Abstract
Background
Open surgery effectively treats mesial temporal lobe epilepsy (MTLE), but carries risks of neurocognitive deficits, which may be reduced with minimally invasive alternatives.
Objective
To describe technical and clinical outcomes of stereotactic laser amygdalohippocampotomy (SLAH) with real-time magnetic resonance thermal imaging (MRTI) guidance.
Methods
Under general anesthesia and utilizing standard stereotactic methods, 13 adult patients with intractable MTLE (with and without mesial temporal sclerosis, MTS) prospectively underwent insertion of a saline-cooled fiber-optic laser applicator into amygdalohippocampal structures from an occipital trajectory. Computer-controlled laser ablation was performed during continuous MRTI followed by confirmatory contrast-enhanced anatomic imaging and volumetric reconstruction. Clinical outcomes were determined from seizure diaries.
Results
A mean 60% volume of the amygdalohippocampal complex was ablated in 13 patients (9 with MTS) undergoing 15 procedures. Median hospitalization was one day. With follow-up ranging from 5-26 (median 14) months, 77% (10/13) of patients achieved meaningful seizure reduction, of which 54% (7/13) were free of disabling seizures. Of patients with preoperative MTS, 67% (6/9) achieved seizure freedom. All recurrences were observed by<6 months. Variances in ablation volume and length did not account for individual clinical outcomes. Whereas no complications of laser therapy itself were observed, one significant complication, a visual field defect, resulted from deviated insertion of a stereotactic aligning rod, which was corrected prior to ablation.
Conclusion
Real-time MR-guided SLAH is a technically novel, safe, and effective alternative to open surgery. Further evaluation with larger cohorts over time is warranted.
Keywords: Epilepsy, laser therapy, magnetic resonance imaging, minimally invasive surgical procedures, stereotactic techniques, temporal lobe, thermometry
Introduction
Surgical resection is the gold standard treatment for drug-resistant focal epilepsy, including mesial temporal lobe epilepsy (MTLE) or other focal cortical lesions with correlated electrophysiological features. Anterior temporal lobectomy with amygdalohippocampectomy (ATLAH) has been shown to be more efficacious than continued medical therapy in a randomized, control trial.1 Focal resections, including ATLAH and selective amygdalohippocampectomy (SAH) yield 60-80% seizure freedom rates in highly selected patients, such as those found to have mesial temporal sclerosis (MTS) on preoperative imaging, but resections are associated with cognitive impairments or focal neurological deficits.2–7
Minimally invasive approaches to treating MTLE might achieve seizure freedom while minimizing adverse effects. MR-guided stereotactic laser ablation is a minimally invasive alternative that utilizes small applicators amenable to stereotactic delivery. Heating is dependent upon source wavelength such that a source laser can be chosen to produce rapid and localized heating of tissue with sharp boundaries at relatively low powers.8 Because optical fibers and laser energy are MRI compatible, simultaneous magnetic resonance thermal imaging (MRTI), with accuracy on the order of ±0.2°C in a number of tissue types enables real-time feedback control of laser output and tissue ablation. MR-guided stereotactic laser ablation has been safely utilized for ablation of intracranial lesions including tumors, and certain epileptogenic foci in children,9–15 and the requisite device has been cleared by the FDA for tissue ablation in neurosurgery.
Utilizing standard stereotactic methods, including either a rigid stereotactic head frame or an MR-guided trajectory frame, we describe our technical approach and early clinical results utilizing minimally invasive MR-guided stereotactic laser ablation of the amygdala and hippocampus (stereotactic laser amygdalohippocampotomy, SLAH). We report our first 15 ablations in 13 adult patients with MTLE, including cases both with and without MTS on preoperative imaging.
Methods
Patient selection
All patients were evaluated by a standard protocol of noninvasive studies including 3 Tesla MRI, 18-fluorodeoxyglucose (18-FDG) positron emission tomography (PET), neuropsychological testing, and inpatient video-EEG monitoring. Functional MRI and intracarotid amobarbital (Wada) testing were often performed to lateralize language dominance and predict risk of postoperative memory deficits. A multidisciplinary committee of epilepsy neurologists, neuropsychologists, and neurosurgeons reviewed results for level of concordance of noninvasive studies and to provide consensus recommendations regarding surgery. Additional intracranial electrode monitoring was performed on two patients in this series. All patients in whom preoperative studies were consistent with focal unilateral seizure onsets within mesial temporal structures were considered candidates for mesial temporal lobe surgery. These included patients with diverse preoperative MRI findings including MTS (mesial temporal atrophy with T2/FLAIR sequence signal change), mesial temporal atrophy only (MTA), mesial temporal signal change only (MT-T2), or normal. Patients with prior temporal lobe surgery, depth electrode intracranial monitoring, or other intracranial abnormalities were not excluded. Both standard open temporal lobe surgery and minimally invasive SLAH were offered to 17 consecutive patients, 13 of which chose SLAH. Twelve SLAH patients provided informed written consents for prospective research procedures (collection of patient information such as seizure diaries, additional imaging, neuropsychological testing, etc.); one SLAH patient underwent limited retrospective examination only. Over the same time period, 4 of 17 patients offered SLAH actually selected open procedures, and 7 additional patients that underwent craniotomies for subdural grid electrodes placement followed by open temporal lobe resections were not offered SLAH. The Emory University Institutional Review Board approved all procedures. Separate standard informed surgical consent was obtained for SLAH itself, which is an ablative procedure using an FDA-cleared surgical device. Two surgeons (JTW and REG) performed SLAH on patients between July 2011 and June 2013 (with one patient undergoing a second procedure in December 2013) using MRI-guided laser treatment following laser applicator placement by either of two standard stereotactic approaches:
Laser Applicator Placement Utilizing a Standard Stereotactic Frame
Nine of 13 patients underwent placement of an MR-compatible stereotactic head frame (CRW, Integra Neurosciences, Plainsboro, NJ) following general endotracheal anesthesia. The frame was affixed to the skull in standard fashion with four skull pins, but with 10-15 degrees of axial rotation and inferior placement of ipsilateral posterior skull pin into the mastoid region to avoid collision with an occipital approach. The MRI fiducial localizer was secured to the base frame, and volumetric image series (T2 FLAIR and T1 post contrast) were acquired using a 1.5-Tesla MRI scanner (Magnetom Espree, Siemens Medical Solutions).
Each patient was transported to the operating room, positioned supine semi-sitting with the neck flexed, and the CRW base ring was affixed to the operating table via a Mayfield adaptor. Stereotactic planning was performed using FrameLink® software (Stealth Treon® or S7® workstations, Medtronic, Louisville, CO). Linear trajectories from the lateral occipital region (starting approximately 4-6 cm superior to the inion and 4-6 cm lateral to midline) through the long axis of the hippocampal body and into the pes hippocampus were selected, terminating in the amygdala. The posterior extent of hippocampal penetration extended at least to the level of the lateral mesencephalic sulcus. Optimal trajectories avoided cortical and sulcal vasculature and the choroid plexus; ventricle penetration was occasionally required, but could generally be avoided by creating inferior or lateral approaches.
The planned entry site was minimally clipped and widely prepped. The sterile CRW arc set to appropriate frame coordinates was mounted over the draped base ring, the entry site was infused with local anesthetic, a small stab incision was made, and a 3.2 mm inner diameter drill guide tube was lowered into the incision and braced against the bone. A craniostomy-durotomy was performed with a 3.2 mm twist drill. The guide was replaced with a 1.9 mm reducing cannula, through which a 1.6-mm stereotactic alignment rod was inserted and used to guide insertion into the twist-drill hole of a threaded polycarbonate anchor bolt with 3.2 mm outer diameter and 1.8 mm inner diameter (Figure 1A; Visualase® Inc., Houston, TX). The alignment rod was then inserted through the anchor bolt and into the brain during live lateral fluoroscopic visualization to verify alignment with stereotactic frame center. The distance to target from the top of the bolt was calculated. The laser applicator assembly (Visualase® Inc., Houston, TX) is comprised of an outer 1.6-mm diameter clear (light transmitting) polycarbonate cooling catheter and inner 0.73-mm diameter flexible laser optical fiber with10-mm long diffuser tip (Figure 1A). After removing the alignment rod from the anchor bolt, the cooling catheter (marked at the distance-to-target) containing a stainless steel stiffening stylet (Figure 1A) was inserted to target under lateral fluoroscopic visualization (Figure 1B). The stylet was then removed and the laser optical fiber with diffuser tip was inserted to the end of the closed-ended cooling catheter. The laser applicator assembly was secured in place via a Touhy-Borst adapter on the proximal end of the anchor bolt. The stereotactic arc was detached, the drapes were removed, and the patient was then transported to the MRI suite and placed supine within the magnet with the head turned to situate the treatment side up, to protect the laser assembly and anchor bolt.
Figure 1. Laser thermal therapy system for stereotactic targeting and focused ablation.
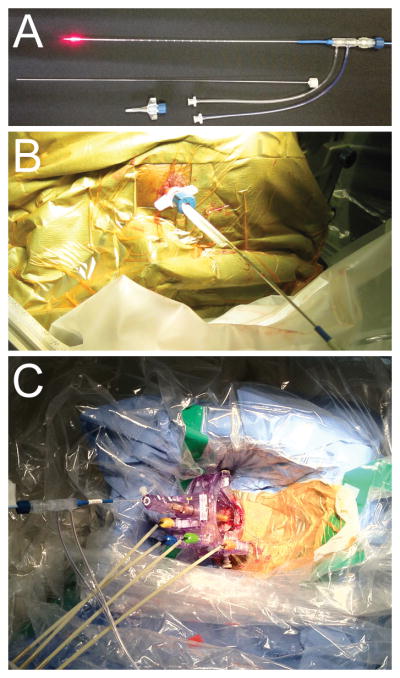
A, A 15 W 980 nm diode laser (Visualase®) is directed through a saline-cooled laser applicator (top), consisting of a 400 μm core silica optical fiber, capped with a cylindrical diffusing tip (red). The optical fiber is housed within a 1.65 mm diameter polycarbonate saline-cooled cannula. A threaded polycarbonate bone anchor (bottom) is placed over a stiffening stylet (middle) via various stereotactic methods to target deep brain structures. B, Targeting of the laser applicator from an occipital approach with a bone anchor and a CRW stereotactic frame (Integra®). C, Use of an MRI-guided stereotactic trajectory frame (Clearpoint SmartFrame®) with the Visualase® applicator enables implantation and real-time localization of the laser applicator within an MRI suite.
Laser Applicator Placement Utilizing an MRI-Guided Trajectory Frame
Five patients underwent endotracheal general anesthesia in the MRI suite and were placed into a prone position with the head secured to the table of the interventional MR scanner by 4 skull pins using a proprietary head coil fixation system (MRI Interventions, Inc.). The exposed occipital region was clipped, prepped, and draped. A sterile self-adhesive fiducial grid (SmartGrid®, MRI Interventions, Inc.) was placed over the approximate entry. Planning scans were then obtained and targeting performed as described above except for use of the MRI Interventions, Inc. workstation (rather than FrameLink®). An entry marking divot in the skull was created by puncturing through the grid and scalp into bone using a marking trocar. The grid was removed, the scalp infused with local anesthetic, and a minimal linear incision was performed with a scalpel. An approximately 14-mm burr hole centered on the marking divot was drilled using an MR-compatible high-speed air drill. The MRI-guided trajectory frame base was affixed overlying the burr hole with self-tapping screws. A minimal durotomy was opened in cruciate fashion. The trajectory frame components were assembled including a hand controller. A series of short planning scans were obtained and the workstation computed suggested adjustments in x, y, pitch and roll that were used to align the frame cannula with the planned trajectory. A ceramic rod was inserted part way to confirm accurate trajectory alignment, and then the Visualase® cooling cannula with stiffening stylet was inserted through a reducing cannula, locked in place (Figure 1C) and confirmed with volumetric imaging. The stylet was removed and replaced with the optical fiber.
MR-guided Laser Treatment
In all cases, the laser fiber and cooling lines were routed from the patient to the control room and connected to the proprietary Visualase® workstation, which combines a 15 W 980 nm diode laser with an image-processing workstation for real-time MRTI and modeled estimates of the thermal necrosis zone. Initially, volumetric 3-D T1 weighted sequences were acquired to verify proper positioning through the hippocampus and to select appropriate imaging planes for monitoring during ablation. User-defined specific temperature safety limits were set relative to the monitoring image, in the inferior lateral thalamus, basal ganglia, and lateral mesencephalon (Figure 2A) to automatically terminate laser delivery if these structures exceeded 45°C, avoiding off-target thermal injury (Figure 2B). The initial lesion was made anteriorly in the amygdalohippocampal complex during real-time MRTI and modeling of the necrotizing zone (Figure 2C-D). The laser fiber was then retracted in approximately 1.0 cm increments, and up to 5 overlapping focal ablations were created, resulting in a confluent tubular ablation zone (Figure 2F) encompassing the amygdalohippocampal complex posteriorly to at least the lateral mesencephalic sulcus (posterior landmark visualized on axial imaging, Figure 2G).
Figure 2. Illustrative treatment cycle.
A, Patient 4 was stereotactically implanted from an occipital approach along the long axis of the amygdalohippocampal complex. Using the Visualase® workstation, multiple points (three shown in this plane) were demarcated for intraoperative MRI thermal measurement – red circle (in the ablation zone), white square (anterior mesencephalon), and blue diamond (lateral mesencephalon). B, Measurement of the temperature at the three points during the ablation. Pink hashed area indicates ablation temperatures >90°C, above which thermal spread is considered unpredictable,19,20 and which triggers cycle termination by the workstation. Note that the brainstem temperature does not increase during the procedure. Real-time temperature measurements (left) and estimated ablation areas (right) at 25 seconds (C), 75 seconds (D), and 130 seconds (E). F, After retracting the laser applicator and ablating at a second site, the final estimate of the total ablated area was calculated. Immediate post-operative T1-weighted imaging with contrast highlights the borders of the lesion in the axial (G, red arrow) and coronal (H) planes. I-J, 6 months after the procedure, the amygdalohippocampal complex demonstrates well-circumscribed non-enhancing pseudocystic atrophy.
Post-procedure MRI including DWI, FLAIR, and T1 post gadolinium contrast (Figure 2G-H) were acquired, verifying the final lesion location and volume. Laser applicators and anchor bolts were completely removed, a titanium burr hole cover was affixed, incisions were closed with resorbable suture, and stereotactic frames were removed. Patients were typically admitted to the hospital for observation, administered dexamethasone for 24 hours, and discharged with a one-week oral dexamethasone taper.
Clinical and imaging follow-up
Patients were evaluated at 2, 6, 12, and 24 months post treatment. Follow-up visits included collection of patient seizure diaries, evaluation for complications, verification of medication status, and quality of life surveys. Repeat MRI was performed at 6 months post treatment (Figure 2I-J). Partial neuropsychological evaluation was performed at 6 months and full evaluation at 12 months. A full description of neuropsychological outcomes for SLAH in this cohort is the subject of additional reports.16
Image processing
Utilizing a BrainLab workstation and the iPlan 3.0 application, the amygdala and hippocampus on the targeted side were manually segmented and volumes reconstructed from pre-procedure T1 gadolinium-contrasted volumetric images using standard anatomic definitions in three planes as described by Pruessner et al,17 with slight modification (alveus and fimbria were not included in the hippocampal volume, as these structures were not universally visualized). Likewise, postoperative ablation volumes were generated by manually segmenting the ring-enhanced ablation zone. Pre- and post-procedure image sets were spatially co-registered, allowing direct volumetric comparison of ablation zones versus anatomic targets (Figure 3). The amount of each target showing evidence of ablation was calculated and expressed as a percent.
Figure 3. Volumetric reconstruction of ablation zone with respect to amygdala and hippocampus.

A, Axial segmentation of the amygdala (yellow boundary), hippocampus (green), and ablation zone (red) of patient 4. Laser applicator trajectory (blue and white dashed line) is also marked. B, Sagittal segmentation of the same tissue, as well as the applicator track (blue). C, 3-D reconstruction of the amygdala (yellow) and hippocampus (green), as well as the path of the fiber optic (blue) through the tissue. D, 3-D reconstruction from segmented sections of the ablated tissue (red) within the amygdala and hippocampus.
Results
Demographics and diagnostic findings
Patient demographics and preoperative diagnostic findings are displayed in Table 1. Seventeen consecutive MTLE patients deemed to be candidates for mesial temporal resections (without prior subdural grid electrode placement) were offered either a standard open temporal resection or minimally invasive SLAH. Whereas 4 patients elected to undergo open surgery, 13 selected SLAH and were enrolled (6 male, 7 female, ages range 16-64 y, age median 24 y). Two subjects (patients 6 and 7) had undergone placement of vagal nerve stimulators prior to current surgical evaluation, and 2 subjects (patients 10 and 12) had undergone previous open temporal lobe surgeries at other institutions. Specifically, patient 10 had remote craniotomy for complete resection of a juvenile pilocytic astrocytoma in the posterior lateral temporal lobe, but subsequently developed epilepsy; intracranial monitoring (ICM) with subdural strip and intraparenchymal depth electrodes confirmed seizures emanating from preserved mesial temporal structures (anterior hippocampus) rather than encephalomalacia contiguous with the resection cavity. Patient 12 had undergone attempted SAH with sparing of mesial temporal structures, after which seizures persisted. Two subjects in this series with equivocal preoperative imaging or other discordant features (patients 10 and 13, Table 1) underwent ICM (bilateral strip and depth electrodes) for localization of seizure onsets prior to laser ablation.
Table 1. Patient demographics and preoperative diagnostic results.
| Patient | Sex | Age at SLAH (y) | Age at onset (y) | Seizure Type | MRI | 18-FDG PET hypo-metabolism | Memory impairment | Video EEG temporal lobe seizure onset | |
|---|---|---|---|---|---|---|---|---|---|
| Neuropsychological testing | Intracarotid amobarbital (Wada) test | ||||||||
| 1 MB | Female | 45 | 36 | CPS | R MT-T2 | R | Bilateral (mild deficits in complex learning only) | R>L | R |
| 2 MC | Male | 22 | 14 | CPS | MT-Normal | R≫L | R>L | R | R |
| 3 RA | Male | 30 | 3 | CPS | L MTS | L | L>R | L | L |
| 4 BS | Male | 18 | 6 | CPS | L MTS | L | L | L | L |
| 5 ML | Female | 24 | 21 | CPS + PNES | L MTS | R>L | L>R | L | L> R |
| 6 TP | Female | 55 | 9 | CPS | Bilateral MTS (L>R) | L | L | L | L |
| 7 BO | Female | 64 | 5 | CPS | R MTS | R | R | Not performed | R |
| 8 SD | Female | 58 | 13 | CPS | L MTS | L | L | L (mild) | L |
| 9 EE | Male | 30 | 1 | CPS + GTC | L MTS | L | L (mild) | L | L |
| 10 MP | Female | 21 | 16 | CPS | MT-normal; atrophic R fornix and MB; R posterior temporalencephalomalacia | L (mild) | L (mild) | R | R (ICM with depth and strip electrodes implicated hippocampus) |
| 11 GA | Male | 23 | <1 | CPS + GTC | L MTS | L>R | Bilateral | Not performed | L |
| 12 MM | Male | 16 | 12 | CPS + GTC | R MTS, prior SAH sparing AHC | R | Unavailable | Not performed | R |
| 13 TS | Female | 18 | 10 | CPS + GTC | L>R MTA; R parietal encephalomalacia | L | L>R | L | L (ICM with depth and strip electrodes implicated hippocampus) |
R, Right; L, Left; CPS, complex partial seizures; MRI, magnetic resonance imaging; MT-T2, mesial temporal signal change on T2 sequence MRI (without atrophy); MT-normal, no mesial temporal atrophy or signal change; MTS, mesial temporal sclerosis (T2 signal change and atrophy); MB, mammillary body; AHC, amygdalohippocampal complex; MTA, mesial temporal atrophy (without signal change); SAH, open selective amygdalohippocampectomy; PNES, psychogenic nonepileptic seizures; 18-FDG PET, 18-fluorodeoxyglucose positron emission tomography; ICM, intracranial electrode monitoring used for confirmation.
Subjects had sufficient concordance of seizure semiology (complex partial seizures) and diagnostic studies (ie, MRI, 18-FDG-PET hypometabolism, neurocognitive profile, and EEG onset) to recommend surgical treatment of MTLE (Table 1). Nine of 13 subjects met strict radiological criteria for ipsilateral MTS (both hippocampal atrophy and increased T2/FLAIR signal intensity) including patient 6 with bilateral MTS (electroencephalographic onset from the more radiologically abnormal left side). Four subjects did not meet strict radiological criteria for MTS: patient 1 (mesial temporal T2/FLAIR signal change without atrophy), patient 2 (neither signal change nor atrophy, whose MRI could be characterized as ‘normal’), patient 10 (‘normal’ mesial temporal structures, but with contiguous posterior lateral temporal encephalomalacia), and patient 13 (mesial temporal atrophy without signal change). Except patient 5, remaining subjects had unilateral or predominant 18-FDG-PET hypometabolism on the side concordant with other findings. Similarly, neurocognitive deficits were concordant in all subjects except patient 12, in whom preoperative data were not available. EEG recorded seizure onsets unilaterally in all subjects except patient 5, who had preoperative evidence of bilateral temporal (left > right) seizure onsets (concordant with left MTS but right > left 18-FDG-PET hypometabolism).
Technical and imaging results
Applicator placement and thermal imaging were accomplished in all cases. Applicator placement relative to location of specific safety temperature limits, a complete thermal treatment cycle, and final acute and chronic ablation results are illustrated specifically for patient 4 (Figure 2). Illustrative ablation imaging is demonstrated for all 15 procedures in 13 patients in the Figure, Supplemental Content 1. In all but the first procedure (patient 1, discussed below) the laser assembly was accurately placed within the amygdala and anterior hippocampus extending posteriorly to a point parallel to the lateral mesencephalic sulcus from a single trajectory.
Patient group treatment summaries are displayed in Table 2; individual patients treatment details are displayed in the Table, Supplemental Content 2. Overall, patients were treated with a median of 3 contiguous ablation zones (Table 2) along a single trajectory per surgical session to achieve a total ablation area involving the amygdala and hippocampus. The hippocampal ablation extended at least as posterior as the lateral mesencephalic sulcus in all subjects (see Figure, Supplemental Content 1). Ongoing thermal imaging maps and integrated estimates of ablation zone were viewed in real time on the Visualase® workstation. The desired treatment areas were generally achieved with laser power of 12W (rarely up to 14 W) and mean exposure time 9.6 +/- 6.4 min (range 3.4-26.1 min) (Table 2; Supplemental Content 2). Cycle time was user defined to balance the spread of ablation while maintaining tissue treatment temperature < 90°C.
Table 2. Summary of 15 treatments in 13 patients.
| Side treated | Stereotactic platform | Laser treatment zones (number) | Laser treatment time (min)* | Length of hippocampus ablated (cm) | Ablation volume (cm3) | Proportion of structure (s) ablated (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Hippocampus | Amygdala | AHC | |||||||
| 6 Right, 9 Left | CRW 10 procedures in 9 patients, CPSF 5 procedures in 5 patients | Mean +/- SD: | 3.1 +/- 1.2 | 9.6 +/- 6.4 | 2.5 +/- 0.44 | 5.3 +/- 1.1 | 63 +/- 10 | 54 +/- 27 | 60 +/- 9.7 |
| Median: | 3 | 7.7 | 2.6 | 5.32 | 62.5 | 57.5 | 59.5 | ||
| Range: | 2 - 5 | 3.4 - 26.1 | 1.55 - 3.07 | 3.52 - 7.59 | 54 - 86 | 4 - 88 | 46 - 76 | ||
Data presented as mean +/- standard deviation of the mean. Ablation length and volumes estimates were generated post hoc from manually segmented volumetric T1 post contrast sequence MRI obtained immediately following treatment and compared to preoperative manually segmented structures.
Laser therapy almost exclusively performed at power = 12 Watt; CRW, Cosman-Roberts-Wells rigid frame (Integra, Inc.); CPSF, ClearPoint SmartFrame MRI-guided trajectory frame (MRI Interventions, Inc.); AHC, amygdalohippocampal complex.
During the SLAH procedure, the lateral ventricle appeared to have an insulating heat sink effect, with ablation zones generally spreading to conform to the superior surface of the hippocampus without thermal spread across CSF spaces (temporal horn, transverse cerebral fissure, or ambient cistern), sparing medial structures (basal ganglia, thalamus, optic tract, or midbrain). Moreover, ablation zones (as confirmed by immediate post procedure FLAIR, DWI and contrast-enhanced T1 signal intensity) tended to incorporate large portions of the hippocampus, medial to lateral, at any given coronal location, forming an eccentric volume with respect to the laser assembly, apparently delimited by the ependymal lining (superiorly and laterally), the transverse cerebral fissure (medially) and, more variably, the hippocampal sulcus or subiculum (inferiorly). Indeed, safety limit set points, which were user-defined in the software interface to terminate laser delivery if any point on the brainstem, optic apparatus and/or thalamus exceeded 45°C, never changed from baseline temperatures (Figure 2A-B). Individual cycles were terminated when on-target temperatures reached 90°C, thus avoiding the risk of char and thermal gassing in target tissues.
Thermal and anatomic imaging overlays yielded integrated ablation zone estimates that correlated well with immediate post-procedure gadolinium contrasted T1 images (Figure 2; Supplemental Content 1). Likewise, MRI scans obtained at 6 months post-procedure (in all but patients 5 and 12) revealed evidence of non-enhancing pseudocystic atrophy confined to treated mesial temporal structures as illustrated in patient 4 (Figure 2I, J).
From immediate post-procedure gadolinium contrasted T1 images, final mean hippocampal length ablated was 2.5 +/- 0.44 cm (Table 2; range 1.55-3.07 cm, Supplemental Content 2) and final mean total ablation volume was 5.3 +/- 1.1 cm3 (Table 2; range 3.52-7.59 cm3, Supplemental Content 2). Post-hoc anatomically segmented volumetric reconstruction of hippocampus and amygdala (as illustrated in Figure 3) revealed mean percent ablations of these individual structures of 63 +/- 10% (range 49-86%) and 54 +/- 27% (range 4-88%), respectively, with a mean cumulative amygdalohippocampal complex (AHC) ablated volume of 60 +/- 9.7% (range 49-76%) (Table 2; Supplemental Content 2).
Patients 1 and 13 in the series had initial ablations of AHC that were judged by imaging to be insufficient after seizures persisted; both underwent repeat SLAH with the aim of additional AHC ablation. In the case of the first procedure in patient 1, the laser fiber assembly had been passed inferiorly through the parahippocampal region rather than the hippocampus, which was recognized upon MR imaging following insertion. We nevertheless proceeded with ablation within the parahippocampal gyrus anticipating superior spread into the amygdalohippocampal complex (AHC). This ablation, however, appeared to be limited superiorly by the hippocampal sulcus, encompassing 50% of the AHC including 59% of the hippocampus but only 4.4% of the amygdale (see Table, Supplemental Content 2). Six months later, SLAH was repeated with hippocampus-centered fiber placement, achieving an AHC ablation of 69% (67% of hippocampus and 66% of amygdala, Supplemental Content 2). Likewise, in the case of patient 13, despite an initial 66% AHC ablation (55% hippocampus ablated and 83% amygdala ablated, Supplemental Content 2), seizures persisted, and 6-month imaging indicated a rim of spared mesial temporal tissue. We therefore repeated SLAH more medially, increasing ablation volumes to 76% (AHC), 69% (hippocampus), and 88% (amygdala).
Patient 5 underwent a technically successful SLAH (Supplemental Content 2) but seizures persisted, and she underwent open ATLAH at 5 months post SLAH, yielding pathological specimens (hippocampus and parahippocampal gyrus, Figure 4A, B). Whereas laser-treated hippocampal tissue was completely infarcted, parahippocampal tissue appeared viable. Likewise, another surgical specimen of mesial temporal lobe was obtained 3 months after stereotactic laser ablation of the parahippocampal region in a patient who did not participate in the prospective study (Figure 4C, D). Contiguous intact and infarcted tissues were sharply demarcated from each other by a pial border containing arterioles. This confirms the impression from imaging that laser ablation can be relatively delimited by anatomical pia/arachnoid boundaries.
Figure 4. H&E stained pathological specimens resected from mesial temporal lobes of patients following stereotactic laser ablations.

A, Section of ablated hippocampus of patient 5, resected during an open surgery 5 months following SLAH procedure, showing complete infarction. Note the absence of viable nuclei and the presence of congested vessels with mildly thickened vascular walls. B, Section of adjacent intact parahippocampal gyrus of patient 5 with normal cellularity and microvasculature. C, Section of en bloc specimen from the parahippocampal region of another patient, resected 3 months following stereotactic laser ablation of the parahippocampal gyrus. Note the cortical layers and the distinct pial boundary (thin black arrows), which contains several blood vessels (block blue arrows) demarcating an intact neocortical gyrus from the ablated parahippocampal gyrus. White square outline marks the area further magnified in D. D, Higher magnification image from C demonstrating perinecrotic microglial infiltrate at the margin of the infarcted tissue (yellow arrowhead). Again note the pial boundary (thin black arrows) containing blood vessels (block blue arrows) between intact and ablated tissues. Subpial gliosis was noted in this and other sections of the specimen, consistent with chronic epilepsy. Scale bars in inferior right corners of each panel.
Seizure Outcomes
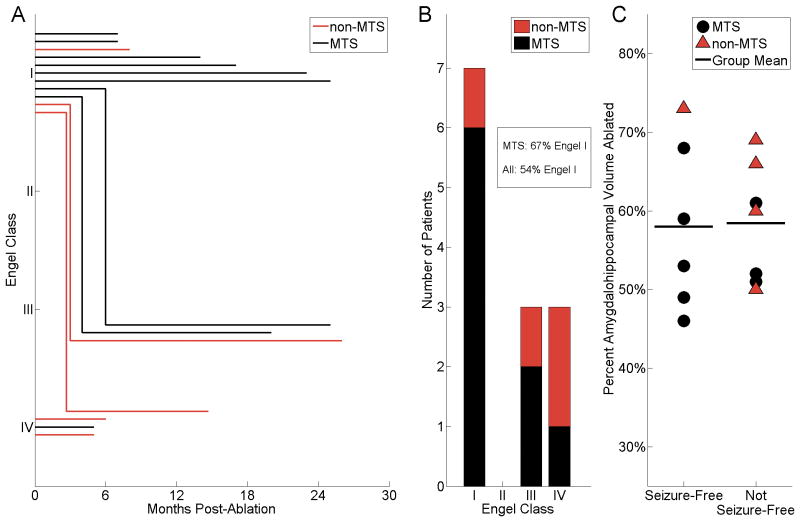
Thirteen patients had post-SLAH follow-up ranging from 5-26 (median 14) months (Table 3). The two procedures (in patients 1 and 5) with only 5 months of outcome are presented, as neither subject was ever seizure-free, and each underwent subsequent procedures (repeat SLAH and open ATLAH, respectively) at the 5-month time points; all others procedures presented have greater than 6 months outcome. Individual outcome duration curves demonstrate time to failure (loss of seizure freedom) for 14 procedures in 13 patients (Figure 5A). This analysis demonstrates that all failures to achieve seizure freedom occurred either immediately or within an interval of no more than 6 months in this series.
Table 3. Clinical Outcomes of SLAH for each Patient.
| Patient | ICU stay (days) | Total hospital stay (days) | Complications or adverse events within 30 days | F/U (mo) | Engle class following SLAH | Comments |
|---|---|---|---|---|---|---|
| 1 | 1 | 2 | None (1st procedure) | 6 | IVB | 1st SLAH: Continued frequent seizures after incomplete amygdalohippocampotomy. |
| 0 | 1 | Homonymous hemianopia (2nd procedure)* | 14 | IVB | 2nd SLAH: Continued frequent seizures despite adequate amygdalohippocampotomy. Underwent open right ATLAH at 14 mo post 2nd SLAH procedure: currently seizure free. |
|
| 2 | 1 | 2 | Emergency visit for seizure (missed AED) | 26 | IIIA | Initially seizure free (5 mo), then emergence of new semiology (less frequent) with new contralateral onset by video-EEG. |
| 3 | 1 | 1 | None | 25 | IIIA | Minimal amygdalotomy. Seizure recurrence (same semiology, reduced frequency) by 6 mo postop. |
| 4 | 0 | 1 | Emergency visit for seizure (missed AED) | 25 | ID | Seizures <1 mo postop with improper AED discontinuation only. |
| 5 | 0 | 1 | Multiple emergency visits and readmissions for PNES | 5** | IVB | Poor medication compliance, continued bilateral temporal seizures and PNES. Underwent open left ATLAH at 5 mo post SLAH: still not seizure free. |
| 6 | 1 | 3 | Acute SDH (no neurologic deficits)* | 20 | IIIA | Seizure recurrence (same semiology, reduced frequency) at 4 mo postop. |
| 7 | 0 | 1 | None | 23 | IB | Non-disabling auras only. |
| 8 | 0 | 1 | None | 17 | IA | |
| 9 | 0 | 3 | None | 14 | IA | |
| 10 | 0 | 2 | None | 8 | IA | |
| 11 | 0 | 1 | None | 7 | IA | |
| 12 | 0 | 1 | None | 7 | ID | Single GTC at 3 mo postop with improper AED wean. |
| 13 | 0 | 1 | None (1st procedure) | 6 | IVB | 1st SLAH: Continued frequent seizures after incomplete amygdalohippocampotomy. |
| 0 | 1 | None (2nd procedure) | 1 | too early to determine | 2nd SLAH: Adequate amygdalohippocampotomy achieved. Outcome <2 mo. |
See text for details.
Patient underwent ATL at 5-mo post-SLAH. ATLAH, anterior temporal lobectomy and amygdalohippocampectomy.
Figure 5. Patient outcomes with respect to time, preoperative MRI, and postoperative amygdalohippocampal ablation volume.
A, Patient outcome in Engel class (I, free of disabling seizures; II, rare disabling seizures; III, worthwhile improvement; IV, no worthwhile improvement18) is presented over time following each of 14 ablation procedures in 13 patients with adequate follow-up. Note that all failures to maintain seizure freedom (Engel I) were apparent by <6 months. Thus, seizure freedom beyond 6 months was a predictor of ongoing therapeutic persistence in our cohort. Three subjects with immediate Engel IV outcomes and <6 months presented went on to undergo additional procedures. B, Summary of the number of patients in each Engel outcome class and subdivided by preoperative MRI findings (MTS or non-MTS). Seven of all 13 (54%) patients and 6/9 (67%) of the select MTS patients were Engel I at last follow-up (median 14 months). Only 1/4 (25%) of non-MTS patients maintained Engel I outcome. C, Patient outcomes (seizure-free versus not seizure-free) with respect to proportional volume of the amygdalohippocampal complex that was acutely ablated. Individual subjects are denoted by symbols (black circles, MTS; red triangles, non-MTS); bold horizontal lines indicate group means. The ablation volumes achieved did not appear to predict seizure freedom. MTS, mesial temporal sclerosis, coded in black (lines, bars, and symbols); non-MTS coded in red.
Overall, at the time of last follow-up, 10 subjects (77%) achieved meaningful seizure reductions, with 7 subjects (54%) classified as Engel I (free of disabling seizures), 3 (23%) as Engel III (worthwhile improvement), and 3 (23%) as Engel IV (no worthwhile improvement)18 (Figure 5A, Table 3). Subjects with Engel III outcomes saw significant seizure reductions by 85-92% per patient seizure diaries. Notably, one subject (patient 2) with an Engel IIIA outcome (worthwhile seizure reduction) had a seizure-free interval of < 2 months followed by recurrence with a change in seizure semiology (preceding déjà vu) and well-documented onsets emerging exclusively contralaterally to the treated side (preoperative studies had not indicated bilaterality; Tables 1, 3). When taken into account, an overall 8 of 13 patients (62%) appeared to maintain seizure freedom from the ablated side.
Three subjects were Engel IVB (no appreciable change, Figure 5A, Table 3). Of these, patient 1, who preoperatively suffered daily seizures, underwent two laser ablation procedures (separated by 5 months) ultimately encompassing both the amygdalohippocampal complex (Supplemental Content 2) and parahippocampal region, resulting in a 3-month seizure-free interval before recurrence of daily seizures (Table 3, Figure 5A). She underwent open ATLAH 15 months following repeat SLAH (with >6 months seizure freedom since; Table 3). Likewise, patient 13 underwent repeat SLAH targeting residual mesial tissue (see Table, Supplemental Content 2), but follow-up of the second procedure was too early (<2 months) to determine outcome. Patient 5 was medically noncompliant and continued to have self-reported events after SLAH (Table 3). Repeated video-EEG examinations documented frequent psychogenic non-epileptic seizures and, with reduction in medications, infrequent bilateral temporal onset epileptic seizures. Given the inability to self-report reliable seizure frequency data, she was classified as Engel IVB and underwent open left ATLAH at 5 months post-SLAH. Following this procedure and despite improved medical compliance, she has not achieved seizure freedom. Thus, two subjects (patients 2 and 5) in this series failed to achieve seizure freedom in part due to bilateral temporal lobe epilepsy.
When analyzed by preoperative imaging status (MTS versus non-MTS, Figure 5B), 6 of 7 patients with Engel I outcome had preoperative MTS, whereas 3 patients who failed to achieve seizure freedom (Engel III and IV) had preoperative MTS. Thus, in the select MTS population, 6 of 9 patients (67%) achieved Engel I outcome, whereas 2 (22%) were Engel III and 1 (11%) was Engel IV.
Whereas the volumes ablated of the amygdala, hippocampus, and amygdalohippocampal complex in each procedure are shown in the Table, Supplemental Content 2, the distribution of volumes of amygdalohippocampal ablation are specifically categorized by clinical outcome in Figure 5C. Notably, the mean ablation volumes of the procedures associated with each outcome (seizure-free, 58.0 +/- 10.7%; not seizure-free, 58.4 +/- 7.6%) were not significantly different (T-test, p=0.42). Likewise, length of hippocampus ablated for each procedure (see Table, Supplemental Content 2) did not correlate to clinical outcome (seizure-free, 2.41 +/- 0.39 cm; not seizure-free, 2.54 +/- 0.49 cm; T-test, p=0.63). Thus, neither amygdalohippocampal ablation volumes nor lengths of hippocampus ablated were particularly predictive of outcome in this series.
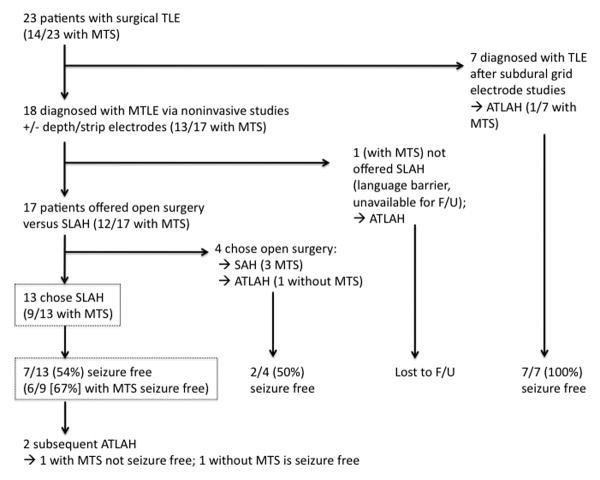
Over the 2.5-year period of this study, a total of 23 patients underwent therapeutic temporal lobe procedures for nonlesional epilepsy and MTS at our tertiary care institution (Figure 6). Seven of these patients were diagnosed with temporal lobe epilepsy in the context of having craniotomies for the placement of diagnostic subdural grid electrode arrays followed immediately by open grid removal and resection. The remaining 18 patients diagnosed with MTLE were eligible for surgical resection on either the basis of noninvasive studies alone or following intraparenchymal depth and subdural strip electrodes or percutanous trans-foramen ovale subarachnoid electrodes. 17 of these subjects were capable of consenting for and participating in the prospective study, and all were offered the choice of open temporal lobe surgery versus SLAH. 4 of these chose open surgery (3 with MTS), and 13 selected SLAH (9 with MTS). Overall, 7/13 (54%) SLAH patients were free of disabling seizures (Engel I), and 6/9 (67%) of SLAH patients with MTS were free of disabling seizures (Figure 6). Thus, our only exclusions from consideration for SLAH were inability to provide research consent (n=1) or having already undergone craniotomy for subdural grid electrodes (n=7). The SLAH cohort represents a ‘real world’ sample of temporal lobe epilepsy patients presenting for surgical consideration.
Figure 6. Surgical treatment and outcome of temporal lobe epilepsy patients.
Flowchart of 23 patients who underwent temporal lobe surgery for nonlesional epilepsy and MTS over 2.5 years. Seven underwent craniotomies for intracranial subdural grid electrode studies followed by open ATLAH. Apart from one patient unable to provide research consent due to a language barrier, the remaining 18 patients with MTLE were offered the choice of open temporal lobe surgery versus SLAH. 4 of these chose open surgery (3 with MTS), and 13 selected SLAH (9 with MTS). The SLAH cohort represents a ‘real world’ sample of temporal lobe epilepsy patients presenting for surgical consideration.
ICU Admission and Hospital Length of Stay
Following the first three procedures, patients were admitted to the intensive care unit (ICU) for observation (Table 3). All three patients denied headaches or other neurological symptoms, and hospital courses were unremarkable. Subsequently, patients were admitted directly to the hospital ward with the exception of patient 6, whose procedure was complicated by a small acute subdural hematoma (see below). Thus, length of hospital admissions averaged 1.6 days (median 1, range 1-3, Table 3). This compares favorably to the routine ICU admissions (median 2 days) and longer postoperative hospital admission (median 5 days) for the last 12 open temporal lobe resections at our institution over an overlapping period of time.
Adverse Events
One major complication (homonymous hemianopia) and one minor complication (small acute subdural hemorrhage) were associated with the stereotactic insertion procedure in this series (Table 3). During the second SLAH procedure on patient 1, failure to adequately thread the anchor bolt into bone resulted in initial superior deviation of the stereotactic alignment rod, which was recognized on lateral fluoroscopy. The deviation was corrected, the remainder of the procedure was technically successful, and MR imaging confirmed an ablation confined to the mesial temporal structures. The patient awoke with a left subtotal homonymous hemianopia, presumably from the initial errant alignment rod placement into the lateral geniculate nucleus or optic tract. Nevertheless, no post-operative hematoma or edema could be identified in these off-target structures by MRI, nor was their evidence of contrast enhancement or DWI signal change that would suggest encroachment of the ablation zone into these structures. The deficit was persistent at last follow-up.
In patient 6, MR imaging during the laser ablation revealed a slowly expanding acute occipital SDH localized near the applicator entry site (Table 3). Following completion of a technically successful SLAH, we elected to evacuate the SDH via a small craniotomy, which revealed bleeding from a superficial cortical artery. Hemostasis was achieved and the hematoma evacuated without neurological sequelae.
Discussion
Stereotactic laser thermal ablation is a minimally invasive alternative to open epilepsy surgery. We report the technical and early clinical results of MR-guided real time stereotactic laser amygdalohippocampotomy for MTLE. Two surgeons (JTW or REG) performed 15 procedures on 13 patients; all but one was studied prospectively. This cohort belonged to a larger group of 23 temporal lobe epilepsy patients surgically treated at our tertiary referral center over the same time period (Figure 6). Thus, SLAH candidates did not require subdural grid electrode arrays to be considered for a definitive resection. The 13 subjects presented in this study include all of the 17 patients that selected SLAH over open temporal lobe surgery. This patient sample represents a ‘real world’ cross-section of patients presenting for epilepsy surgery and includes patients with and without MTS (Figure 6).
We found that ablation of the amygdalohippocampal complex is safe, associated with brief hospitalizations, and effective: most patients achieved seizure freedom. This study is novel in several respects: it constitutes the largest series of stereotactic laser ablation for epilepsy to date, it is the first reported series of SLAH for MTLE, and it is the first reported convergence of two independent FDA-cleared technologies – an MRI-guided stereotactic laser ablation system (Visualase, Inc.) and an MRI-guided trajectory frame (MRI Interventions, Inc.).
Technical considerations
The stereotactic laser ablation method, using a diffusion-tipped 15 W 980 nm diode laser applicator system, appears readily adaptable to delivery via distinct types of stereotactic platforms in current practice, including traditional rigid fixation headframes (eg, CRW® and Lexsell®), image-guided frameless stereotaxis (eg, Medtronic Stealth® and BrainLab®), and MRI-guided miniframe systems (eg, ClearPoint SmartFrame®). Our technique required a median 3 treatment cycles to conform to the amygdalohippocampal complex with direct imaging confirmation of ablation. We achieved a mean hippocampal treatment length of 2.5 cm with a mean 60% of the total amygdalohippocampal volume ablated. The use of MR thermometry via the Visualase® workstation to monitor both target and collateral tissue temperatures provides automatic feedback control. Whereas time-dependent tissue injury occurs at tissue temperatures of 45-60°C, rapid irreversible ablation occurs at tissue temperatures >60°C.19 Feedback control prevented superheating (>100°C), which is associated with charring, tissue gassing, and unpredictable heat spread to off-target tissue.20 Finally, immediate post-procedure confirmation by direct anatomical imaging is facilitated prior to removing the laser applicator. The result is a powerful approach that is measured, safe, and effective for neural tissue ablations in the setting of MTLE.
We observed that ablation zones tended to conform to anatomical boundaries of the targeted structures, and can be delimited by pial boundaries; an impression from imaging that was confirmed in pathological specimens. The ablation dynamics in our study resembled those experienced by Curry et al.14 when using the same laser ablation system. This conformal result is not immediately intuitive given that the laser applicator emits light circumferentially around the distal 1 cm of the optical fiber, and the amygdalohippocampal volume is curved and tubular rather than spherical. The thermodynamic factors most likely responsible for this respect for anatomical boundaries include the presence of surrounding cerebrospinal fluid-containing ventricle and cisterns, acting as a heat sink around the hippocampus, and either small amounts of CSF within arachnoidal boundaries (eg, the hippocampal sulcus), or, more likely, light reflectance at such boundaries. Our ability to ablate the amygdalohippocampal complex posteriorly at least to the level of the lateral mesencephalic sulcus, along the natural curved anatomy of the hippocampus, and using only a single trajectory was likely feasible due to these favorable local anatomic-thermodynamic features.
In this series, we successfully performed the procedure using either a traditional stereotactic frame (CRW®) or an MRI-guided trajectory frame (ClearPoint SmartFrame®). The latter enables performance of the entire procedure within an interventional MRI (iMRI) environment. More importantly, it provides an ideal technique for MRI-based radiological verification of accurate localization of the laser fiber assembly during insertion. In contrast, use of the stereotactic frame, in our hands, only allowed for 2-D fluoroscopic radiological control, which does not provide any medial-lateral information or information with respect to tissue anatomy. In fact, it is likely that stereotactic technique, at least in part, contributed to the one major adverse effect in this series. This complication, a subtotal homonymous hemianopia, likely resulted from inadequately maintaining the stereotactic trajectory by improper placement of the anchor bolt. The alignment rod inserted through the anchor bolt deviated superiorly as visualized on fluoroscopy. This experience resulted in better recognition of the tactile feedback of a fully threaded anchor bolt during subsequent procedures. However, improved 3-D visualization during insertion (which was not available with the CRW-based deployment) could have helped detect the deviation prior to fully deploying the rod. In contrast, the MRI-based trajectory frame allows more confident deployment with 3-D anatomical control of the laser assembly. Notably, neither this complication, nor that of a small subdural hematoma, appeared to result from the laser ablation step itself, but rather from either the stereotactic approach or craniostomy method employed, and both are known complications of analogous procedures such as stereotactic depth electrode insertion and deep brain stimulation.
Preoperative Patient Factors Predict Seizure Outcome
Overall, 7/13 (54%) SLAH patients were free of disabling seizures (Engel I), and 6/9 (67%) SLAH patients with MTS were free of disabling seizures. All SLAH patients had been diagnosed preoperatively with unilateral MTLE except one (patient 5), who had evidence of bilateral temporal epilepsy but was considered for surgical palliation, given the less invasive nature of the proposed intervention. However, our clinical results with respect to seizure freedom (Table 3) may correlate to preoperative patient factors (Table 1). All subjects with Engel I outcomes (patients 4, 7, 8, and 9-12), with the exception of patient 10, had in common unilateral seizure onset and MTS with concordant lateralized 18-FDG-PET hypometabolism and memory dysfunction. Patient 10 did not have MTS, but did have prior confirmation of seizure onset to mesial structures via depth electrodes.
Potential explanations for SLAH failures
There are known or potential explanations for unfavorable outcomes in the patients in this series with Engel III/IV outcomes (patients 1-3, 5, 6 and 13; Table 3), including discordant or bilateral findings on preoperative workup (Table 1) and possibly suboptimal ablation volumes.
Low ablation volumes
Overall, total proportions of the entire amygdalohippocampal complex ablated did not correlate to seizure outcomes, as represented in Figure 5C. Likewise, the volumes of hippocampus ablated did not differentiate patients who achieved seizure freedom (seizure-free: 63.2 +/- 7.8% of volume, versus not seizure-free: 62.8 +/- 11.5% of volume). Regarding amygdala, the proportions ablated were generally more variable with smaller mean ablation volumes than those achieved in hippocampus (Table 2). When amygdala ablation volumes were compared by outcome (seizure-free: 53.5 +/- 21% of volume, versus not seizure-free: 54.8 +/- 32% of volume), no overall difference was observed. However, two subjects did not achieve seizure freedom after notably low volume amygdala ablations (4% after first ablation in patient 1 [Engel IV] and 12% in patient 3 [Engel III]). When patient 1 underwent a second ablation procedure, her amygdala ablation increased to 66%, but she still did not achieve seizure freedom (remained Engel IV until undergoing open ATLAH, which resulted in seizure freedom). Thus, no firm conclusions can be drawn from these preliminary observations regarding relationships of outcome to ablation volumes.
[Patient 6 (Engel III) had left-sided onset seizures with left 18-FDG-PET hypometabolism and left memory dysfunction, and bilateral MTS on MRI. After ablation, seizures recurred at reduced frequency without change in semiology, possibly suggesting failure to adequately ablate the seizure network rather than bilaterality of seizures, although post-op video-EEG monitoring has not been performed. Of note, this subject had the second lowest ablation volumes in the series with only 41% of amygdala ablated and 51% of the total amygdalohippocampal complex.]
Bilateral seizure onsets
Patient 2 (Engel III) had 9 seizures recorded from the right anterior temporal region on preoperative video scalp EEG, in the setting of bilateral temporal hypometabolism on PET. Seizures were reduced 85%. Postoperative video-EEG demonstrated a left-sided temporal seizure (with a distinct semiology), contralateral to the ablated right side.
Patient 5 (Engel IV) had left MTS, but bilateral 18-FDG-PET hypometabolism and bilateral memory dysfunction (left > right). Bilateral seizures onsets were recorded pre-operatively, although right-sided onsets were only observed after weaning antiepileptic medications, and psychogenic non-epileptic seizures were recorded as well. It was decided to offer palliative surgery and SLAH was performed. Postoperative video-EEG demonstrated left and right onsets and PNES. Left ATLAH was performed after 5 months, but seizures have continued and not been further characterized. This patient highlights the consequences of studying a new procedure in the ‘real world’ context, that is, SLAH was offered to all patients for whom resective temporal lobe surgery was determined to be the recommended treatment course at our comprehensive epilepsy surgery conference.
More extended seizure network
SLAH alone, mostly sparing the parahippocampal region, was sufficient to produce seizure freedom in the majority of patients in this small series. Similarly, Malikova et al. concluded that the parahippocampal region was not critical for seizure freedom following RF ablation, suggesting the amygdala and hippocampus was generally sufficient.21 Nevertheless, the patients without MTS in our series were less likely to be seizure-free following SLAH alone. Notably, patient 1 (non-MTS; Engel IV), underwent two ablation sessions ultimately encompassing both the amygdalohippocampal complex and also the parahippocampal gyrus without eliminating seizures; seizure freedom was achieved only following open ATLAH (follow-up >6 months). Indeed, a recent meta-analysis showed that 8% more patients became seizure-free after ATLAH as compared to SAH, suggesting that in some patients, resection of neocortical structures beyond the mesial temporal region (amygdala, hippocampus, and parahippocampal/entorhinal cortices) achieve greater likelihood of seizure freedom.22 Perhaps generating more widespread ablation zones via two or more laser trajectories to encompass the parahippocampal region (entorhinal cortex, perirhinal cortex, and parahippocampal gyrus) might improve the chance of seizure freedom in select patients. However, it is also possible that ablating additional regions will adversely affect cognitive outcome.16
Comparison of SLAH to other surgical approaches to MTLE
This small series shows promise for the effectiveness of SLAH, which we expect to improve with more technical experience and appropriate patient selection. Likewise, a full assessment of the true risk of adverse effects requires greater case numbers, making detailed comparisons to other surgical approaches premature. With these caveats, some potential advantages and disadvantages include the following:
Open surgical resection (ATLAH, SAH)
Seizure-free rates from open surgical resection have varied from 60 - 80%,2 the benchmark being 64% as found in the only randomized controlled study of highly select patients.1 It would not be surprising to find in a larger series a somewhat lower rate of seizure freedom as compared to ATLAH in light of findings in a recent meta-analysis that ATLAH is associated with an 8% greater rate of seizure freedom as compared to the more limited SAH resection.22 However, the possibly greater effectiveness of open resection must be balanced against the potentially greater risk of neuropsychological decline from collateral injury to the temporal stem.6,23,24 In fact, we have observed improved outcomes on neuropsychological testing at 6 months and 1 year with respect to naming and object recognition (left and right SLAH, respectively) as compared to open resections.16 An additional advantage of SLAH over open resective procedures is minimal recovery times and decreased short-term health care utilization with respect to ICU and hospital lengths of stay.25
Stereotactic radiosurgery
While SRS for MTLE has produced seizure freedom rates comparable to open resection,26,27 therapeutic effects are delayed up to two years, as compared to the immediate results from SLAH, which does not involve ionizing radiation. Moreover, potential complications from radiation necrosis following SRS require close postoperative monitoring and may require intensive medical or surgical management. A recent prospective randomized clinical trial of SRS for MTLE was discontinued due to low enrollment.
Stereotactic radiofrequency ablation
RF ablation is the most comparable procedure to SLAH. While earlier results were disappointing,28 a more recent series utilizing an occipital approach and length of hippocampus ablation similar to SLAH demonstrated 78% of 32 MTLE patients were seizure-free over 2 years.29 Complications were low, and the cohort exhibited no evidence of cognitive decline with standard testing.21,30 Limitations of RF ablation include difficulty conforming thermal energy to sensitive anatomic structures, the need to perform a large number of lesions, and lack of real time imaging verification. Moreover, the ‘string electrode’ used in this recent series is no longer commercially available. Stereotactic delivery of laser thermal energy not only facilitates MRI real-time monitoring, but may also offer superior thermodynamic properties for more safely and effectively conforming ablations to anatomically distinct structures. 9–11,14
In sum, SLAH has significant potential advantages in comparison to alternative procedures, including gold-standard open resection, SRS and RF ablation. Whether seizure-free rates are high enough and the complication rate low enough to justify this minimally invasive procedure must await a larger series, and a multicenter study is underway to examine this question.31 Since neuropsychological sequelae appear to be lower compared to open resection,16 a reasonable approach may be to perform SLAH first, as the majority of patients can expect to become seizure free. Should a patient fail to achieve seizure freedom following SLAH, the patient may still be a candidate for more extensive ablation or open resection, an approach we took in three of our patients.
Conclusion
This report demonstrates the technical feasibility and encouraging early results of SLAH, a novel approach to eliminating seizures while minimizing collateral injury in patients with MTLE. Efficacy appears to approach that of open resection, especially in patients with MTS. Such minimally invasive techniques may be more desirable to patients and result in increased utilization of epilepsy surgery among the large number of medically intractable epilepsy patients. A larger, longer-term multicenter study of seizure and cognitive outcomes following SLAH is currently underway.
Supplementary Material
Supplemental Content 1: Figure: Laser applicator placement, final integrated ablation estimates, and actual ablation for all 15 SLAH procedures in 13 patients. First row: Axial T1 MR images demonstrating placement of laser applicator along the long axis of the hippocampus for each procedure. Second row: Final ablation estimate integrated from thermographic images and overlaid digitally within an anatomic plane as determined in real time using the Visualase® workstation. Third row: Immediate post-operative imaging (contrast enhanced T1 sequences) reveals rim-enhancing area of acute ablation in the axial and coronal (fourth row) planes. Note the close correspondence between the estimated ablation and the actual ablation. Subjects underwent a single SLAH procedure with the exceptions of patients 1 and 13 who each underwent two separate procedures (see text for details).
Supplemental Content 2: Table: Individual and Group SLAH Treatment Summaries
Acknowledgments
We thank Brad Fernald, PhD and Anil Shetty, PhD for providing additional assistance with data collection and analysis, and Emilee Holland for clinical research coordination.
Disclosure: Funding was provided to Emory University by way of a clinical study agreement from Visualase, Inc., which develops products related to the research described in this paper. In addition, Dr. Gross serves as a consultant to Visualase and receives compensation for these services.
Dr. Ashok Gowda is an employee and stockholder in Visualase, Inc.
Dr. Daniel Drane receives funding from the NIH/NINDS (K02 NS070960), which provides support for his work.
Footnotes
The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 2.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 3.Lutz MT, Clusmann H, Elger CE, Schramm J, Helmstaedter C. Neuropsychological outcome after selective amygdalohippocampectomy with transsylvian versus transcortical approach: a randomized prospective clinical trial of surgery for temporal lobe epilepsy. Epilepsia. 2004;45:809–816. doi: 10.1111/j.0013-9580.2004.54003.x. [DOI] [PubMed] [Google Scholar]
- 4.Von Rhein B, et al. Neuropsychological outcome after selective amygdalohippocampectomy: subtemporal versus transsylvian approach. J Neurol Neurosurg Psychiatr. 2012;83:887–893. doi: 10.1136/jnnp-2011-302025. [DOI] [PubMed] [Google Scholar]
- 5.Crane J, Milner B. Do I know you? Face perception and memory in patients with selective amygdalo-hippocampectomy. Neuropsychologia. 2002;40:530–538. doi: 10.1016/s0028-3932(01)00131-2. [DOI] [PubMed] [Google Scholar]
- 6.Drane DL, et al. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46:1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seidenberg M, et al. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology. 1998;12:303–316. doi: 10.1037//0894-4105.12.2.303. [DOI] [PubMed] [Google Scholar]
- 8.McNichols RJ, et al. MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surg Med. 2004;34:48–55. doi: 10.1002/lsm.10243. [DOI] [PubMed] [Google Scholar]
- 9.Carpentier A, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63:ONS21–28. doi: 10.1227/01.neu.0000335007.07381.df. discussion ONS28–29. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier A, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43:943–950. doi: 10.1002/lsm.21138. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier A, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44:361–368. doi: 10.1002/lsm.22025. [DOI] [PubMed] [Google Scholar]
- 12.Jethwa PR, Lee JH, Assina R, Keller IA, Danish SF. Treatment of a supratentorial primitive neuroectodermal tumor using magnetic resonance-guided laser-induced thermal therapy. J Neurosurg Pediatr. 2011;8:468–475. doi: 10.3171/2011.8.PEDS11148. [DOI] [PubMed] [Google Scholar]
- 13.Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71:ons133–145. doi: 10.1227/NEU.0b013e31826101d4. [DOI] [PubMed] [Google Scholar]
- 14.Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012;24:408–414. doi: 10.1016/j.yebeh.2012.04.135. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013;113:495–503. doi: 10.1007/s11060-013-1142-2. [DOI] [PubMed] [Google Scholar]
- 16.Drane DL, et al. Temporal lobe epilepsy surgical patients undergoing MRI-Guided stereotactic laser ablation exhibit better episodic memory outcome as compared to standard surgical approaches. Epilepsia. 2013;54:20. [Google Scholar]
- 17.Pruessner JC, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 18.Engel J, Van Ness PC. In: Surgical Treatment of the Epilepsies. Jerome Engel J, editor. Raven Press; 1993. pp. 609–21. [Google Scholar]
- 19.Henle KJ, Dethlefsen LA. Time-temperature relationships for heat-induced killing of mammalian cells. Ann N Y Acad Sci. 1980;335:234–253. doi: 10.1111/j.1749-6632.1980.tb50752.x. [DOI] [PubMed] [Google Scholar]
- 20.Panescu D, et al. Three-dimensional finite element analysis of current density and temperature distributions during radio-frequency ablation. IEEE Trans Biomed Eng. 1995;42:879–890. doi: 10.1109/10.412649. [DOI] [PubMed] [Google Scholar]
- 21.Malikova H, et al. Stereotactic radiofrequency amygdalohippocampectomy: Does reduction of entorhinal and perirhinal cortices influence good clinical seizure outcome? Epilepsia. 2011;52:932–940. doi: 10.1111/j.1528-1167.2011.03048.x. [DOI] [PubMed] [Google Scholar]
- 22.Josephson CB, et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80:1669–1676. doi: 10.1212/WNL.0b013e3182904f82. [DOI] [PubMed] [Google Scholar]
- 23.Helmstaedter C. Temporal lobe resection--does the prospect of seizure freedom outweigh the cognitive risks? Nat Clin Pract Neurol. 2008;4:66–67. doi: 10.1038/ncpneuro0657. [DOI] [PubMed] [Google Scholar]
- 24.Drane DL, et al. Famous face identification in temporal lobe epilepsy: support for a multimodal integration model of semantic memory. Cortex. 2013;49:1648–1667. doi: 10.1016/j.cortex.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmers SL, Gross RE, Willie JT. Short-term healthcare utilization in stereotactic MRI-guided laser ablation for treatment of mesial temporal lobe epilepsy. Epilepsia. 2013;54:190. [Google Scholar]
- 26.Régis J, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504–515. doi: 10.1111/j.0013-9580.2004.07903.x. [DOI] [PubMed] [Google Scholar]
- 27.Bartolomei F, et al. Long-term efficacy of gamma knife radiosurgery in mesial temporal lobe epilepsy. Neurology. 2008;70:1658–1663. doi: 10.1212/01.wnl.0000294326.05118.d8. [DOI] [PubMed] [Google Scholar]
- 28.Parrent AG, Blume WT. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. Epilepsia. 1999;40:1408–1416. doi: 10.1111/j.1528-1157.1999.tb02013.x. [DOI] [PubMed] [Google Scholar]
- 29.Liscak R, et al. Stereotactic radiofrequency amygdalohippocampectomy in the treatment of mesial temporal lobe epilepsy. Acta Neurochir (Wien) 2010;152:1291–1298. doi: 10.1007/s00701-010-0637-2. [DOI] [PubMed] [Google Scholar]
- 30.Vojtěch Z, et al. Cognitive outcome after stereotactic amygdalohippocampectomy. Seizure. 2012;21:327–333. doi: 10.1016/j.seizure.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Willie JT, et al. Multicenter experience with minimally invasive sterotactic laser thermal amygdalohippocampotomy for mesial temporal lobe epilepsy. Epilepsia. 2013;54:290. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Content 1: Figure: Laser applicator placement, final integrated ablation estimates, and actual ablation for all 15 SLAH procedures in 13 patients. First row: Axial T1 MR images demonstrating placement of laser applicator along the long axis of the hippocampus for each procedure. Second row: Final ablation estimate integrated from thermographic images and overlaid digitally within an anatomic plane as determined in real time using the Visualase® workstation. Third row: Immediate post-operative imaging (contrast enhanced T1 sequences) reveals rim-enhancing area of acute ablation in the axial and coronal (fourth row) planes. Note the close correspondence between the estimated ablation and the actual ablation. Subjects underwent a single SLAH procedure with the exceptions of patients 1 and 13 who each underwent two separate procedures (see text for details).
Supplemental Content 2: Table: Individual and Group SLAH Treatment Summaries