Abstract
Background
Anemia has been associated with worse outcomes in patients with chronic heart failure (HF). We aimed to characterize the clinical profile and postdischarge outcomes of hospitalized HF patients with anemia at admission or discharge.
Methods and Results
An analysis was performed on 3731 (90%) of 4133 hospitalized HF patients with ejection fraction ≤40% enrolled in the Efficacy of Vasopressin Antagonist in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial with baseline hemoglobin data, comparing the clinical characteristics and outcomes (all-cause mortality and cardiovascular mortality or HF hospitalization) of patients with and without anemia (hemoglobin <12 g/dL for women and <13 g/dL for men) on admission or discharge/day 7. Overall, 1277 patients (34%) were anemic at baseline, which persisted through discharge in 73% and resolved in 27%; 6% of patients without baseline anemia developed anemia by discharge or day 7. Patients with anemia were older, with lower blood pressure, and higher creatinine and natriuretic peptide levels compared with those without anemia (all P<0.05). After risk adjustment, anemia at discharge, but not admission, was independently associated with increased all-cause mortality (hazard ratio, 1.30; 95% confidence interval, 1.05–1.60; P=0.015; and hazard ratio, 0.94; 95% confidence interval, 0.76–1.15; P=0.53, respectively) and cardiovascular mortality plus HF hospitalization early postdischarge (≤100 days; hazard ratio 1.73; 95% confidence interval, 1.37–2.18; P<0.001; and hazard ratio, 0.92; 95% confidence interval, 0.73–1.16; P=0.47, respectively). Neither baseline nor discharge anemia was associated with long-term cardiovascular mortality plus HF hospitalization (>100 days) on adjusted analysis (both P>0.1).
Conclusions
Among hospitalized HF patients with reduced ejection fraction, modest anemia at discharge but not baseline was associated with increased all-cause mortality and short-term cardiovascular mortality plus HF hospitalization.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00071331.
Keywords: anemia, heart failure, hemoglobin, hospitalization
Anemia is common in patients with ambulatory and hospitalized heart failure (HHF) with prevalence estimates of ≈70% in some studies, depending on the definition used.1 Although a large body of data suggests that anemia is associated with worse outcomes in patients with HF,2–5 earlier studies found conflicting results after rigorous adjustment for baseline characteristics and in elderly cohorts as well as those with recent-onset HF or advanced disease.6–9 Most of these studies were conducted in outpatients with chronic HF as opposed to HHF where hemodilution may explain a larger component of anemia.10,11 Thus, less is known about the clinical profile and outcomes of contemporary HHF patients with anemia on admission12,13 versus discharge. In addition, the neutral results of trials investigating the treatment of anemia in patients with HF,14,15 including the recent Reduction of Events with Darbepoetin alfa in Heart Failure (RED-HF) trial,16 highlight the need to more fully characterize the phenotype of anemic patients with HF. We had 2 main objectives: (1) to determine the clinical characteristics of HHF patients with reduced ejection fraction with anemia on admission and discharge, and (2) to explore the association of baseline versus discharge anemia with postdischarge morbidity and mortality.
Methods
Study Design and Patient Population
The design and results of the Efficacy of Vasopressin Antagonist in Heart Failure Outcome Study with Tolvaptan (EVEREST) program have been reported previously.17,18 In brief, the EVEREST trial was a prospective, multicenter, double-blinded, placebo-controlled clinical trial that randomized patients <48 hours of admission to oral tolvaptan, a vasopressin-2 receptor antagonist, or placebo. Patients aged >18 years who were hospitalized for worsening HF with ejection fraction ≤40% and New York Heart Association class III or IV functional status presenting with ≥2 signs/symptoms of fluid overload (ie, dyspnea, edema, and jugular venous distension [JVD]) were eligible for enrollment. Relevant exclusion criteria included a hemoglobin <9 g/dL, hemofiltration or dialysis, serum creatinine >3.5 mg/dL, and conditions with expected survival <6 months. Background HF therapy was left to the discretion of the treating physician, with guideline-based recommendations included in the study protocol. The coprimary end points were all-cause mortality (ACM) and cardiovascular mortality (CVM)/HF hospitalization. Median follow-up was 9.9 months. EVEREST was conducted in accordance with the Declaration of Helsinki; the protocol was independently approved by the institutional review board or ethics committee at each participating center; and written informed consent was obtained from all participants.
Study Definitions
Anemia was defined at baseline laboratory assessment based on the World Health Organization criteria (hemoglobin <12 g/dL for women and <13 g/dL for men). Renal insufficiency was defined at baseline assessment by investigators based on past medical history and laboratory data. Data on in-hospital bleeding were not collected in EVEREST. Causes of death and hospitalization were adjudicated by a blinded clinical events committee. Mode of death was classified as cardiovascular, noncardiovascular, or unknown as previously described.19 In brief, cardiovascular death comprised sudden cardiac death, HF, acute myocardial infarction, stroke, or other. Noncardiovascular death was because of a specific noncardiovascular event, whereas unknown death was defined as a death for which no information surrounding the event was available. Cardiovascular hospitalizations included HF, arrhythmia, acute myocardial infarction, and stroke as previously defined.19 Noncardiovascular hospitalizations were those without a cardiovascular classification.
Study End Points and Statistical Analysis
Post hoc analyses were performed on the entire EVEREST population with available baseline hemoglobin data with patients grouped as anemic or nonanemic. Those patients with hemoglobin data at discharge or day 7 were further grouped based on whether they remained anemic (ie, persistent anemia), had resolution of anemia, developed anemia during hospitalization, or were not anemic at both baseline and discharge at day 7 (ie, no anemia; Figure 1).
Figure 1.
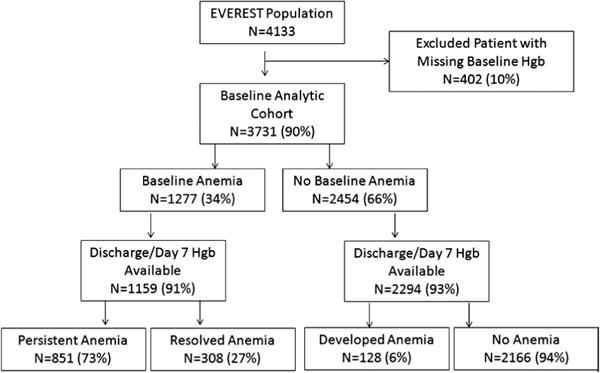
Analytic cohort. EVEREST indicates Efficacy of Vasopressin Antagonist in Heart Failure Outcome Study with Tolvaptan; and Hgb, hemoglobin.
Baseline characteristics were compared using the Student t test or Wilcoxon rank-sum test for continuous variables and χ2 tests for categorical variables as appropriate in patients with baseline anemia versus no baseline anemia, persistent anemia versus resolved anemia, and anemia at discharge versus no anemia at discharge. We plotted the median hemoglobin values during the follow-up period in those with and without discharge anemia. A cause-specific analysis of the cause of rehospitalization and death, based on adjudicated end points, was prespecified in the original trial design and was performed based on anemia status. The primary end points for the present analyses were ACM and CVM/HF hospitalization based on baseline or discharge anemia status. Univariate time-to-event comparisons between those with versus without anemia were made using log-rank tests. Kaplan–Meier estimates of the event rates were calculated for the entire follow-up period. Hazard ratios (HRs) and corresponding confidence intervals (CIs) were calculated relative to anemia status using a Cox proportional hazards model with and without adjustment for baseline covariates. Bivariable analyses were performed with both baseline and discharge anemia status in the model to ascertain independent relative predictive value of anemia as obtained at these time points. Patients who died in the hospital and patients with missing hemoglobin measures at either time point were excluded. Proportional hazards assumption was tested; this was violated for the CVM/HF hospitalization end point such that the follow-up period was divided into 2 periods: ≤100 and >100 days. Thus, we assessed the baseline characteristics of patients surviving through 100 days stratified by their discharge anemia status. Adjustment covariates in the multivariable model included randomization group and clinically relevant demographic (age, sex, region), clinical (admission systolic blood pressure, ejection fraction, QRS duration, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, β-blocker use, mineralocorticoid receptor antagonist use, digoxin use, intravenous inotrope use, previous HF hospitalization, diabetes mellitus, hypertension, coronary artery disease, chronic obstructive pulmonary disease, ischemic cause, and renal insufficiency), and laboratory values (admission B-type natriuretic peptide [BNP]/N-terminal pro-BNP, sodium and blood urea nitrogen) as in previous EVEREST analyses.20 Independent predictors of baseline anemia were explored from a candidate variable list including baseline covariates with a univariate association with baseline anemia at a significance level of P<0.1. Odds ratios and 95% CIs were presented for those variables with a P value <0.05. The association between natriuretic peptide level as a continuous variable and the odds of baseline anemia was analyzed further. We also investigated the association between baseline hemoglobin as a continuous variable and adjusted outcomes. Statistical significance was assessed using 2-sided P values. A P value <0.05 was considered statistically significant. All analyses were run in SASv9.3 (Cary, NC).
Results
Clinical Characteristics
Baseline hemoglobin was unavailable for 402 patients (10%). Only 1 patient in EVEREST received a blood transfusion during index hospitalization. Of the 3731 patients in EVEREST with hemoglobin data, 1277 (34%) were anemic at baseline (Figure 1). Of the patients with baseline anemia and discharge hemoglobin data at day 7 available (n=1159), 73% remained anemic (n=851) and 27% (n=308) were no longer anemic. Few patients who were nonanemic at baseline were anemic at discharge or day 7 (n=128; 6%). There were 109 in-hospital deaths. Of the 278 patients with baseline anemia status who had missing discharge anemia status, 98 (35%) died in the hospital. Figure 2 presents the median hemoglobin values during the follow-up period in those with and without discharge anemia.
Figure 2.

Hemoglobin values during the follow-up period in those with and without discharge anemia.
Independent predictors of baseline anemia included age, blood urea nitrogen, baseline JVD ≥10 cm, and natriuretic peptide level (Table I in the Data Supplement). Additional predictors of baseline anemia were previous myocardial infarction or coronary artery bypass grafting and clopidogrel use. Analyzing the association between natriuretic peptide level and the odds of baseline anemia demonstrated that each 1 log-unit increase in N-terminal pro-BNP or BNP was associated with an odds ratio for baseline anemia of 1.50 (95% CI, 1.40–1.60; P<0.001; Table II in the Data Supplement).
Patient characteristics based on anemia status are shown in Table 1. Compared with nonanemic patients (at admission and discharge), anemic patients were older, with more comorbidities, previous revascularization, previous HF hospitalization, lower blood pressure, more JVD, and higher blood urea nitrogen, creatinine, and N-terminal pro-BNP levels. Anemic patients were less likely to receive angiotensin-converting enzyme inhibitors/angiotensin receptor blockers or mineralocorticoid receptor antagonists. Similar between-group differences in those with versus without baseline/discharge anemia were seen when comparing those with persistent anemia versus resolved anemia. Notably, the resolved anemia group (n=308) had the largest percentage of patients with baseline JVD ≥10 cm. Compared with other patient groups, the resolved anemia group experienced the most weight loss during hospitalization (median, 3.6 kg) and had the largest reduction in BNP (all P values for comparisons <0.05; Table III in the Data Supplement).
Table 1.
Baseline Clinical and Demographic Characteristics of Patients With HF With Systolic Dysfunction With and Without Anemia at Baseline and Discharge
| Baseline Anemia Status
|
P Value | By Discharge Anemia Status in Patients Anemic at Baseline |
P Value | By Discharge Anemia Status in Entire Cohort |
P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Anemia (n=1277) |
No Anemia (n=2454) |
Persistent Anemia (n=851) |
Resolved Anemia (n=308) |
Anemia (n=979) | No Anemia (n=2474) |
||||
| Age, y | 70 (62–77) | 65 (56–73) | <0.001 | 70 (62–77) | 68 (62–75) | 0.012 | 70 (62–77) | 65 (57–73) | <0.001 |
| Male sex | 78% | 73% | <0.01 | 79% | 77% | 0.39 | 78% | 74% | 0.003 |
| NYHA class IV | 43% | 38% | 0.01 | 43% | 44% | 0.68 | 42% | 39% | 0.13 |
| Ejection fraction, % | 28 (20–35) | 29 (20–35) | 0.52 | 28 (20–35) | 29 (20–35) | 0.75 | 28 (20–35) | 29 (20–35) | 0.72 |
| Previous HF hospitalization | 82% | 78% | <0.01 | 84% | 78% | 0.022 | 83% | 77% | <0.001 |
| Coronary artery disease | 77% | 67% | <0.001 | 79% | 72% | 0.016 | 79% | 67% | <0.001 |
| Previous MI | 59% | 47% | <0.001 | 62% | 51% | 0.001 | 61% | 47% | <0.001 |
| Previous PCI | 25% | 14% | <0.001 | 28% | 18% | <0.001 | 28% | 14% | <0.001 |
| Previous coronary bypass | 33% | 14% | <0.001 | 36% | 25% | 0.001 | 35% | 15% | <0.001 |
| Hypertension | 73% | 70% | 0.08 | 72% | 74% | 0.53 | 73% | 70% | 0.103 |
| Hypercholesterolemia | 56% | 45% | <0.001 | 59% | 45% | <0.001 | 59% | 44% | <0.001 |
| Diabetes mellitus | 47% | 34% | <0.001 | 49% | 40% | 0.010 | 49% | 34% | <0.001 |
| Chronic kidney disease | 42% | 18% | <0.001 | 47% | 26% | <0.001 | 45% | 19% | <0.001 |
| Peripheral vascular disease | 27% | 18% | <0.001 | 28% | 22% | 0.069 | 28% | 18% | <0.001 |
| COPD | 13% | 8% | <0.001 | 15% | 9% | 0.011 | 14% | 8% | <0.001 |
| Weight, kg | 81 (70–94) | 81 (71–96) | 0.15 | 80 (70–94) | 80 (70–91) | 0.34 | 81 (69–94) | 81 (71–95) | 0.25 |
| SBP, mm Hg | 116 (102–130) | 120 (108–135) | <0.001 | 115 (102–130) | 120 (105–130) | 0.011 | 115 (102–130) | 120 (108–135) | <0.001 |
| Heart rate, beats/min | 78 (68–86) | 79 (70–90) | <0.01 | 77 (68–86) | 78 (68–88) | 0.76 | 76 (68–86) | 79 (70–90) | 0.001 |
| JVD ≥10 cm | 32% | 25% | <0.001 | 31% | 35% | 0.25 | 30% | 26% | 0.011 |
| Rales | 82% | 82% | 0.87 | 82% | 81% | 0.55 | 82% | 82% | 0.81 |
| Peripheral edema | 81% | 80% | 0.74 | 82% | 79% | 0.38 | 81% | 80% | 0.63 |
| Hemoglobin, g/dL | 11.5 (10.7–12.2) | 14.4 (13.5–15.3) | <0.001 | 11.3 (10.4–11.9) | 12.2 (11.7–12.7) | <0.001 | 11.5 (10.5–12.2) | 14.3 (13.2–15.2) | <0.001 |
| White blood cell count, ×109 | 6.9 (5.6–8.6) | 7.3 (6.1–8.7) | <0.001 | 6.9 (5.8–8.6) | 6.8 (5.6–8.1) | 0.28 | 6.9 (5.6–8.7) | 7.2 (6.0–8.6) | 0.018 |
| Serum BUN, mg/dL | 31 (22–45) | 24 (18–31) | <0.001 | 32 (24–46) | 26 (19–36) | <0.001 | 32 (24–46) | 24 (19–32) | <0.001 |
| Serum creatinine, mg/dL | 1.4 (1.1–1.9) | 1.2 (1–1.5) | <0.001 | 1.5 (1.1–2.0) | 1.2 (1.0–1.6) | <0.001 | 1.4 (1.1–1.9) | 1.2 (1.0–1.5) | <0.001 |
| Serum sodium, mg/dL | 139 (137–142) | 140 (137–143) | <0.001 | 139 (137–142) | 140 (138–142) | 0.001 | 139 (137–142) | 140 (137–143) | <0.001 |
| NT-proBNP, pg/mL | 6136 (3113–12 843) | 3909 (1668–7939) | <0.001 | 6878 (3419–13 028) | 4675 (2473–10 236) | 0.005 | 6695 (3378–12 888) | 3909 (1692–7819) | <0.001 |
| Albumin, g/dL | 3.5 (3.2–3.9) | 3.9 (3.6–4.2) | <0.001 | 3.5 (3.2–3.8) | 3.6 (3.3–3.9) | 0.005 | 3.6 (3.2–3.9) | 3.9 (3.5–4.2) | <0.001 |
| QRS duration, ms | 126 (102–154) | 121 (96–148) | <0.001 | 127 (102–155) | 123 (97–149) | 0.050 | 126 (101–155) | 121 (96–148) | <0.001 |
| ICD | 22% | 11% | <0.001 | 24% | 13% | <0.001 | 23% | 10% | <0.001 |
| Baseline medication use | |||||||||
| ACE inhibitors/ARBs | 81% | 87% | <0.001 | 80% | 86% | 0.014 | 80% | 87% | <0.001 |
| β-Blockers | 71% | 71% | 0.99 | 71% | 71% | 0.92 | 72% | 70% | 0.31 |
| Aldosterone-blocking agents | 48% | 59% | <0.001 | 47% | 57% | 0.002 | 47% | 61% | <0.001 |
| Diuretic | 97% | 98% | 0.74 | 98% | 96% | 0.25 | 97% | 98% | 0.59 |
| Amiodarone | 20% | 17% | 0.01 | 20% | 18% | 0.34 | 20% | 17% | 0.037 |
| Digoxin | 44% | 51% | <0.001 | 42% | 49% | 0.050 | 42% | 51% | <0.001 |
| Calcium channel blockers | 10% | 11% | 0.33 | 10% | 10% | 0.87 | 11% | 11% | 0.65 |
| Statin | 43% | 29% | <0.001 | 45% | 37% | 0.014 | 45% | 30% | <0.001 |
| Aspirin | 58% | 56% | 0.24 | 57% | 60% | 0.27 | 57% | 56% | 0.67 |
| Clopidogrel | 12% | 5% | <0.001 | 13% | 10% | 0.19 | 14% | 6% | <0.001 |
| Coumadin | 39% | 34% | 0.01 | 39% | 37% | 0.48 | 37% | 35% | 0.14 |
Data expressed as percentage or median (interquartile range). ACE indicates angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; HF, heart failure; ICD, implantable cardioverter-defibrillator; JVD, jugular venous distension; MI, myocardial infarction; NT-proBNP, N-terminal pro-BNP; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; and SBP, systolic blood pressure.
Patients who developed anemia (n=128) had significantly higher baseline BNP values compared with patients who did not have anemia during hospitalization (P<0.0001; Table III in the Data Supplement). Despite high baseline BNP values, only 25% of these patients had baseline JVD ≥10 cm. In patients who developed anemia, the weight reduction from baseline to discharge was significantly less than all other groups (all P values <0.0001). Compared with those with resolved anemia, patients who developed anemia had more modest reductions in BNP from baseline to discharge (median change in log [BNP] of −0.2 versus −0.4; P<0.0001].
Outcomes
The event rates based on anemia status are presented in Table 2. Compared with those without baseline anemia, patients with baseline anemia had higher event rates for mortality (33.8% versus 22.1%; P<0.001) and CVM plus HF hospitalization (50.8% versus 35.5%; P<0.001). The CVM rate was higher in the group with baseline anemia because of a higher rate of death from HF. Similar event rates were seen when comparing patients with persistent versus resolved anemia and discharge anemia versus no anemia.
Table 2.
Event Rates in Patients With HF With Systolic Dysfunction With and Without Anemia at Baseline and Discharge
| Outcome | Baseline Anemia Status
|
P Value | By Discharge Anemia Status in Patients Anemic at Baseline |
P Value | By Discharge Anemia Status in Entire Cohort |
P Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Anemia (n=1277) |
No Anemia (n=2454) |
Persistent Anemia (n=851) |
Resolved Anemia (n=308) |
Anemia (n=979) | No Anemia (n=2474) |
||||
| All-cause mortality | 431 (33.8) | 542 (22.1) | <0.001 | 307 (36.1) | 71 (23.1) | <0.001 | 380 (35.3) | 556 (20.8) | <0.001 |
| CV mortality or HF hospitalization | 649 (50.8) | 871 (35.5) | <0.001 | 464 (54.5) | 118 (38.3) | <0.001 | 584 (54.2) | 936 (35.1) | <0.001 |
| CV mortality | 321 (25.1) | 424 (17.3) | <0.001 | 225 (26.4) | 55 (17.9) | 0.003 | 283 (26.3) | 444 (16.6) | <0.001 |
| HF | 195 (15.3) | 208 (8.5) | <0.001 | 136 (16) | 26 (8.4) | 0.001 | 165 (15.3) | 206 (7.7) | <0.001 |
| Sudden cardiac death | 91 (7.1) | 156 (6.4) | 0.37 | 63 (7.4) | 23 (7.5) | 0.97 | 83 (7.7) | 178 (6.7) | 0.26 |
| Stroke | 8 (0.6) | 15 (0.6) | 0.96 | 4 (0.5) | 3 (1.0) | 0.39 | 5 (0.5) | 15 (0.6) | 0.81 |
| Acute MI | 8 (0.6) | 16 (0.7) | 0.93 | 7 (0.8) | 1 (0.3) | 0.69 | 9 (0.8) | 17 (0.6) | 0.51 |
| Other CV mortality | 19 (1.5) | 29 (1.2) | 0.43 | 15 (1.8) | 2 (0.6) | 0.37 | 21 (1.9) | 28 (1.0) | 0.028 |
| Non-CV mortality* | 71 (5.6) | 63 (2.6) | <0.001 | 59 (6.9) | 7 (2.3) | 0.002 | 68 (6.3) | 60 (2.2) | <0.001 |
| Clinically worsening HF | 597 (46.8) | 747 (30.4) | <0.001 | 439 (51.6) | 96 (31.2) | <0.001 | 544 (50.5) | 790 (29.6) | <0.001 |
| CV hospitalization | 740 (57.9) | 1021 (41.6) | <0.001 | 437 (51.4) | 122 (39.6) | <0.001 | 547 (50.8) | 923 (34.6) | <0.001 |
| HF hospitalization | 450 (35.2) | 579 (23.6) | <0.001 | 337 (39.6) | 82 (26.6) | <0.001 | 421 (39.1) | 634 (23.8) | <0.001 |
| MI hospitalization | 19 (1.5) | 20 (0.8) | 0.06 | 13 (1.5) | 4 (1.3) | 1.00 | 17 (1.6) | 22 (0.8) | 0.040 |
| Stroke hospitalization | 15 (1.2) | 31 (1.3) | 0.82 | 12 (1.4) | 2 (0.6) | 0.38 | 13 (1.2) | 33 (1.2) | 0.94 |
| Arrhythmia hospitalization | 45 (3.5) | 69 (2.8) | 0.23 | 21 (2.5) | 19 (6.2) | 0.002 | 31 (2.9) | 84 (3.1) | 0.67 |
| Other CV hospitalization | 76 (6) | 134 (5.5) | 0.54 | 54 (6.3) | 15 (4.9) | 0.35 | 65 (6.0) | 150 (5.6) | 0.62 |
Expressed as events (%). CV indicates cardiovascular; HF, heart failure; and MI, myocardial infarction.
CV plus non-CV mortality does not add up to total mortality because there were deaths that were not successfully adjudicated.
Figure 3 presents the Kaplan–Meier curves for the primary end points based on anemia status. On bivariable analysis, discharge but not baseline anemia was associated with increased ACM and CVM/HF hospitalization (Table 3). After multivariable risk adjustment, discharge anemia, but not baseline anemia, was independently associated with increased ACM (HR, 1.30; 95% CI, 1.05–1.60; P=0.015; and HR, 0.94; 95% CI, 0.76–1.15; P=0.53, respectively) and CVM/HF hospitalization early postdischarge (≤100 days; HR, 1.73; 95% CI, 1.37–2.18; P<0.001; and HR, 0.92; 95% CI, 0.73–1.16; P=0.47, respectively). Neither baseline nor discharge anemia was associated with long-term CVM/HF hospitalization (>100 days) on adjusted analysis (both P>0.1). Given the differential association between anemia and the composite end point ≤100 versus >100 days, we assessed the baseline characteristics of patients surviving through 100 days stratified by their discharge anemia status. The EVEREST population that was alive at 100 days demonstrated similar between-group differences to the baseline cohort when comparing patients with and without anemia (data not shown). When analyzing the association between baseline hemoglobin as a continuous variable and adjusted outcomes, there was a trend toward reduced ACM with increasing hemoglobin (HR, 0.97; 95% CI, 0.93–1.00 for 1 g/dL increase in hemoglobin; P=0.053; Table IV in the Data Supplement). Increasing hemoglobin was associated with reduced CVM/HF hospitalization during the early period after hospitalization (≤100 days; HR, 0.92; 95% CI, 0.89–0.96; P<0.001) but not >100 days (P=0.52).
Figure 3.
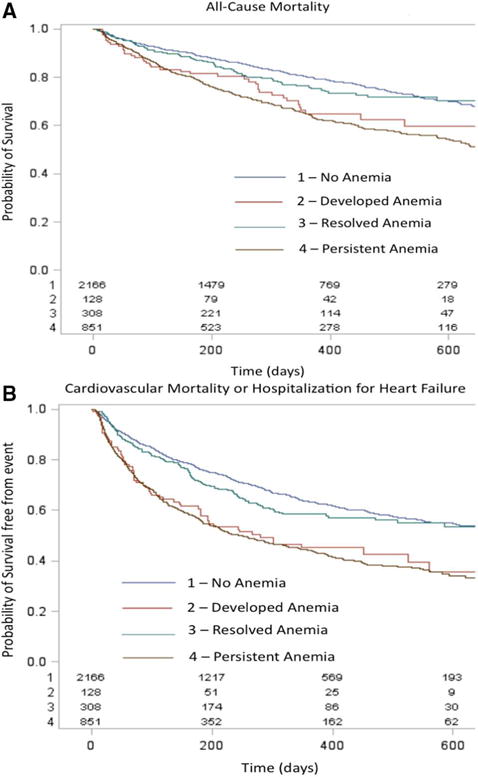
Kaplan–Meier survival curves for (A) all-cause mortality and (B) cardiovascular mortality or heart failute (HF) hospitalization in patients with HF based on whether anemia was (1) absent, (2) developed during hospitalization, (3) present at baseline but resolved by discharge or d 7, or (4) persisted from admission through discharge or d 7. Log-rank P value <0.001 for both figures.
Table 3.
Effects of Baseline and Discharge Anemia on Outcomes for Patients With HF With Systolic Dysfunction
| Outcomes | Independent Bivariable*
|
Adjusted for EVEREST Covariates† Plus Discharge Anemia Status
|
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| All-cause mortality | ||||
| Baseline anemia | 1.13 (0.93–1.38) | 0.21 | 0.94 (0.76–1.15) | 0.53 |
| Discharge anemia | 1.65 (1.35–2.02) | <0.001 | 1.30 (1.05–1.60) | 0.015 |
| CV mortality or HF hospitalization | ||||
| Baseline anemia | ||||
| ≤100 d | 1.09 (0.87–1.36) | 0.45 | 0.92 (0.73–1.16) | 0.47 |
| >100 d | 1.13 (0.91–1.41) | 0.28 | 0.95 (0.76–1.19) | 0.65 |
| Discharge anemia | ||||
| ≤100 d | 2.15 (1.72–2.69) | <0.001 | 1.73 (1.37–2.18) | <0.001 |
| >100 d | 1.42 (1.13–1.78) | 0.003 | 1.22 (0.96–1.54) | 0.11 |
CI indicates confidence interval; CV, cardiovascular; EVEREST, Efficacy of Vasopressin Antagonist in Heart Failure Outcome Study with Tolvaptan; HF, heart failure; and HR, hazard ratio.
Both baseline and discharge anemia status were simultaneously analyzed in this model to assess their predictive value independent of the other measurement. Patient who died in-hospital were excluded from this analysis.
Adjusted for variables noted in the Methods section.
Discussion
Approximately one third of patients in this large trial of patients hospitalized for HF had baseline anemia, which persisted through day 7/discharge in 73% of patients and resolved in 27%. Compared with those without anemia, anemic patients differed significantly with regard to baseline clinical and laboratory characteristics. Notably, anemic patients had higher natriuretic peptide levels and were more likely to have evidence of significant congestion. Anemic patients exhibited an increased comorbidity burden and were less likely to receive guideline-recommended HF therapies. Discharge anemia, but not baseline anemia, was independently associated with a 30% increased risk of mortality and a 73% increased risk of early postdischarge CVM/HF hospitalization.
These findings confirm and extend previous research by demonstrating the distinct characteristics of anemic patients with HF at admission and discharge and by documenting the prognostic significance of discharge, but not baseline anemia status. Furthermore, these data add support for the hemodilution hypothesis as a cause of anemia in a subset of patients with acute HF. They also highlight the importance of other nondilutional causes of anemia, because anemia persisted through hospitalization in 73% of patients, and a subgroup of patients developed anemia during hospitalization.
The main finding of our analysis was the significant increased risk of mortality in patients with anemia at discharge from HF hospitalization. Although previous studies have suggested increased risk associated with anemia in patients with HF,1,3 many of these studies were conducted in the chronic HF population before contemporary HF therapies. The current analysis highlights the independent prognostic value of discharge anemia status after acute HF hospitalization. Potential explanations for worse outcomes in anemic patients with HF include adverse left ventricular remodeling effects,21 increased neurohormonal and proinflammatory cytokine levels,22 and the association with poor nutritional status and cardiac cachexia.23 Previous studies have also characterized the role of cardiorenal syndrome in association with adverse events in patients with HF and anemia.24
Previous work has suggested that non–iron-deficient anemia in patients with HF may be categorized into 2 major types: true reduction in red blood cell content, or dilutional anemia with relatively preserved red blood cell content but excess intravascular volume.25 Our finding that 27% of patients had a resolution in anemia during hospitalization supports the hemodilution hypothesis in a subgroup of patients. Hemoconcentration during hospitalization, as characterized by increases in hemoglobin or hematocrit, albumin, and total protein, represents a marker of decongestion. Patients who undergo hemoconcentration experience greater weight loss during treatment and have improved postdischarge outcomes.10,11 Our results support these previous data. Consistent with previous studies, the resolved anemia group (ie, patients who hemoconcentrate) had significant congestion at baseline and experienced the largest reductions in weight and BNP with therapy. The patients who had resolution of anemia also had similar postdischarge outcomes to those without anemia. Moreover, it is notable that patients with persistent anemia were more likely to have a history of renal insufficiency and higher baseline creatinine and blood urea nitrogen. Thus, these patients may have had a reduced response or tolerability to aggressive diuresis, which translated into less hemoconcentration.
In addition to the hemodilution hypothesis, our data suggest the presence of additional mechanisms for anemia in patients with HF. Despite inpatient therapy targeting decongestion in EVEREST, anemia persisted through hospitalization in 73% of patients. The majority of patients with anemia at baseline remained anemic at discharge. Previous data support a high prevalence of iron-deficient anemia in patients with HF.26 Another potential cause of anemia in this population may relate to underlying chronic kidney disease and reduced renal erythropoietin production. Anemic patients in our analysis consistently had worse baseline renal function compared with those without anemia. Volume overload with passive renal venous congestion is an established mechanism for renal dysfunction in patients with HF.27 Whether this renal dysfunction was related to increased baseline congestion in anemic patients is unclear. The increased prevalence of diabetes mellitus among anemic patients is another possible contributor. In sum, low hemoglobin values should not be routinely attributed to hemodilution in the broad HF population; thorough investigation for alternative causes of anemia should be performed.
We provide data characterizing the small group of patients that developed anemia during hospitalization. Although the specific reason for the development of anemia in these patients is unclear, the clinical characteristics of this population may inform future study. Patients who developed anemia during hospitalization tended to have high BNP values, yet baseline elevated JVD ≥10 cm was relatively uncommon. These findings may represent a mismatch between left- and right-sided filling pressures.28 High left-sided filling pressures may be responsible for BNP elevations out of proportion to JVD. A mismatch between ventricular filling pressures where patients have low right-sided pressures despite elevated wedge pressures may lead clinicians to limit diuresis, thereby minimizing weight loss during hospitalization. We found that patients who developed anemia had the least weight loss during hospitalization. Alternatively, it is possible that physicians may have underestimated the JVD in these patients, which led to less intense decongestive strategies. In sum, we identified a small group of patients with acute HF that developed anemia during hospitalization and seemed to have a distinct phenotype from other patients. Future study is necessary to better characterize the clinical characteristics and outcomes of these patients. An improved understanding of this population may provide insight into the pathophysiologic mechanisms of anemia in patients with HF in addition to the mechanisms of hemodilution, iron deficiency, and chronic disease.
Our observation of increased morbidity in the early period after discharge has important clinical implications. Hospitalizations for HF have continued to increase over time, and costs related to hospitalization account for ≈75% of the total cost of HF care.29 If anemia in fact represents a mediator of risk and not merely a marker of disease severity, there is the potential for a reduction in the burden of HF care through anemia treatment in appropriately selected patients.
At present, it is unclear whether anemia is truly a prognostic marker or a mediator of risk. It has been postulated that anemia is a marker of disease severity for HF, or that other factors associated with anemia are responsible for the increased events.16 Multiple randomized trials, including the recent RED-HF trial,16 investigating the treatment of anemia in patients with HF failed to demonstrate significant benefit on mortality or HF hospitalizations.14,15 Future research is needed to better define the role of anemia as purely prognostic versus a target for HF therapy. Taken together, 1 hypothesis based on RED-HF and our results is that a subsequent study could be considered in patients after hospitalization for HF who remain anemic after aggressive inpatient decongestive therapy. This population may be the HF subgroup to derive the most robust benefit from iron therapy or recombinant erythropoietin in terms of long-term symptoms, HF re-admissions, and potential mortality.
Our findings also identify targets to improve care in patients with HF with anemia. Anemic patients were undertreated with evidence-based chronic HF therapies. Specifically, we demonstrated significant room for improvement in the use of angiotensin-converting enzyme inhibitors and mineralocorticoid receptor antagonists. The between-group differences in HF medication use may be even more marked in routine clinical practice. Although these differences may have been due, in part, to increased kidney disease and lower blood pressure in those with anemia, recent analyses have suggested that those with decreased renal function may experience the greatest benefit from such therapies.30
In EVEREST, anemic patients hospitalized with HF had a modest in-hospital increase in hemoglobin level, which continued to increase during the follow-up period before reaching a plateau at 32 weeks. Interestingly, these longitudinal data also demonstrated that at 16 weeks (ie, 112 days), the median hemoglobin in the cohort with discharge anemia reached ≈12 g/dL. These findings of an improvement in hemoglobin at ≈100 days may explain, in part, the observation that the prognostic utility of discharge anemia status was no longer significant for cardiovascular mortality or HF hospitalization after 100 days. Alternatively, these data may demonstrate the overall modest prognostic utility of anemia status during HHF given the multitude of factors that influence the biomarker. Targeting anemia in the broad population with HHF may not be a viable strategy until there is an improved understanding of how anemia directly affects outcomes.
Limitations
This was a retrospective analysis from a clinical trial. Outside the context of a prospective, randomized, controlled trial, definitive cause-and-effect relationships are difficult to determine. Not all patients in the EVEREST data set had documentation of anemia status at baseline and discharge. The study population had strict inclusion and exclusion criteria, such that these findings may not apply to those with different baseline characteristics. Specifically, patients with significant anemia at the time of enrollment (hemoglobin <9 g/dL) and severe renal disease (serum creatinine >3.5 mg/dL) were excluded from the EVEREST trial. Thus, these data apply only to patients with modest reductions in hemoglobin and without severe chronic kidney disease. Despite covariate adjustment, other measured and unmeasured factors may have influenced these findings. Furthermore, adjustment covariates such as renal insufficiency were based on investigator documentation. Thus, future prospective studies are necessary to externally validate these associations.
Conclusions
In patients hospitalized for worsening HF with reduced ejection fraction, anemia is prevalent and associated with older age, chronic kidney disease, higher natriuretic peptide levels, and worse outcomes. Our characterization of the clinical profiles and outcomes of patients hospitalized for worsening HF with different classifications of anemia, including persistent and developed anemia, provide a framework for future studies exploring the underlying pathophysiology and outcomes in the high-risk acute HF population.
Supplementary Material
CLINICAL PERSPECTIVE.
Anemia has been associated with worse outcomes in patients with chronic heart failure (HF). We aimed to characterize the clinical profile and outcomes of hospitalized HF patients with anemia at admission or discharge. We found that 34% of hospitalized HF patients were anemic at baseline, which persisted through discharge in 73% and resolved in 27%; 6% of patients without baseline anemia developed anemia during hospitalization. Patients with anemia were older, with lower blood pressure, and higher creatinine and natriuretic peptide levels. Anemic patients were less likely to receive guideline-recommended HF therapies. Discharge anemia, but not baseline anemia, was independently associated with a 30% increased risk of mortality and a 73% increased risk of early postdischarge cardiovascular mortality or HF hospitalization. These findings confirm and extend previous research by demonstrating the distinct characteristics of anemic HF patients at admission and discharge and by documenting the prognostic significance of discharge, but not baseline anemia status. These data add support for the hemodilution hypothesis as a cause of anemia in a subset of patients with acute HF. They also highlight the importance of other nondilutional causes of anemia, because anemia persisted through hospitalization in 73% of patients, and a subgroup of patients developed anemia during hospitalization. Our characterization of the clinical profiles and outcomes of patients hospitalized for worsening HF with different classifications of anemia, including persistent and developed anemia, provides a framework for future studies.
Acknowledgments
Sources of Funding
Financial and material support for Efficacy of Vasopressin Antagonist in Heart Failure Outcome Study with Tolvaptan (EVEREST) was provided by Otsuka, Inc (Rockville, MD). Database management was performed by the sponsor. H.P. Subacius conducted all analyses for this report with funding from the Center for Cardiovascular Innovation, Northwestern University. Dr Mentz is supported by the National Institute of General Medical Sciences (T32GM086330). The authors are solely responsible for the design and conduct of this study, drafting, and editing of the article and its final contents.
Footnotes
Guest Editor for this article was W.H. Wilson Tang, MD.
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.113.000840/-/DC1.
Disclosures
Dr Swedberg received research grants from AstraZeneca, Servier, and Amgen; honoraria from AstraZeneca, Otsuka, Servier, and Amgen; and is consultant to Cytokinetics, Servier, and Novartis. Dr Maggioni received honoraria from Otsuka. Dr Gheorghiade is consultant to Abbott Laboratories, Astellas, AstraZeneca, Bayer Schering PharmaAG, CorThera Inc, Cytokinetics Inc, DebioPharm S.A., Errekappa Terapeutici, GlaxoSmithKline, JNJ, Medtronic, Novartis Pharma AG, Otsuka, Sigma Tau, Solvay Pharmaceuticals, and Pericor Therapeutics. Dr Butler is consultant to Amgen. The other authors report no conflicts.
References
- 1.Anand IS. Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol. 2008;52:501–511. doi: 10.1016/j.jacc.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J. Prevalence of anemia and effects on mortality in patients with heart failure. Am Heart J. 2005;149:391–401. doi: 10.1016/j.ahj.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 3.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, van der Meer P. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Tang YD, Katz SD. The prevalence of anemia in chronic heart failure and its impact on the clinical outcomes. Heart Fail Rev. 2008;13:387–392. doi: 10.1007/s10741-008-9089-7. [DOI] [PubMed] [Google Scholar]
- 5.van der Meer P, Groenveld HF, Januzzi JL, Jr, van Veldhuisen DJ. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart. 2009;95:1309–1314. doi: 10.1136/hrt.2008.161091. [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Curtis JP, Wang Y, Smith GL, Masoudi FA, Foody JM, Havranek EP, Krumholz HM. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med. 2005;165:2237–2244. doi: 10.1001/archinte.165.19.2237. [DOI] [PubMed] [Google Scholar]
- 7.Maraldi C, Volpato S, Cesari M, Onder G, Pedone C, Woodman RC, Fellin R, Pahor M, Investigators of the Gruppo Italiano di Farmacoepidemiologia nell’Anziano Study Anemia, physical disability, and survival in older patients with heart failure. J Card Fail. 2006;12:533–539. doi: 10.1016/j.cardfail.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Felker GM, Gattis WA, Leimberger JD, Adams KF, Cuffe MS, Gheorghiade M, O’Connor CM. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol. 2003;92:625–628. doi: 10.1016/s0002-9149(03)00740-9. [DOI] [PubMed] [Google Scholar]
- 9.Kalra PR, Collier T, Cowie MR, Fox KF, Wood DA, Poole-Wilson PA, Coats AJ, Sutton GC. Haemoglobin concentration and prognosis in new cases of heart failure. Lancet. 2003;362:211–212. doi: 10.1016/S0140-6736(03)13912-8. [DOI] [PubMed] [Google Scholar]
- 10.van der Meer P, Postmus D, Ponikowski P, Cleland JG, O’Connor CM, Cotter G, Metra M, Davison BA, Givertz MM, Mansoor GA, Teerlink JR, Massie BM, Hillege HL, Voors AA. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol. 2013;61:1973–1981. doi: 10.1016/j.jacc.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 11.Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, Subacius H, Maggioni AP, Nodari S, Konstam MA, Butler J, Filippatos G. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: Insights from the EVEREST trial. Eur J Heart Fail. 2013;61:1973–1981. doi: 10.1093/eurjhf/hft110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Takeshita A, Yokoshiki H, Tsutsui H. Anemia is an independent predictor of long-term adverse outcomes in patients hospitalized with heart failure in japan. A report from the japanese cardiac registry of heart failure in cardiology. Circ J. 2009;73:1901–1908. doi: 10.1253/circj.cj-09-0184. [DOI] [PubMed] [Google Scholar]
- 13.von Haehling S, Schefold JC, Hodoscek LM, Doehner W, Mannaa M, Anker SD, Lainscak M. Anaemia is an independent predictor of death in patients hospitalized for acute heart failure. Clin Res Cardiol. 2010;99:107–113. doi: 10.1007/s00392-009-0092-3. [DOI] [PubMed] [Google Scholar]
- 14.van Veldhuisen DJ, Dickstein K, Cohen-Solal A, Lok DJ, Wasserman SM, Baker N, Rosser D, Cleland JG, Ponikowski P. Randomized, double-blind, placebo-controlled study to evaluate the effect of two dosing regimens of darbepoetin alfa in patients with heart failure and anaemia. Eur Heart J. 2007;28:2208–2216. doi: 10.1093/eurheartj/ehm328. [DOI] [PubMed] [Google Scholar]
- 15.Ghali JK, Anand IS, Abraham WT, Fonarow GC, Greenberg B, Krum H, Massie BM, Wasserman SM, Trotman ML, Sun Y, Knusel B, Armstrong P, Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation. 2008;117:526–535. doi: 10.1161/CIRCULATIONAHA.107.698514. [DOI] [PubMed] [Google Scholar]
- 16.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O’Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJ, RED-HF Committees; RED-HF Investigators Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiade M, Orlandi C, Burnett JC, Demets D, Grinfeld L, Maggioni A, Swedberg K, Udelson JE, Zannad F, Zimmer C, Konstam MA. Rationale and design of the multicenter, randomized, double-blind, placebo-controlled study to evaluate the Efficacy of Vasopressin antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) J Card Fail. 2005;11:260–269. doi: 10.1016/j.cardfail.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) Investigators Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor CM, Miller AB, Blair JE, Konstam MA, Wedge P, Bahit MC, Carson P, Haass M, Hauptman PJ, Metra M, Oren RM, Patten R, Pina I, Roth S, Sackner-Bernstein JD, Traver B, Cook T, Gheorghiade M. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from EVEREST program. Am Heart J. 2010;159:841–849. doi: 10.1016/j.ahj.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Mentz RJ, Schmidt PH, Kwasny MJ, Ambrosy AP, O’Connor CM, Konstam MA, Zannad F, Maggioni AP, Swedberg K, Gheorghiade M. The impact of chronic obstructive pulmonary disease in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST Trial. J Card Fail. 2012;18:515–523. doi: 10.1016/j.cardfail.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Anand I, McMurray JJ, Whitmore J, Warren M, Pham A, McCamish MA, Burton PB. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110:149–154. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 22.Anand IS, Chandrashekhar Y, Ferrari R, Poole-Wilson PA, Harris PC. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J. 1993;70:357–362. doi: 10.1136/hrt.70.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Meara E, Clayton T, McEntegart MB, McMurray JJ, Lang CC, Roger SD, Young JB, Solomon SD, Granger CB, Ostergren J, Olofsson B, Michelson EL, Pocock S, Yusuf S, Swedberg K, Pfeffer MA. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: Results of the CHARM program. Circulation. 2006;113:986–994. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 24.Scrutinio D, Passantino A, Santoro D, Catanzaro R. The cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long-term survival. Eur J Heart Fail. 2011;13:61–67. doi: 10.1093/eurjhf/hfq167. [DOI] [PubMed] [Google Scholar]
- 25.Androne AS, Katz SD, Lund L, LaManca J, Hudaihed A, Hryniewicz K, Mancini DM. Hemodilution is common in patients with advanced heart failure. Circulation. 2003;107:226–229. doi: 10.1161/01.cir.0000052623.16194.80. [DOI] [PubMed] [Google Scholar]
- 26.Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou-Nana MI. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48:2485–2489. doi: 10.1016/j.jacc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 27.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drazner MH, Velez-Martinez M, Ayers CR, Reimold SC, Thibodeau JT, Mishkin JD, Mammen PP, Markham DW, Patel CB. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail. 2013;6:264–270. doi: 10.1161/CIRCHEARTFAILURE.112.000204. [DOI] [PubMed] [Google Scholar]
- 29.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 30.Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: Insights from RALES. J Am Coll Cardiol. 2012;60:2082–2089. doi: 10.1016/j.jacc.2012.07.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


