Abstract
The homeostasis of multicellular organisms requires terminally differentiated cells to preserve their lineage specificity. However, it is unclear if mechanisms exist to actively protect cell identity in response to environmental cues that confer functional plasticity. Regulatory T (Treg) cells, specified by the transcription factor Foxp3, are indispensable for immune system homeostasis. Here, we report that conserved non-coding sequence 2 (CNS2), a CpG-rich Foxp3 intronic cis-element specifically demethylated in mature Tregs, helps maintain immune homeostasis and limit autoimmune disease development by protecting Treg identity in response to signals that shape mature Treg functions and drive their initial differentiation. In activated Tregs, CNS2 helps protect Foxp3 expression from destabilizing cytokine conditions by sensing TCR/NFAT activation, which facilitates the interaction between CNS2 and Foxp3 promoter. Thus, epigenetically marked cis-elements can protect cell identity by sensing key environmental cues central to both cell identity formation and functional plasticity without interfering with initial cell differentiation.
Introduction
In multicellular organisms, terminally differentiated cells need to preserve their lineage identity as well as exerting a certain degree of functional plasticity in response to different environment cues to optimally function. Whether the environment cues that confer differentiated cells functional plasticity can also compromise cell identity and whether mechanisms exist to actively guard against potential loss of cell identity while preserving functional plasticity is not clear. This is particularly relevant in the immune system, where various immune cells have to cope with a wide range of challenging conditions, including pathogen invasion of hosts, inflammatory conditions, and host tissue damages. Immune cells may be informed by these environmental cues through an abundance of sensors to meet these challenges. Yet failure to safeguard immune cell lineage identity can lead to either compromised immune responses or autoimmune diseases.
Regulatory T (Treg) cells are a distinct lineage of CD4+ T cells that are essential for maintaining immune system homeostasis by promoting self-tolerance and restraining excessive immune responses (Sakaguchi et al., 2008). Foxp3, an X-chromosome-encoded transcription factor belonging to the forkhead family, plays an indispensable role in Treg lineage specification and function (Josefowicz et al., 2012a). Expression of Foxp3 can be induced in Treg precursors either in the thymus or in the periphery by a combination of TCR and cytokine signals. Mutation of Foxp3 in mice results in severe lymphoproliferative disorder and widespread tissue inflammation due to paucity of Treg cells (Fontenot et al., 2003; Khattri et al., 2003). Additionally, ablation or attenuation of Foxp3 expression in mature Treg cells leads to dysregulation of Foxp3 target genes and compromised Treg suppressor function (Wan and Flavell, 2007; Williams and Rudensky, 2007), indicating the requirement of constant Foxp3 expression for maintaining Treg lineage identity and function.
Fate-mapping studies have demonstrated that Foxp3 expression in Treg cells is remarkably stable in steady state and a number of inflammatory conditions, although moderate loss of Treg Foxp3 expression owing to limited IL-2 availability has been observed (Rubtsov et al., 2010). It is also possible that Foxp3 expression could be destabilized in Treg cells under other pathological conditions (Miyao et al., 2012; Rubtsov et al., 2010). Despite the apparent stability of the Tregs, several lines of evidence suggest that Tregs also possess a great degree of functional plasticity (Sakaguchi et al., 2013). Expression of certain polarizing transcription factors can endow Tregs with the ability to specifically suppress effector CD4 T cells that express the same polarizing transcription factor. For example, IRF4 expression in Tregs is essential for Tregs to control T helper type 2 (TH2) inflammation (Zheng et al., 2009). Expression of other transcription factors such as T-bet, BLIMP1, GATA3, and STAT3 in Tregs also affects specific aspects of Treg functions (Chaudhry et al., 2009; Cretney et al., 2011; Koch et al., 2009; Wang et al., 2011; Wohlfert et al., 2011). Tregs present in tissues (tissular Tregs) have also been shown to have gene expression programs distinct from Tregs in lymphoid organs, likely resulting from residing in unique tissue environments that also confer unique functions (Burzyn et al., 2013; Cipolletta et al., 2012). Therefore, whether mechanisms exist to actively preserve the identity of Tregs exhibiting these types of functional plasticity is unclear.
Our previous investigation showed that among three evolutionarily conserved non-coding sequences (CNSs) located in the Foxp3 intronic regions, CNS2 (also named TSDR for Treg specific demethylated region) plays a unique role in maintaining Foxp3 expression in mature Treg cells (Zheng et al., 2010). In comparison to other conserved cis-elements in the Foxp3 locus, CNS2 is uniquely enriched with CpG motifs, which are specifically demethylated during thymic Treg cell differentiation (Floess et al., 2007). A number of transcription factors, including CREB, NF-kB, Runx1, STAT5, Gata3, Foxo1, Ets1, and Foxp3 itself, can interact with CNS2, although whether CNS2 plays a non-redundant role for these transcription factors to regulate Foxp3 expression is not clear (Bruno et al., 2009; Kim and Leonard, 2007; Kitoh et al., 2009; Long et al., 2009; Ouyang et al., 2010; Ruan et al., 2009; Rudra et al., 2009; Wang et al., 2011; Wohlfert et al., 2011; Yao et al., 2007).
Here we took advantage of the remarkable capability of Tregs to maintain their identity while exhibiting a great degree of functional plasticity to test if an active mechanism exists to prevent potential conflicts between these two properties. We examined the physiological consequences of CNS2 deletion and explored the mechanisms underlying CNS2-dependent regulation of Foxp3 expression and Treg cell identity protection. We found that the CNS2 knockout (CNS2−) mice developed spontaneous lymphoproliferative disease and were more susceptible to experimental autoimmune encephalomyelitis (EAE), although Treg cell numbers in most lymphoid organs were not reduced in CNS2 mice. By performing unbiased gene expression profiling, we revealed that genes highly expressed in the CNS2-dependent Treg subset fit the profiles of activated Treg cells that receive strong TCR and cytokine stimulation, implicating the role of CNS2 in protecting Treg identity in highly activated “effector” Tregs. Mechanistically, we found that CNS2 protected Treg identity from challenging cytokine conditions that were known to impede initial Treg identity formation. CNS2 helped maintain mature Treg identity by sensing TCR activation that is central for both mature Treg functions and initial Treg lineage specification, whereas CNS2 methylation before Treg identity formation prevented undesired induction of Foxp3 expression. We identified NFAT as a novel CNS2-binding transcription factor in response to TCR activation. Upon TCR stimulation, CNS2 interacted with the Foxp3 promoter in an NFAT-dependent manner to stabilize Foxp3 transcription in Treg cells. These results demonstrated that a cis-element epigenetically switched on during lineage differentiation can protect the identity of differentiated cells in challenging environments by sensing signals central to both lineage differentiation and mature cell functional plasticity, without undesired perturbation of initial lineage differentiation.
Results
CNS2 deletion leads to spontaneous lymphoproliferative disease
We analyzed CNS2− (KO) and wild-type (WT) littermate control mice to determine if CNS2 is required for regulatory T cells to control immune homeostasis in vivo. CNS2− mice appeared to be normal up to 2 months of age when they started to develop moderate lymphoproliferative disease with increased cellularity in spleen and lymph nodes, correlated with lower body weight compared to littermates as they aged (Figures 1A, 1B, and 1C). Histopathology analysis of 6- to 9-month-old CNS2− mice revealed multifocal, moderate to severe perivascular and peribronchiolar lympho-plasmacytic infiltration in the lung with mild thickening of alveolar walls (Figure 1D). Lesions mostly consisting of lympho-plasmacytic infiltration were also observed frequently in liver and kidney of CNS2− mice, but not in littermate control mice (Figure 1D). Furthermore, CNS2− mice also showed evidence of lympho-plasmacytic enteritis in small intestines, with multifocal infiltrations of the lamina propria with lymphocytes, plasma cells, and neutrophils (Figure 1D). Thus, CNS2− mice spontaneously develop lymphoproliferative disease manifesting multi-organ inflammatory lesions.
Figure 1.

CNS2 deletion in mice leads to spontaneous lymphoproliferative disease.
(A, B, D, H) Body weights (A), spleen weights (B), representative hematoxylin and eosin staining of small intestine, liver, kidney, and lung sections (D), and concentration of IgE, IgG2c, IgG1, and IgA in serum, determined by enzyme linked immunosorbent assay (H) of 6-9-month-old CNS2− (knockout, KO) and littermate control (WT) mice. n = 5–7.
(C, E, F, and G) Cellularity (C), CD4+ and CD8+ T cell numbers (E), frequency of CD62Llo CD44hi cells in Foxp3− CD4+ T cells (F), and frequency of IFN-γ+ and IL-4+ cells in CD4+ T cells (G) in spleens and peripheral lymph nodes (LN) from 9-month-old KO and WT mice. Numbers in top right quadrants (F, left) indicate percent gated cells. n = 5–6.
All data are representative of three experiments. Mean ± s.d.
See also Figure S1.
Consistent with the role of Tregs in controlling T cell proliferation and activation (Josefowicz et al., 2012a), the numbers of CD4+ T cells in the spleen and lymph nodes of CNS2− mice increased compared to littermates (Figure 1E). Frequencies of activated CD4+ T cells (CD44hi CD62Llo) also significantly increased in the spleens, peripheral lymph nodes, mesenteric lymph nodes, lamina propria of small and large intestines, and lungs of CNS2− mice (Figures 1F and S1A). Moreover, frequencies of both IFN-γ+ and IL-4+ CD4+ T cells increased in the spleen and lymph nodes from CNS2− mice (Figure 1G), suggesting that both T helper type 1 (TH1) and T helper type 2 (TH2) responses contributed to the pathology of CNS2− mice. IFN-γ production by CD4+ T cells from Peyer's patch and lung and splenic CD8+ T cells also increased in CNS2− mice (Figures S1B and S1C). In agreement with these results, serum concentrations of IgE and IgG2c which are associated with TH2 and TH1 responses, respectively, were significantly higher in CNS2− mice compared to WT littermates (Figure 1H). Although frequency of IL-17A secreting T helper type 17 (TH17) cells decreased in lymph nodes from CNS2− mice compared to littermate controls, frequency of TH17 cells significantly increased in Peyer's patch and small intestines (Figure S1D). These findings demonstrate that CNS2− mice have a general defect in controlling excessive CD4+ T cell activation and proliferation, as well as TH1, TH2, and TH17 cell differentiation.
CNS2-dependent Tregs are enriched in the Foxp3lo subset of Tregs from CNS2− mice
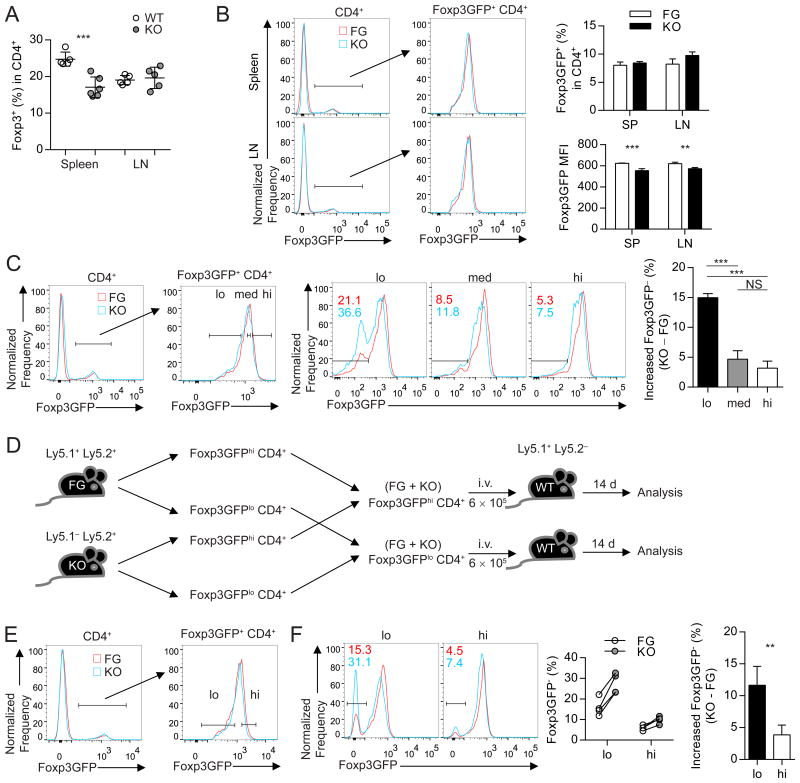
Because the main function of CNS2 is likely to regulate Foxp3 expression, we examined if CNS2 deletion led to numerical deficiency of Tregs or overall decrease of Foxp3 expression in Tregs. CNS2− mice had a moderate decrease of Treg cell frequency in spleen, but not in peripheral lymph nodes, mesenteric lymph node, Peyer's patch, lung, and lamina propria of small and large intestines (Figures 2A and S2A). Interestingly, on a per cell basis, Tregs in CNS2− and WT littermates had comparable overall Foxp3 expression levels (Figure S2B). Since a more lymphoproliferative environment in aged CNS2− mice might affect Foxp3 expression, we analyzed Tregs in 6-week-old CNS2− and CNS2wild-type Foxp3GFP (FG) mice. Frequency of Tregs in spleen and lymph nodes of young CNS2− and Foxp3GFP mice was comparable, whereas a small but significant decrease of Foxp3 expression was observed in CNS2− Tregs (Figure 2B). These results suggest that, at any given moment, CNS2 likely regulates Foxp3 expression in a small subset of Tregs in unprovoked mice, thus minimizing its effect on the overall Foxp3 expression level in the total Treg population.
Figure 2.
CNS2-dependent Tregs are enriched in the Foxp3lo Treg subset from CNS2− mice.
(A) Frequency of Foxp3+ cells in CD4+ T cells in spleen and peripheral lymph nodes (LN) from 9-month-old CNS2−(KO) and littermate control (WT) mice. n = 5–6.
(B) Flow cytometry analysis of Foxp3GFP expression in cells in spleen and LN from 6-week-old young CNS2− (KO) and CNS2wild-type Foxp3GFP (FG) mice. MFI, mean fluorescence intensity. n = 4.
(C) Foxp3GFPlo (lo), Foxp3GFPmed (med), and Foxp3GFPhi (hi) Tregs from KO and FG mice (left) were cultured in vitro for 3 days before flow cytometry analysis of Foxp3GFP expression (middle and right). Numbers in top left quadrants (middle) indicate percent Foxp3GFP− cells.
(D, E, and F) Equal numbers of Foxp3GFPlo (lo) and Foxp3GFPhi (hi) Tregs sort-purified (E) from Ly5.1+ Ly5.2+ FG and Ly5.1− Ly5.2+ KO mice were mixed and injected into Ly5.1+ Ly5.2− wild-type recipient mice intravenously. 14 days later, transferred cells were analyzed by flow cytometry (F). n = 4.
All data are representative of three experiments. Mean ± s.d.
See also Figure S2.
We sought to use an unbiased approach to identify the subset of Tregs that require CNS2 for stable Foxp3 expression. We hypothesized that CNS2-dependent Tregs should express lower levels of Foxp3 in CNS2− mice than in WT mice, resulting in their enrichment in the Foxp3lo population and depletion in the Foxp3hi population in CNS2− mice. To test this, we sorted Foxp3GFP high, medium, and low populations from Foxp3GFP and CNS2− mice, and compared the stability of Foxp3 expression after they were cultured for 3 days in vitro. Although all three subsets of CNS2− Tregs lost more Foxp3 expression compared to the corresponding subsets of Foxp3GFP Tregs, the difference was markedly higher in Foxp3lo Tregs than in Foxp3med and Foxp3hi subsets (Figure 2C). To recapitulate this observation in vivo, we co-transferred equal numbers of Foxp3hi (or Foxp3lo) Tregs sorted from Ly5.1− Ly5.2+ CNS2− and Ly5.1+ Ly5.2+ Foxp3GFP mice into Ly5.1+ Ly5.2− wild-type recipient mice and analyzed transferred cells 14 days later (Figure 2D, 2E, and S2C). Again, CNS2 deletion led to significantly greater loss of Foxp3 expression in the Foxp3lo subset than in the Foxp3hi subset of Tregs (Figure 2F). Thus, the Foxp3lo subset of Tregs in CNS2− mice are the most defective Treg subset in maintaining Foxp3 expression and are likely enriched with CNS2-dependent Tregs.
CNS2 is required for activated “effector” Tregs to maintain high levels of Foxp3 expression
To further characterize Tregs that depend on CNS2 to maintain Foxp3 expression (enriched in the Foxp3lo Tregs in CNS2− mice), we performed RNA-sequencing analysis to compare gene expression profiles of Foxp3lo Tregs isolated from CNS2− and Foxp3GFP mice. To minimize and control variations caused by different inflammation levels between CNS2− and Foxp3GFP mice, we used two individual six- to eight-week-old mice for each genotype and also profiled Foxp3hi Tregs as controls (Figure 3A). Interestingly, clustering analysis indicated that the gene expression profile of CNS2− Foxp3lo Tregs was more similar to Foxp3hi Tregs from both CNS2− and Foxp3GFP mice, than to Foxp3lo Tregs from Foxp3GFP mice (Figure 3B), consistent with the notion that CNS2− Foxp3lo Tregs included some Tregs that would have higher levels of Foxp3 expression were it not for CNS2 deficiency.
Figure 3.
CNS2 is required for “effector” Tregs to maintain high levels of Foxp3 expression.
(A) Foxp3GFPlo (lo) and Foxp3GFPhi (hi) Tregs sort-purified from 6-week-old CNS2− (KO) and Foxp3GFP (FG) mice (A) were used for RNA sequencing (RNA-seq) analysis (B and C). n = 2.
(B) Clustering of RNA-seq samples based on gene expression.
(C) Genes enriched in the Foxp3lo subset and/or depleted in the Foxp3hi subset of KO Tregs relative to FG Tregs, shown in groups based on their functions.
(D, E, and F) Ki67+ cell frequency (D) and expression of CTLA4 (E) and ICOS (F) in Foxp3lo and Foxp3hi Tregs from 2-month-old KO and FG mice. Numbers in histograms indicate percent, Ki67+ cell (D) and mean fluorescence intensity (MFI) of CTLA4 (E) and ICOS (F). n = 5–6.
(G) Suppression of proliferation of CellTrace Violet-labeled wild-type naive (CD62L+ CD44−) CD4+ T responder cells (Tresp) by KO and FG Tregs, presented as dilution of CellTrace Violet in Tresp cells cultured with Tregs at indicated ratio (left).
Data are representative of two (A, B, C, and G) and three (D, E, and F) experiments. Mean ± s.d.
See also Figure S3 and Table S1.
There were clusters of genes that were generally up-regulated or down-regulated in CNS2− Tregs compared with Foxp3GFP Tregs regardless of Foxp3 expression levels (Figure 3B and Table S1), likely influenced by changes in environmental factors in CNS2− mice, such as cytokine milieus, that could affect Treg gene expression. To search for features of gene expression more directly associated with CNS2-dependency, we identified genes whose expression levels were higher in CNS2− Foxp3lo Tregs than in Foxp3GFP Foxp3lo Tregs and/or lower in CNS2− Foxp3hi Tregs than in Foxp3GFP Foxp3hi Tregs (Figure 3C). One group of genes encode factors associated with cell growth and division, including the cell proliferation marker Ki-67 (Mki67), cyclin and cyclin-dependent kinase (Ccnb2, Ccnb1, and Cdk1), and others involved in T cell activation and proliferation (Cdc25, Ttk, Cdkn2, Bub1, and Espl1) (Figure 3C). A second group of genes encode chemokine receptors (Ccr2, Ccr5, Ccr3, Cxcr6, Cxcr3, and Ccr4), cytokine receptors (Il23r, Il9r, Il12rb1, and Il12rb2), and transcription factors playing key roles in CD4+ T cell polarization (Rorc, Tbx21, and Prdm1) (Cretney et al., 2011; Koch et al., 2009; Manel et al., 2008) (Figure 3C), suggesting that CNS2-dependent Tregs were responsive to chemotaxis and cytokine signals, and differentiating into activated “effector” Tregs. The third group of genes were Treg “signature” genes, many of which are essential for Treg suppressor function, including Il10, Ctla4, and Icos (Herman et al., 2004; Rubtsov et al., 2008; Wing et al., 2008) (Figure 3C). Thus, CNS2 is required for activated “effector” Tregs to maintain higher levels of Foxp3 expression.
We next examined protein expression levels of several key genes identified by gene expression profiling, including Ki-67, CTLA4, and ICOS, in CNS2− and Foxp3GFP Tregs by flow cytometry. The expression patterns of these genes in Foxp3lo and Foxp3hi Tregs in CNS2− and Foxp3GFP mice were in concordance with the gene expression profiles generated by the RNA-seq analysis (Figures 3D, 3E, and 3F). Consistent with key roles of CTLA4 and ICOS in Treg function, the suppressor function of CNS2− Tregs was much lower compared to Foxp3GFP control Tregs (Figure 3G). Interestingly, Foxp3hi Tregs had much higher suppression capacity than Foxp3lo Tregs and the small increase in suppressor function of CNS2− Foxp3lo Tregs apparently could not compensate for the decrease of suppressor function in CNS2− Foxp3hi Tregs compared to their wild-type counterparts (Figure S3A). In addition, at the end of the in vitro suppression assay, more CNS2− Tregs lost Foxp3 expression compared to Foxp3GFP Tregs irrespective of their original Foxp3 expression levels (Figure S3B). Taken together, these results indicated that CNS2 plays a critical role in maintaining high levels of Foxp3 expression and normal suppressor capacity in activated “effector” Tregs.
CNS2 deletion exacerbates experimental autoimmune encephalomyelitis development
We hypothesized that the inability of CNS2− “effector” Tregs to maintain Foxp3 expression could lead to exacerbation of autoimmune diseases in CNS2− mice. To test this, we challenged CNS2− mice with the experimental autoimmune encephalomyelitis (EAE) disease model. During EAE development, strong TCR stimulation and abundant inflammatory cytokines including IL-6 lead to the activation, expansion, and differentiation of CD4+ cells into TH1 and TH17 cells (Korn et al., 2009). CNS2− mice developed more severe disease and lost more body weight compared to WT littermates during the course of the EAE (Figure 4A). This was accompanied with increased IFN-γ secreting TH1 and IL-17A secreting TH17 cells in central nervous system (CNS) of CNS2− mice (Figure 4B). Importantly, CNS2− mice had severely reduced frequency of Tregs within CD4+ T cells in CNS (Figure 4C), whereas the reduction of Treg frequency in the spleen was comparable to that observed in unchallenged mice and much smaller than in the CNS (Figures 2A, 4C, and S4A). In addition, Foxp3 expression in Tregs was also much lower in CNS2− mice in the CNS, but not in the spleen (Figures 4D and S4B). These results indicate a critical role for CNS2 in maintaining Tregs stability and numbers in an autoimmune disease setting.
Figure 4.
CNS2 deletion exacerbates experimental autoimmune encephalomyelitis (EAE) development in mice.
(A) Disease progression (left) and body weight (right) of CNS2− (KO) and littermate control mice (WT) after EAE induction. n = 6–7.
(B, C, and D) Numbers of IL-17A+ and IFN-γ+ CD4+ T cells (B), frequency of Foxp3+ CD4+ T cells (C), and mean fluorescence intensity (MFI) of Foxp3 in Foxp3+ CD4+ T cells (D) in CNS 22 days after EAE induction.
All data are representative of three experiments. Mean ± s.d.
See also Figure S4.
CNS2 helps maintain Foxp3 expression in Tregs in destabilizing cytokine environments
We next investigated the molecular mechanisms underlying the requirement of CNS2 for “effector” Tregs to maintain Foxp3 expression. Given that CNS2-dependent Tregs were highly capable of travelling to inflammation sites and being stimulated by pro-inflammatory cytokines (Figure 3C), we examined if CNS2 helps protect Treg identity in the presence of T cell polarizing cytokines. Addition of IL-4 or IL-6, but not IL-12 to cultured Foxp3GFPmed-hi Tregs greatly down-regulated Foxp3 expression in CNS2−, but not Foxp3GFP Tregs (Figure 5A), indicating that CNS2 is critical for Tregs to maintain Foxp3 expression in the presence of TH2 and TH17 related cytokines. Addition of IL-4 and IL-6 to Treg cultures did not negatively affect cell survival and proliferation (Figure S5A and data not shown). In addition, reduction of Foxp3 expression in CNS2− Tregs treated with IL-4 or IL-6 could still be observed when only proliferating Foxp3GFP positive Tregs were examined (Figure S5A and S5B), suggesting that they have a direct inhibitory effect on Tregs, not through negatively affecting cell health. The muted effect of IL-12 on CNS2− Tregs is possibly due to compromised expression of IL-12Rb2 (Koch et al., 2012). As IL-2 neutralization can mildly destabilize Foxp3 expression in mature WT Tregs in vivo (Rubtsov et al., 2010), we asked if CNS2 is required for maintaining Foxp3 expression in Tregs when IL-2 is limited. Both Foxp3GFP and CNS2− Tregs cultured without exogenous IL-2 had increased loss of Foxp3 expression (Figure 5A). Addition of a mouse IL-2 neutralizing antibody destabilized Foxp3 expression in CNS2− Tregs to a similar, if not greater extent than in wild-type FG Tregs (Figure 5B). Taken together, these findings showed an important function for CNS2 to maintain Foxp3 expression in Tregs exposed to cytokines IL-4 and IL-6 or when IL-2 is limited.
Figure 5.
CNS2 helps stabilizing Foxp3 expression in Tregs exposed to challenging cytokine conditions.
(A and B) Foxp3GFP expression in CNS2− (KO) and Foxp3GFP (FG) Tregs cultured in the absence or presence of cytokines (IL-2, IL-12, IL-4, or IL-6) (A) or mouse IL-2 neutralizing antibody (B) for 3 days. Numbers in histograms (A) indicate percent Foxp3GFP− cells.
(C and D) Foxp3GFP expression (D) in in vivo generated FG and KO induced Tregs (iTregs)
(C) cultured in the presence of IL-2 for 3 days.
(E) Foxp3GFP expression in FG and KO natural Tregs (nTreg, Nrp1+) and iTregs (Nrp1−) cultured in the presence of IL-2 for 3 days.
All data are representative of at least two experiments. Mean ± s.d.
See also Figure S5.
Foxp3 expression can be induced extrathymically in naive CD4+ T cells to generate induced Tregs (iTregs) (Chen et al., 2003). Whereas iTregs generated in vitro are not stable in the absence of TGF-β (Floess et al., 2007), iTregs generated in vivo can acquire stable Foxp3 expression and perform essential functions (Josefowicz et al., 2012b; Polansky et al., 2008). CNS2 deletion did not affect the induction and stability of in vitro generated iTreg as expected (Figures S5C and S5D). To examine if CNS2 contributes to stable Foxp3 expression in iTregs generated in vivo, we transferred Ly5.1− Ly5.2+ Foxp3GFP− naive CD4+ T cells from Foxp3GFP or CNS2− mice together with cogenically marked Ly5.1+ Ly5.2− wild-type Tregs into Rag1 knockout recipient mice (Figure 5C). 12 days later, Foxp3GFP and CNS2− iTregs (Ly5.1− Ly5.2+ Foxp3GFP+) were isolated from recipient mice and activated in vitro to test Treg stability (Figure 5C). Only ∼20% of CNS2− iTregs remained Foxp3GFP positive compared to ∼60% of wild-type iTregs (Figure 5D). We also took advantage of Neuropilin 1 (Nrp1) as a marker for natural Tregs (nTregs) to sort both nTregs (Nrp1+) and iTregs (Nrp1−) from unprovoked Foxp3GFP and CNS2− mice to test their lineage stability (Figure S5E) (Weiss et al., 2012; Yadav et al., 2012). Again, CNS2 deletion significantly destabilized Foxp3 expression in both nTregs and iTregs after 3 days of culture in vitro (Figure 5E). Taken together, these results demonstrated an essential role of CNS2 in stabilizing Foxp3 expression in both nTregs and iTregs.
CNS2 is required for Tregs receiving strong TCR stimulation to maintain elevated Foxp3 expression in vitro and in vivo
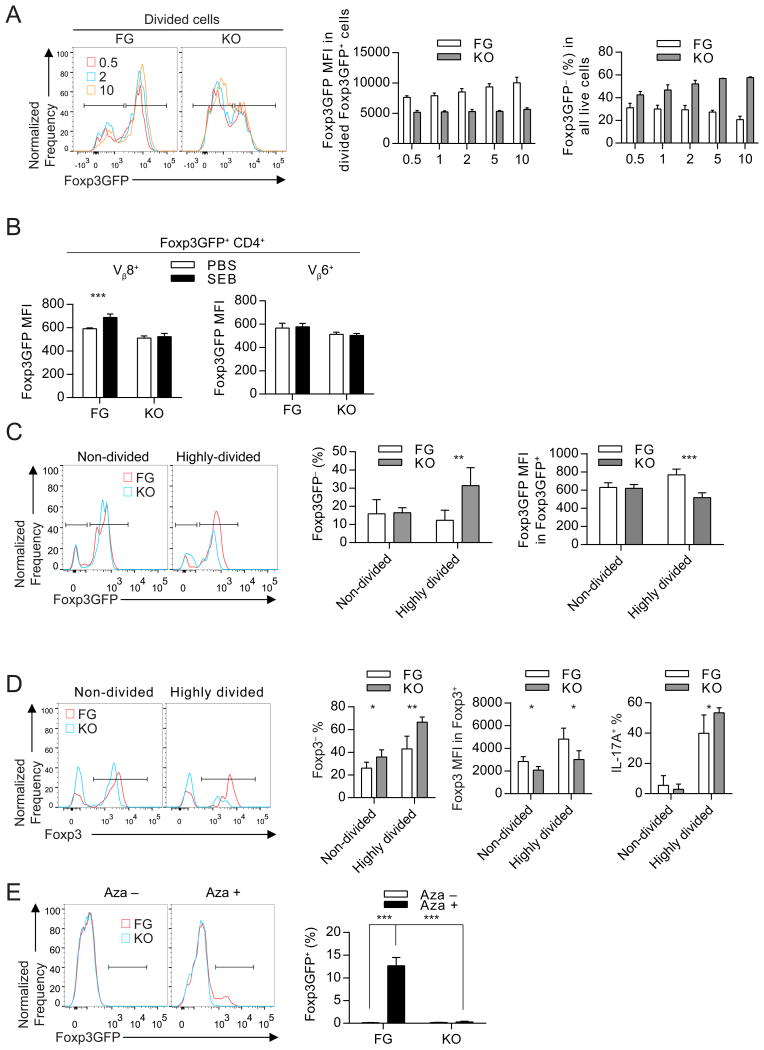
We next asked what signals promote stable Foxp3 expression through CNS2. Given increased expression of proliferation-associated genes in CNS2-dependent Tregs (Figure 3C), increased loss of Foxp3 expression in divided cells (Zheng et al., 2010), and the central role of TCR signaling in activation and proliferation of Tregs (Walker et al., 2003), we evaluated the effects of different TCR stimulation strengths on Foxp3 expression in CNS2− and Foxp3GFP Tregs. Increasingly stronger TCR stimulation by anti-CD3 antibody progressively increased and stabilized Foxp3 expression in Foxp3GFP Tregs. In contrast, CNS2− Tregs failed to up-regulate Foxp3 and more CNS2− Tregs lost Foxp3 expression after receiving stronger TCR stimulation (Figure 6A).
Figure 6.
CNS2 is required for Tregs receiving strong TCR stimulation to maintain elevated Foxp3 expression in vitro and in vivo.
(A) Foxp3GFP expression in CellTrace Violet-labeled CNS2− (KO) and Foxp3GFP (FG) Tregs cultured in wells coated with 0.5, 1, 2, 5, or 10 μg/ml of anti-CD3 and 10 μg/ml of anti-CD28 for 3 days.
(B) Foxp3GFP expression in staphylococcal enterotoxin B (SEB)-responsive Vβ8+ and SEB-non-responsive Vβ6+ Tregs in spleens from 6-week-old KO and FG mice 4 days after intravenous injection with 100 μg SEB or vehicle (PBS). n = 4.
(C) Foxp3 expression in cogenically marked CellTrace Violet-labeled KO and FG Tregs 14 days after being co-transferred into wild-type mice. n = 5.
(D) Foxp3 expression and IL-17A secretion in CellTrace Violet-labeled FG or KO Tregs 7 days after being transferred into WT mice immunized with MOG and CFA 7 days before cell transfer. n = 5.
(E) Foxp3GFP induction in KO and FG naive (Foxp3GFP– CD62Lhi CD44lo) CD4+ T cells activated for 4 days in the absence or presence of 1 μM 5-Aza-2′-deoxycytidine (Aza) (added 1 day after start of cell culture for the duration of 24 h).
Mean ± s.d.
See also Figure S6.
To examine how TCR stimulation regulates Foxp3 expression in wild-type and CNS2-deficient Tregs in vivo, we injected superantigen staphylococcal enterotoxin B (SEB) or PBS into CNS2− and Foxp3GFP mice and examined Foxp3 expression in SEB-responsive TCR Vβ8+ and SEB-unresponsive TCR Vβ6+ Tregs 3 days later. SEB treatment increased Foxp3 expression in Vβ8+ Tregs, but not in Vβ6+ Tregs in Foxp3GFP mice (Figure 6B and S6A). Importantly, SEB treatment failed to up-regulate Foxp3 expression in Vβ8+ Tregs in CNS2− mice (Figure 6B and S6A).
To assess if CNS2 helps maintain stable Foxp3 expression in Tregs receiving strong TCR stimulation in vivo, we also tested if CNS2 deletion leads to preferential loss of Foxp3 expression in proliferative Tregs than in non-proliferative Tregs, since proliferative Tregs likely receive stronger TCR stimulation than non-proliferative Tregs. We co-transferred equal numbers of Ly5.1− Ly5.2+ CNS2− and Ly5.1+ Ly5.2+ Foxp3GFPmed-hi Tregs stained with CellTrace Violet into Ly5.1+ Ly5.2− wild-type mice and analyzed cell division and Foxp3 expression in transferred cells 14 days after transfer (Figures S6B and 6C). CNS2− Tregs divided less compared with WT Tregs (Figure S6C), in agreement with their decreased levels of Ki-67 expression (Figure 3D). Importantly, CNS2 deletion led to significantly increased loss of Foxp3 expression and significantly reduced Foxp3 expression in cells that remained Foxp3GFP+ in highly-divided, but not in non-divided Tregs (Figure 6C). Thus, both in vitro and in vivo, CNS2-dependent signal is important for Tregs to up-regulate and maintain Foxp3 expression levels upon strong TCR stimulation.
Under chronic inflammatory conditions, a subset of Tregs can lose Foxp3 expression and become ex-Tregs contributing to disease pathology (Bailey-Bucktrout et al., 2013; Komatsu et al., 2014). To examine if CNS2− Tregs that had lost Foxp3 expression could become cytokine producing ex-Tregs in vivo, we transferred CellTrace Violet-labeled Ly5.1− Ly5.2+ Foxp3GFPmed-hi Tregs from Foxp3GFP or CNS2− mice into congenically marked wild-type mice that had been immunized with MOG/CFA 7 days before cell transfer, and analyzed transferred cells 7 days after transfer (Figure S6D). CNS2 deletion led to increased loss of Foxp3 expression and reduced Foxp3 expression in Tregs that remained Foxp3 positive, especially in highly divided cells (Figures 6D and S6E). Notably, most of the ex-Tregs became IL-17A secreting cells and higher percentage of CNS2− Tregs became TH17 effector cells, probably because increased ex-Treg conversion (Figure 6D). It remains to be determined if CNS2 deletion can also promote ex-Tregs to Th2 cell conversion in vivo.
Given that TCR also plays a central role in Treg lineage specification (Hsieh et al., 2006; Jordan et al., 2001; Moran et al., 2011), and that CNS2 is only fully demethylated in mature Tregs (Floess et al., 2007), we asked if enforced CNS2 demethylation in naive CD4 T cells activated in vitro can drive aberrant Foxp3 induction (Figure 6E). Although it has been reported that 5-Aza could enhance Foxp3 induction (Lal et al., 2009; Polansky et al., 2008), it is possible that 5-Aza promotes Foxp3 expression through demethylation of other enhancer elements at the Foxp3 locus or through indirectly regulating other transcription factors that modulate Foxp3 expression. As expected, 5-Aza increased Foxp3 expression in naive CD4 T cells from Foxp3GFP mice (Figure 6E). Importantly, CNS2 was required for Foxp3 induction by 5-Aza (Figure 6E). Taken together, these results showed that CNS2 senses TCR signals to increase and to maintain Foxp3 expression in mature Tregs and its methylation prevents undesired Treg generation.
TCR stimulation activates NFAT to facilitate the interaction between CNS2 and Foxp3 promoter to stabilize Foxp3 expression
We next asked what signals downstream of TCR are sensed by CNS2 to promote and to stabilize Foxp3 expression in Tregs. Using retroviral expression of dominant negative inhibitors, we found that CREB and NF-kB can promote Foxp3 expression in a CNS2-dependent manner in activated Tregs (Figures S7A and S7B), in agreement with results from previous studies (Kim and Leonard, 2007; Long et al., 2009; Ruan et al., 2009). These results suggested that other TCR-activated factors may contribute to CNS2-dependent maintenance of Foxp3 expression.
We tested if NFAT, one of the key players in TCR signaling (Muller and Rao, 2010), can promote Foxp3 expression through CNS2. NFAT has been shown to interact with Foxp3 CNS1 region along with Smad3 to facilitate TGF-β-induced Foxp3 expression (Tone et al., 2008), but there was no report linking NFAT to CNS2. Surprisingly, inhibition of the calcineurin-NFAT signaling pathway by Cyclosporin A (CsA) significantly reduced Foxp3 expression in activated wild-type Tregs, but not in CNS2− Tregs (Figures 7A and S7C). Importantly, CNS2 deletion did not further reduce Foxp3 expression in Tregs treated with CsA (Figure 7A). Thus, NFAT plays a critical role in CNS2-dependent regulation of Foxp3 expression.
Figure 7.

TCR stimulation activates NFAT to facilitate the interaction between CNS2 and Foxp3 promoter to promote Foxp3 expression.
(A) Effects of NFAT inhibition with Cyclosporin A (CsA) on Foxp3GFP expression in CNS2− (KO) and Foxp3GFP (FG) Tregs activated by plate-coated anti-CD3 (10 μg/ml) and anti-CD28 (10 μg/ml) for 3 days.
(B) Alignment of a putative NFAT:Foxp3 binding site on mouse CNS2 (mCNS2) and human CNS2 (hCNS2) with the consensus sites in human and mouse CTLA4 gene.
(C) Binding of NFATc1 and NFATc2 to the putative NFAT binding element at CNS2. Wild-type and mutant IL-2 antigen receptor response elements (IL-2 ARRE) served as positive and negative controls, respectively.
(D) Effects of deletion of the putative NFAT binding site on the activity of CNS2 in a luciferase reporter assay in mouse CD4+ T cells.
(E) NFAT binding to Foxp3 promoter and CNS2 analyzed by ChIP in wild-type Tregs activated with Ionomycin in the presence or absence of CsA for 30 minutes. The Ctla4 and the Gmpr gene served as positive and negative controls, respectively.
(F) NFATc1 and NFATc2 binding to CNS2 analyzed by ChIP in WT Tregs stimulated with Ionomycin.
(G) Interaction of Foxp3 promoter with CNS2 and other locations within the Foxp3 locus in Tregs and Foxp3GFP− conventional CD4 T cells (Tconv) from FG and KO mice measured by Chromosome Conformation Capture (3C) assay after cells were activated with Ionomycin for 30 min.
(H) Interaction of CNS2 with Foxp3 promoter in wild-type Tregs measured by 3C assay after cells were activated with Ionomycin in the presence or absence of CsA for 30 min.
(I and K) Nipbl (I) or Med12 (K) binding to Foxp3 promoter, CNS2, and other locations at the Foxp3 locus analyzed by ChIP in WT Tregs and Tconv cells stimulated with PMA and Ionomycin.
(J and L) Foxp3GFP expression in FG and KO Tregs 3 days after cells were transduced with control (Ctrl) RNAi retroviral vector and RNAi vector targeting Nipbl (J) or Med12, (L). All data are representative of at least two experiments. Mean ± s.d.
See also Figure S7 and Tables S2, S3, and S4.
Close examination of the CNS2 locus identified a potential NFAT binding site with high similarity to the consensus NFAT:Foxp3 binding site (Wu et al., 2006) (Figures 7B and S7D). Both NFATc1 and NFATc2 bound to DNA probes containing the putative CNS2 NFAT binding motif, but not to probes containing a mutant NFAT motif in a DNA pulldown assay (Figure 7C). Deletion of this site in CNS2 greatly impaired the transactivation ability of CNS2 in a luciferase reporter assay in both primary mouse CD4+ T cells and Jurkat cells (Figures 7D and data not shown). To determine if NFAT binds to CNS2 in vivo, we performed chromatin immunoprecipitation (ChIP) assay. NFAT bound to CNS2 in addition to Foxp3 promoter in WT Tregs and its binding to both locations was abolished by CsA (Figure 7E). Deletion of CNS1 did not reduce NFAT binding to CNS2 (Figure S7E) and both NFATc1 and NFATc2 could bind to CNS2 (Figure 7F).
Enhancer:promoter looping is one of the key mechanisms by which enhancers regulate gene expression (Levine et al., 2014). To examine if CNS2 can physically interact with Foxp3 promoter through a “looping” mechanism specifically in Tregs, we performed chromatin conformation capture (3C) assay (Dekker et al., 2002). We used a DpnII digestion fragment encompassing the Foxp3 proximal promoter region as the “anchoring point” to assess the interaction frequency between Foxp3 promoter and various regions across the Foxp3 locus (Figure 7G). We placed the 3C qPCR primers for CNS2 at sites next to CNS2 so that we could assess looping of the Foxp3 promoter to the CNS2 proximal region if it occurs in the CNS2− Tregs (Figure 7G). The Foxp3 promoter:CNS2 interaction was higher than the interaction between Foxp3 promoter and other locations examined across the Foxp3 locus in Foxp3GFP Tregs (Figure 7G). Importantly, the Foxp3 promoter:CNS2 interaction was ∼6 fold higher in Tregs than in Foxp3GFP− CD4+ Tconv cells (Figure 7G). In addition, in CNS2− Tregs, the interaction between Foxp3 promoter and regions immediately adjacent to CNS2 was reduced to the same basal level as in Tconv cells (Figure 7G). To determine if NFAT activity plays a role in the looping of CNS2 to Foxp3 promoter, we performed 3C assay with WT Tregs that were untreated, activated with ionomycin, or activated with ionomycin in the presence of CsA. The looping activity between Foxp3 promoter and CNS2 was enhanced by ionomycin activation of NFAT, while the CsA blocked this looping activity (Figure 7H). Thus, NFAT promotes a specific interaction between Foxp3 promoter and CNS2 in Tregs.
To evaluate a possible role of the looping interaction, we first examined if mediator and cohesin factors that were previously implicated in mediating interactions between promoters and distal enhancers could bind to CNS2 and Foxp3 promoter (Kagey et al., 2010). Both Med12 (a component of mediator) and the cohesin loading factor Nipbl bound to both CNS2 and Foxp3 promoter in activated Tregs but not in conventional T cells in ChIP assays (Figures 7I and 7K). Importantly, knockdown of either of these two factors using shRNA vectors targeting previously validated target sequences (Kagey et al., 2010) led to increased loss of Foxp3 expression in wild-type Foxp3GFP Tregs, but not in CNS2− Tregs (Figures 7J and 7L), suggesting that these factors contribute to CNS2-dependent maintenance of Foxp3 expression in mature Tregs. Taken together, these results suggest that CNS2 interacts specifically with Foxp3 promoter in Tregs in an NFAT-dependent manner upon TCR activation to promote stable Foxp3 expression.
Discussion
Terminally differentiated cells such as Tregs can maintain lineage stability while exerting certain degrees of functional plasticity in response to external cues. It is unclear if functional plasticity could jeopardize cell lineage stability and if mechanisms are needed to resolve this potential conflict. Here we explored how Foxp3 CNS2, a CpG-rich cis-element accessible only in mature regulatory T cells, protects Treg identity. We found it somewhat surprising that in unprovoked mice, CNS2 was mainly required for highly activated “effector” Tregs to maintain Foxp3 expression, but not for the majority of Tregs. These activated “effector” Tregs that particularly depended on CNS2 for maintaining Foxp3 expression expressed increased levels of genes that have been previously associated with Treg functional plasticity, such as Tbx21 (encoding T-bet) and Prdm1 (encoding BLIMP-1) (Cretney et al., 2011; Koch et al., 2009). This suggests that Tregs that modulated their gene expression program and functions in response to environmental cues (TCR activation, cytokines etc.) were more vulnerable to losing Treg identity in the absence of CNS2 protection. It would be interesting to assess if any other environmental cues that have been shown to shape Treg functional plasticity also require CNS2 to protect Treg identity.
Our results also provided some tentative explanations of why environmental cues that confer Treg functional plasticity also destabilized CNS2 deficient Tregs. The conditions that promoted loss of Foxp3 expression in CNS2 deficient Tregs bear a striking similarity to those that have been shown by others to influence Treg development in thymus and Treg differentiation in the periphery. For example, the IL-2 signaling pathway has been shown to be important for Treg differentiation (Chen et al., 2003; Fontenot et al., 2005a). IL-4 and IL-6 have also been found by others to inhibit Treg induction (Korn et al., 2008; Mantel et al., 2007). This similarity likely results from methylated CNS2 in precursor cells prior to Treg differentiation functionally mimicking a CNS2 deletion. Inhibition of DNA methylation by 5-Aza has been shown to promote Foxp3 induction, even in the absence of TGF-β or in the presence of cytokines that could inhibit iTreg generation. Although CNS2 has been associated with the enhancement and stabilization of Foxp3 expression by 5-Aza, the causal relationship between CNS2 demethylation and Foxp3 induction has not been clearly established and it is entirely possible that 5-Aza promotes Foxp3 expression by removing DNA methylation on other cis-elements at the Foxp3 locus or regulating the expression levels of other factors that can influence Foxp3 expression. We found that CNS2 was essential for 5-Aza to mediate these effects. Therefore, whereas demethylated CNS2 in mature Tregs protect cell identity, methylated CNS2 may ensure that Treg differentiation can be properly regulated by cytokine environments.
We found that CNS2 functions as a sensor to TCR signal to upregulate and maintain Foxp3 expression in activated Tregs. Among numerous environmental cues that may shape mature Treg functional plasticity, TCR activation might be a central and shared signal in various conditions. TCR signaling also plays a central role in Treg lineage specification (Hsieh et al., 2006; Jordan et al 2001; Moran et al., 2011). Thus sensing TCR activation may provide a simple solution for Tregs to maintain Foxp3 expression. In addition to TCR signal, IL-2 is also a key player involved in keeping stable Foxp3 expression in Tregs, possibly through activation of STAT5 and its interaction with the Foxp3 promoter and CNS2 regions (Burchill et al., 2007; Yao et al., 2007). The interplay between TCR and IL-2 signals in regulating Foxp3 expression might be an adaptation to the complex environment where Tregs survive and function.
In addition to transcription factors identified in previous studies, we were able to establish a critical role for NFAT in CNS2-dependent regulation of Foxp3 expression. We found that NFAT binds to CNS2 both in vitro and in vivo, as well as Foxp3 promoter upon TCR stimulation. Using chromatin conformation capture assay, we showed that CNS2 interacts with Foxp3 promoter specifically in activated Tregs, but not in Tconv cells in which CNS2 is methylated or in CNS2− Tregs, suggesting that specific demethylation of CNS2 in mature Tregs may contribute to the high specificity of the interaction between Foxp3 promoter and CNS2 in these cells. The relative strength of the interaction between Foxp3 promoter and CNS2 among the examined interactions between Foxp3 promoter and other locations spanning the Foxp3 locus also suggests an important role for this particular interaction. Notably, we showed that NFAT signaling is required for the interaction between Foxp3 promoter and CNS2. Although NFAT has been proposed to participate in enhancer looping activity (Tsytsykova et al., 2007), to the best of our knowledge, this is the first demonstration of experimental evidence supporting a key role of NFAT signaling in the establishment of enhancer looping. Importantly, the availability of CNS2 deficient Tregs allowed us to specifically evaluate the function of an enhancer-promoter looping interaction in the regulation of gene expression. We found that both Med12 and Nipbl, two factors that have been shown to play important roles in enhancer-promoter looping interactions, bind to both CNS2 and Foxp3 promoter specifically in Tregs, but not in Tconv cells. More importantly, knockdown of either factor destabilized Foxp3 expression in wild-type Tregs, but not in CNS2 deficient Tregs, suggesting that these looping factors help stabilize Foxp3 expression in activated Tregs in a CNS2-dependent manner.
In conclusion, we show how a cis regulatory element could play a crucial role in maintaining cell identity in response to destabilizing environmental factors. Specifically, our studies indicate that CNS2, which is demethylated during Treg development, helps maintain stable expression of Foxp3, the key Treg lineage specification gene in destabilizing cytokine environments by serving as a sensor to TCR activation, a key signal among various external cues. Furthermore, we define a novel role for NFAT in maintaining gene expression by facilitating enhancer-promoter interaction. We suggest that these interactions provide a framework for understanding how genetic and epigenetic programs establish dynamic processes in cell lineage determination and maintenance.
Experimental Procedures
Mice
The generation of the CNS2− (Foxp3ΔCNS2) mice and Foxp3GFP mice has been described (Fontenot et al., 2005b; Zheng et al., 2010). C57BL/6 and Ly5.1+ B6 congenic mice were purchased from the Jackson Laboratory. All mice were maintained in the Salk Institute of Biological Studies SPF animal facility in accordance with institutional regulations.
Histopathology
Mouse tissues were fixed in 10% phosphate-buffered formalin before being processed for staining with haematoxylin and eosin according to standard procedures.
Serum immunoglobulin ELISA
Serum IgG2c, IgG1 and IgA concentrations were measured using SBA Clonotyping System (Southern Biotech). IgE ELISA was performed using biotinylated anti-IgE detection antibody (BD Pharmingen) and streptavidin-conjugated HRP.
RNA sequencing
RNA was extracted with the TRIzol Reagent (Life Technologies). Multiplexed libraries were constructed using the Illumina TruSeq RNA Sample Prep Kit. Libraries were sequenced on the Illumina HiSeq 2000 according to the manufacturer's instructions. The GEO accession number for RNA-seq data is GSE57272.
In vitro suppression assay
The division of responder T cells co-cultured with irradiated antigen-presenting cells and different amounts of Tregs was assessed by dilution of CellTrace Violet and the division index was calculated using FlowJo software (TreeStar).
Adoptive transfer of Tregs
Tregs were FACS-sorted from total CD4+ T cells isolated from spleens and lymph nodes of 6- to 8-week-old mice before being injected intravenously into recipient mice.
DNA pulldown assay
DNA pulldown assay was performed as previously described with minor modifications using biotinylated dsDNA probes listed in Table S4 (Zheng et al., 2010).
Induction of EAE and isolation of CNS mononuclear cells
EAE was induced and scored as previously described with modifications(Stromnes and Goverman, 2006).
Luciferase reporter assay
CD62L+ CD44− CD4+ T cells were activated for 3 days before transfection with luciferase reporter plasmids. Luciferase activities were measured at 24 h after transfection using Dual-Glo Luciferase Assay System (Promega).
In vitro Treg culture and stability assay
Tregs were activated for 72 h with plate-coated anti-CD3 (145-2C11) and anti-CD28 (37.51) or with Dynabeads Mouse T-activator CD3/CD28 (Life Technologies) before flow cytometry analysis of Foxp3 expression.
Retroviral and lentiviral infection
ACREB expressing retroviral vector was previously described (Zheng et al., 2010). IkBαM coding sequence was cloned into a Thy1.1-expressing retroviral vector.
Chromatin immunoprecipitation and qPCR
NFAT (Santa Cruz Biotechnology) ChIP was performed as previously described (Zheng et al., 2009). Med12 and Nipbl ChIP was performed as described previously (Kagey et al., 2010). qPCRs were performed using primers listed in Table S2.
Chromatin conformation capture (3C) assay and qPCR
3C assay was performed as previously described with modifications(Spilianakis and Flavell, 2004). qPCRs were performed using primers listed in Table S3.
Statistical analysis
Statistical analyses were performed with Prism 6.0 (GraphPad). P values were calculated using two-tailed unpaired Student's t-test or two-way analysis of variance (ANOVA). P values of less than 0.05 were considered significant. * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001 (Student's t-test).
Supplementary Material
Acknowledgments
We would like to thank Y. Zhang, C. Gordon and C. Zhou for mouse colony management, M. Ku and S. Heinz for assistance in RNA-seq experiments, L.-F. Lu, S. Z. Josefowicz and C. S. Hsieh for critical comments on the manuscript, and A. Y. Rudensky for providing critical reagents and helpful discussion. X. L. was supported by a postdoc fellowship from the Nomis Foundation. Y. Z. was supported by the Nomis Foundation, the Rita Allen Foundation, the Emerald Foundation, the Hearst Foundation, the National Multiple Sclerosis Society, and National Institute of Health (AI099295, AI107027). This work was also supported by National Cancer Institute funded Salk Institute Cancer Center core facilities (CA014195) and the James B. Pendleton Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno L, Mazzarella L, Hoogenkamp M, Hertweck A, Cobb BS, Sauer S, Hadjur S, Leleu M, Naoe Y, Telfer JC, et al. Runx proteins regulate Foxp3 expression. J Exp Med. 2009;206:2329–2337. doi: 10.1084/jem.20090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005a;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005b;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012a;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012b;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitoh A, Ono M, Naoe Y, Ohkura N, Yamaguchi T, Yaguchi H, Kitabayashi I, Tsukada T, Nomura T, Miyachi Y, et al. Indispensable role of the Runx1-Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009;31:609–620. doi: 10.1016/j.immuni.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nature medicine. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE, Bromberg JS. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Cattoglio C, Tjian R. Looping back to leap forward: transcription enters a new era. Cell. 2014;157:13–25. doi: 10.1016/j.cell.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Rudra D, Egawa T, Chong MM, Treuting P, Littman DR, Rudensky AY. Runx-CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2009;10:1170–1177. doi: 10.1038/ni.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nat Immunol. 2004;5:1017–1027. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nature protocols. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- Tsytsykova AV, Rajsbaum R, Falvo JV, Ligeiro F, Neely SR, Goldfeld AE. Activation-dependent intrachromosomal interactions formed by the TNF gene promoter and two distal enhancers. Proc Natl Acad Sci U S A. 2007;104:16850–16855. doi: 10.1073/pnas.0708210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–1742. S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.