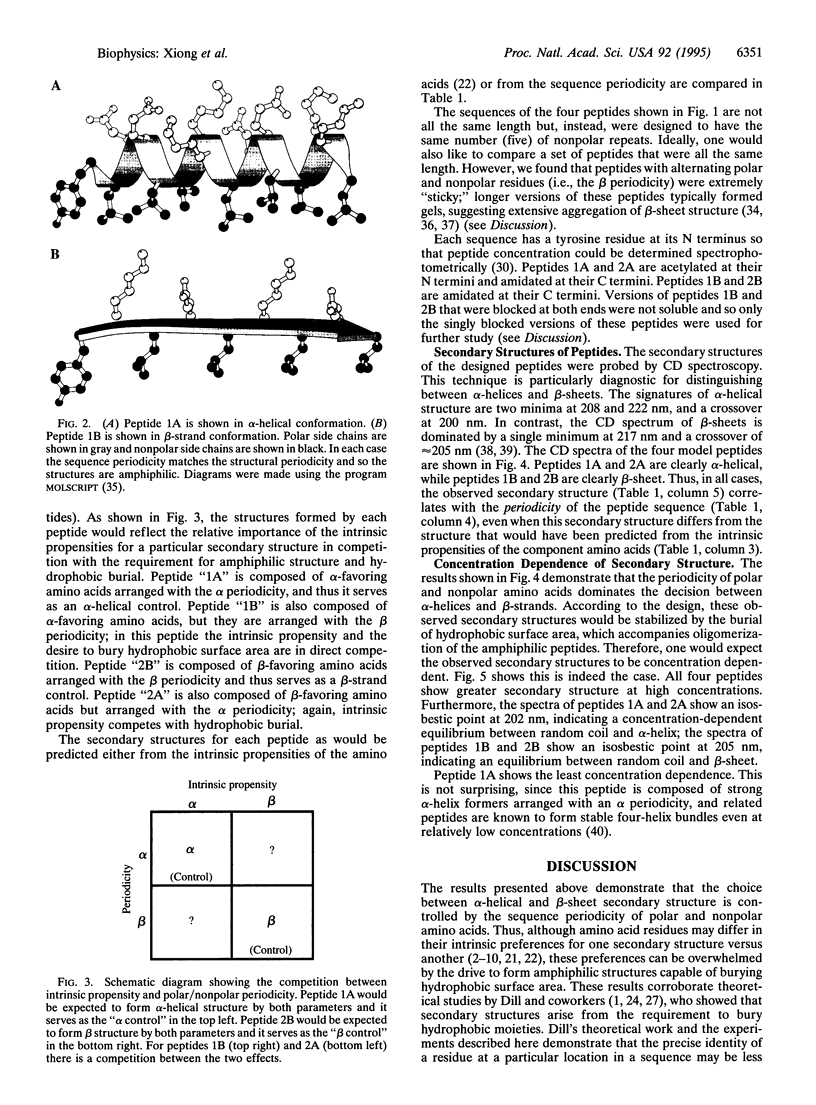
The tendency of a polypeptide chain to form alpha-helical or beta-strand secondary structure depends upon local and nonlocal effects. Local effects reflect the intrinsic propensities of the amino acid residues for particular secondary structures, while nonlocal effects reflect the positioning of the individual residues in the context of the entire amino acid sequence. In particular, the periodicity of polar and nonpolar residues specifies whether a given sequence is consistent with amphiphilic alpha-helices or beta-strands. The importance of intrinsic propensities was compared to that of polar/nonpolar periodicity by a direct competition. Synthetic peptides were designed using residues with intrinsic propensities that favored one or the other type of secondary structure. The polar/nonpolar periodicities of the peptides were designed either to be consistent with the secondary structure favored by the intrinsic propensities of the component residues or in other cases to oppose these intrinsic propensities. Characterization of the synthetic peptides demonstrated that in all cases the observed secondary structure correlates with the periodicity of the peptide sequence--even when this secondary structure differs from that predicted from the intrinsic propensities of the component amino acids. The observed secondary structures are concentration dependent, indicating that oligomerization of the amphiphilic peptides is responsible for the observed secondary structures. Thus, for self-assembling oligomeric peptides, the polar/nonpolar periodicity can overwhelm the intrinsic propensities of the amino acid residues and serves as the major determinant of peptide secondary structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Sun D. P., Wilson K., Wozniak J. A., Cook S. P., Matthews B. W. Contributions of hydrogen bonds of Thr 157 to the thermodynamic stability of phage T4 lysozyme. Nature. 1987 Nov 5;330(6143):41–46. doi: 10.1038/330041a0. [DOI] [PubMed] [Google Scholar]
- Anderson D. E., Becktel W. J., Dahlquist F. W. pH-induced denaturation of proteins: a single salt bridge contributes 3-5 kcal/mol to the free energy of folding of T4 lysozyme. Biochemistry. 1990 Mar 6;29(9):2403–2408. doi: 10.1021/bi00461a025. [DOI] [PubMed] [Google Scholar]
- Argos P. Analysis of sequence-similar pentapeptides in unrelated protein tertiary structures. Strategies for protein folding and a guide for site-directed mutagenesis. J Mol Biol. 1987 Sep 20;197(2):331–348. doi: 10.1016/0022-2836(87)90127-6. [DOI] [PubMed] [Google Scholar]
- Bajaj M., Blundell T. Evolution and the tertiary structure of proteins. Annu Rev Biophys Bioeng. 1984;13:453–492. doi: 10.1146/annurev.bb.13.060184.002321. [DOI] [PubMed] [Google Scholar]
- Blaber M., Zhang X. J., Matthews B. W. Structural basis of amino acid alpha helix propensity. Science. 1993 Jun 11;260(5114):1637–1640. doi: 10.1126/science.8503008. [DOI] [PubMed] [Google Scholar]
- Brack A., Orgel L. E. Beta structures of alternating polypeptides and their possible prebiotic significance. Nature. 1975 Jul 31;256(5516):383–387. doi: 10.1038/256383a0. [DOI] [PubMed] [Google Scholar]
- Chakrabartty A., Kortemme T., Baldwin R. L. Helix propensities of the amino acids measured in alanine-based peptides without helix-stabilizing side-chain interactions. Protein Sci. 1994 May;3(5):843–852. doi: 10.1002/pro.5560030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Dill K. A., Fiebig K. M., Chan H. S. Cooperativity in protein-folding kinetics. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1942–1946. doi: 10.1073/pnas.90.5.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Baase W. A., Zhang X. J., Heinz D. W., Blaber M., Baldwin E. P., Matthews B. W. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992 Jan 10;255(5041):178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Hecht M. H. De novo design of beta-sheet proteins. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8729–8730. doi: 10.1073/pnas.91.19.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M. H., Richardson J. S., Richardson D. C., Ogden R. C. De novo design, expression, and characterization of Felix: a four-helix bundle protein of native-like sequence. Science. 1990 Aug 24;249(4971):884–891. doi: 10.1126/science.2392678. [DOI] [PubMed] [Google Scholar]
- Horovitz A., Matthews J. M., Fersht A. R. Alpha-helix stability in proteins. II. Factors that influence stability at an internal position. J Mol Biol. 1992 Sep 20;227(2):560–568. doi: 10.1016/0022-2836(92)90907-2. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Protein secondary structure and circular dichroism: a practical guide. Proteins. 1990;7(3):205–214. doi: 10.1002/prot.340070302. [DOI] [PubMed] [Google Scholar]
- Johnsson K., Allemann R. K., Widmer H., Benner S. A. Synthesis, structure and activity of artificial, rationally designed catalytic polypeptides. Nature. 1993 Oct 7;365(6446):530–532. doi: 10.1038/365530a0. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kamtekar S., Schiffer J. M., Xiong H., Babik J. M., Hecht M. H. Protein design by binary patterning of polar and nonpolar amino acids. Science. 1993 Dec 10;262(5140):1680–1685. doi: 10.1126/science.8259512. [DOI] [PubMed] [Google Scholar]
- Kim C. A., Berg J. M. Thermodynamic beta-sheet propensities measured using a zinc-finger host peptide. Nature. 1993 Mar 18;362(6417):267–270. doi: 10.1038/362267a0. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. Intermediates in the folding reactions of small proteins. Annu Rev Biochem. 1990;59:631–660. doi: 10.1146/annurev.bi.59.070190.003215. [DOI] [PubMed] [Google Scholar]
- Levitt M. Conformational preferences of amino acids in globular proteins. Biochemistry. 1978 Oct 3;17(20):4277–4285. doi: 10.1021/bi00613a026. [DOI] [PubMed] [Google Scholar]
- Lyu P. C., Liff M. I., Marky L. A., Kallenbach N. R. Side chain contributions to the stability of alpha-helical structure in peptides. Science. 1990 Nov 2;250(4981):669–673. doi: 10.1126/science.2237416. [DOI] [PubMed] [Google Scholar]
- Merutka G., Lipton W., Shalongo W., Park S. H., Stellwagen E. Effect of central-residue replacements on the helical stability of a monomeric peptide. Biochemistry. 1990 Aug 14;29(32):7511–7515. doi: 10.1021/bi00484a021. [DOI] [PubMed] [Google Scholar]
- Minor D. L., Jr, Kim P. S. Measurement of the beta-sheet-forming propensities of amino acids. Nature. 1994 Feb 17;367(6464):660–663. doi: 10.1038/367660a0. [DOI] [PubMed] [Google Scholar]
- Mutter M. Nature's rules and chemist's tools: a way for creating novel proteins. Trends Biochem Sci. 1988 Jul;13(7):260–265. doi: 10.1016/0968-0004(88)90159-4. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990 Nov 2;250(4981):646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- Osterman D. G., Kaiser E. T. Design and characterization of peptides with amphiphilic beta-strand structures. J Cell Biochem. 1985;29(2):57–72. doi: 10.1002/jcb.240290202. [DOI] [PubMed] [Google Scholar]
- Regan L., DeGrado W. F. Characterization of a helical protein designed from first principles. Science. 1988 Aug 19;241(4868):976–978. doi: 10.1126/science.3043666. [DOI] [PubMed] [Google Scholar]
- Smith C. K., Withka J. M., Regan L. A thermodynamic scale for the beta-sheet forming tendencies of the amino acids. Biochemistry. 1994 May 10;33(18):5510–5517. doi: 10.1021/bi00184a020. [DOI] [PubMed] [Google Scholar]
- Yue K., Dill K. A. Inverse protein folding problem: designing polymer sequences. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4163–4167. doi: 10.1073/pnas.89.9.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Holmes T., Lockshin C., Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N. E., Kay C. M., Sykes B. D., Hodges R. S. A single-stranded amphipathic alpha-helix in aqueous solution: design, structural characterization, and its application for determining alpha-helical propensities of amino acids. Biochemistry. 1993 Jun 22;32(24):6190–6197. doi: 10.1021/bi00075a011. [DOI] [PubMed] [Google Scholar]



