Abstract
We studied whether self-reported intent to exert cognitive control over eating was associated with differences in brain response to food cues, independent of genetic background. Subjects were ten pairs of identical twins in which one twin was a restrained eater and the co-twin was unrestrained, as classified by the Herman and Polivy Restraint Scale. Before and after ingestion of a milkshake, we used functional magnetic resonance imaging to measure brain response to photographs of objects, “fattening” food, and “non-fattening” food. At baseline, restrained eaters had greater activation in the left amygdala and the right thalamus in response to fattening food cues than did their unrestrained co-twins. When restrained eaters drank a milkshake, activation in response to fattening food photographs decreased across multiple brain areas, whereas activation induced by non-fattening food photographs increased. As compared to their unrestrained co-twins, restrained eaters who drank a milkshake had greater decreases in activation by fattening food images in the left amygdala and occipital lobe, and greater increases in activation by non-fattening food images in the medial orbitofrontal cortex. Because of the discordant monozygotic twin study design, the findings provide a rigorous level of support for the hypothesis that adopting an intention to restrain eating alters brain response to food cues.
Keywords: Amygdala, Functional MRI, Orbitofrontal cortex, Restrained eating, Twin studies
Body weight and adiposity are strongly genetically determined traits, with estimates of 50–90% for the heritability of body mass index (BMI) [1]. Resting energy expenditure is also influenced by heredity [2, 3]. However, evidence suggests that environmental factors rather than heredity may be important determinants of food intake [4, 5], and humans are apparently the only mammals to attempt volitional control of eating. Such attempts might represent a non-genetic or acquired influence on food intake with the potential to modify an inherited risk of weight gain. However, there is no evidence that adopting a cognitively driven pattern of eating results in acquired differences in the centrally mediated control of food intake.
A co-twin control study is a powerful method of detecting subtle environmental effects on regulation of food intake by the central nervous system (CNS). By enrolling monozygotic twin pairs that differ in the phenotype of interest, a co-twin control study provides an ideal pair that is matched for sex, age, genetics, shared family environment, countless unmeasured experiences and traits, and even brain morphology [6]. Therefore, short of a randomized-controlled trial, a discordant monozygotic twin study is the most rigorous means of approaching causal inference through an observational study [7]. We chose this design to assess acquired differences in CNS response to food cues and food intake among restrained eaters.
As identified by the Herman and Polivy Revised Restraint Scale [8], restrained eaters are characterized by attempted cognitive control over food intake, high concern with weight and dieting, and frequent weight fluctuations. Evidence is mixed as to whether restrained eaters are in a hypocaloric state [9–11], and their success in achieving weight loss varies [12, 13]. Their weight fluctuates and both BMI and maximal lifetime weights are higher than those observed in unrestrained eaters [13]. Paradoxically, some restrained eaters also display a propensity toward overeating [9]. In experimental conditions, this has been demonstrated after ingesting a highly caloric food such as a milkshake [14] or when triggered by stress [15] or anxiety [16].
We recruited identical (monozygotic) female twin pairs who were discordant in restrained eating (i.e., one was classified as a restrained eater by the Restraint Scale and the other was not) and obtained functional magnetic resonance imaging (fMRI) scans of the brain before and after drinking a milkshake. It has been proposed that certain foods can challenge cognitive control and trigger overeating in restrained eaters [17]. Based on this theory, we hypothesized that restrained twins would show reduced prefrontal cortical activation after milkshake consumption, since the prefrontal cortex has previously been implicated in cognitive control of food intake [18, 19]. Alternatively, restrained eaters’ efforts at weight control might stimulate compensatory hormonal responses, resulting in an appetite-stimulated state [20]. Therefore, we also examined critical brain regions that regulate food intake and respond to appetite-regulating hormones [21, 22].
1. Methods
1.1. Participants
All twins were members of the University of Washington Twin Registry, a community-based registry of twin pairs derived from applications for drivers’ licenses in Washington State. The construction and characteristics of the Registry and its sample population are described elsewhere [23]. In August 2006, a written health survey that included the Restraint Scale was mailed to all twins enrolled in the Registry (n=4407), achieving a response rate of 55%. Female mono-zygotic twin pairs aged 18–65 years who were reared together until age 15 and were discordant for restrained eating were included. Exclusion criteria were current smoking; drinking>2 alcoholic beverages per day or using recreational drugs; a lifetime history of weight loss surgery or eating disorders; pregnancy or a major medical problem such as diabetes; current participation in a formal weight loss program; and contraindication to MRI. Study procedures were approved by the University of Washington Human Subjects Committee, and all participants provided written informed consent.
1.2. Measures
1.2.1. Eating behavior
The Revised Restraint Scale is a 10-item self-report questionnaire designed to identify individuals with chronic dieting and weight concerns [8]. Its internal reliability, test-retest reliability, and predictive validity are well established [24]. However, the scale may overestimate restraint in overweight and obese populations [25]. We defined restrained eaters as those with a score of 15 or more [26] and that was 3 or more points higher than their co-twins’ score. Unrestrained eaters scored less than 15. On a day separate from the fMRI studies, twin pairs underwent a study in which they drank a milkshake and then completed an ice cream taste test. Intake was surreptitiously monitored, and twins were separated during all food intake. This study replicated an earlier experiment in which a milkshake triggered overeating among restrained eaters [14]. To further describe twins’ eating behaviors, we administered the Three Factor Eating Questionnaire, Revised 18-item version (TFEQ-R18) to assess uncontrolled and emotional eating [27, 28].
1.2.2. Zygosity assignment
Responses to self-report questions on childhood similarity were used in a multi-step process to assign zygosity. Such questions correctly classify zygosity with an accuracy of approximately 95% compared with zygosity determined by biological indicators [29–32].
1.2.3. Body weight
Weight and height were measured and BMI was calculated (weight/height2). Lifetime maximum weight, excluding pregnancy, was self-reported.
1.2.4. Food appeal ratings
Twins viewed a sample of 41 food photographs that were included as stimuli during the fMRI scans. They were instructed to mark the number that “best describes how appealing the food shown in the photograph appears to you right now” using a 10-point Likert scale, ranging from “not at all” to “extremely” appealing.
1.3. Procedures
All twin pairs participated together, and investigators remained blinded to restraint status throughout the study. Twins were told that the project studied “taste perception” to distract them from focusing on eating patterns and restraint. After an overnight fast starting at 9:30 pm, twins ate an ad libitum breakfast at 8:00 am. In one pair, both members chose to skip breakfast. fMRI sessions took place between 10:00 am and 12:30 pm. The order of fMRI sessions was counterbalanced between restrained and unrestrained twins and assigned by a statistician. Twins viewed a brief sample slideshow to become familiarized with the speed at which photographs would appear. After the initial scan, twins exited the scanner and had 10 min to drink a 10.9 ounce milkshake consisting of 313 kcals (19% protein, 43% carbohydrate, 38% fat). Twins then returned to the scanner and were asked to think about the flavor of the milkshake. The fMRI protocol was then repeated. Food appeal ratings were completed after all fMRI sessions concluded.
1.4. Imaging paradigm
To select the study images, 214 food photographs were initially evaluated by 13 independent raters. For each photograph, raters responded to the following instruction: “If someone was dieting in order to lose weight, rate how acceptable each food would be for them to eat.” Photographs were rated on a Likert scale from 1 to 7. Categories 1–3 were defined as “definitely”, “probably”, and “maybe” should not eat when dieting. Category 4 was defined as “unsure/can’t tell from picture.” Categories 5–7 were defined as “maybe,” “probably,” and “definitely” acceptable to eat when dieting. Photographs with mean scores of ≤2 or ≥6 were selected for inclusion as stimuli in the protocol.
Foods judged to be unacceptable when dieting were universally characterized by high caloric content and were usually high in fat or sugar content. They included candy, desserts, pastries, and high-fat savory foods such as pizza, hamburgers, and French fries. We named this category the “fattening” food group, a term chosen to indicate that such foods are typically regarded as incompatible with achieving weight loss. We chose the term “non-fattening” to describe photographs of foods that were deemed compatible with weight loss. These depicted low-calorie foods, including fruits, vegetables, salads, low-fat meats (e.g., chicken breast), and seafood. We subsequently validated these categories in a sample of 16 adults who did not undergo brain imaging. They appropriately categorized 100% of non-fattening and 98% of fattening food photographs. The current appeal (“how good does the food look to you?”) of images in both groups was rated almost identically on a 10-point Likert scale (fattening 6.4±1.1; non-fattening 6.2±1.1; p=NS). Non-food images designated as controls consisted of common, recognizable large and small objects such as furniture, sundries, toiletries, and electronics.
Each session consisted of a distinct set of 13 blocks of 10 photographs each. All food and object images were commercial-quality stock photographs obtained from Web sites or donated for research use. All photographs were matched for size (600×400), quality, and visual interest, and were group-matched for luminosity (F(2, 257)= 0.00, P=0.99). Quality and visual interest were assessed by 3 authors (ES, NK, KM), and the independent raters provided feedback on food pictures. Each photograph was projected for 2.4 seconds on a screen easily viewed in a mirror by the participant while in the MRI scanner. Non-food blocks alternated with fattening and non-fattening food blocks.
To ensure that participants focused on each image, they were told before the scan that they would be tested regarding which photos they had seen. After the scans, they were given a memory test consisting of images viewed in the scanner mixed with distracter images not previously seen. They were asked to state whether they had seen each image while in the scanner, and the percentage of correct responses was calculated. Thirty-two pictures were included (16 non-food, 8 fattening food, 8 non-fattening food) of which 50% in each category were distracter images.
1.5. Image acquisition
Structural and fMRI exams were performed on a 3 T Philips Achieva MR System (version 1.5, Philips Medical Systems, Best, The Netherlands) with dual Quasar gradients (80 mT/m at a slew rate of 110 mT/m/s or 40 mT/m at a slew rate of 220 mT/m/s) using an 8-channel SENSE head coil. fMRI scans used single-shot echo-planar imaging with a TR/TE=2000/30. The matrix was 64×64 with a 240 mm field of view, yielding an in-plane resolution of 3.75×3.75 mm with slice thickness of 4 mm. The entire brain was imaged with 32 slices for each volume. The total scan duration was 5 min and 24 seconds; 156 dynamic volumes and 5 dummy scans were acquired. Before post-processing, image datasets were normalized to a B0 field map acquired at each imaging session. A sagittal T1-weighted 3D MPRAGE image with isotropic voxels of 1×1×1 mm was acquired after each fMRI session for anatomical co-registration. The SameScan software program, part of the Phillips scanning software platform (Version R2.5.3.0), was used to reposition the twins reproducibly.
1.6. fMRI processing and statistical analysis
Twin pairs were classified by Restraint Scale score, and means and standard deviations were calculated for twin characteristics. Differences according to restrained eating status were tested with paired t-tests. fMRI data analyses were performed by using the Oxford Centre for Functional MRI of the Brain (FMRIB) Software Library version 3.3 (FSL; http://www.fmrib.ox.ac.uk/fsl/). The following preprocessing steps were applied: 1) motion correction was conducted with Motion Correction using FMRIB’s Linear Image Registration Tool; 2) nonbrain structures were removed by using the Brain Extraction Tool; and 3) data were spatially smoothed by using a Gaussian kernel of full width at half maximum=5 mm and temporally smoothed with a high-pass filter of sigma=72 s. Time series statistical analyses were carried out by using FMRIB’s Improved Linear Model with local autocorrelation correction. Condition effects were estimated at each voxel for the following contrasts: 1) fattening food>object; 2) non-fattening food>object; 3) fattening food>non-fattening food; and 4) non-fattening food>fattening food. Individual fMRI data were registered to the high-resolution scan by using fieldmap corrections, warped with an affine transformation to the MNI152 standard image by using FMRIB’s Linear Image Registration Tool, and resampled to 2×2×2 mm voxels.
Using a region-of-interest approach, analyses of group-wise effects were conducted by using FMRIB’s Local Analysis of Mixed Effects, a method for modeling and estimating the random-effects component of the measured inter-session mixed-effects variance. Anatomical masks were either hand-drawn on the MNI152 standard brain or defined by Anatomical Automatic Labeling, an in-house package made by Neurofunctional Imaging Group (GIN, UMR6095, CYCERON, Caen, France). The following 14 brain regions were tested separately: brainstem, hypothalamus, left/right amygdala, left/right inferior frontal cortex, left/right insula, left/right striatum, left/right thalamus, medial orbitofrontal cortex (OFC), and occipital lobe. Brain regions of interest for these analyses were chosen a priori based on anatomical regions known to be involved in energy homeostasis, appetite regulation, and cognitive control of behavior and prior data demonstrating that these regions are selectively responsive to visual food cues [33]. Statistical corrections for multiple comparisons were conducted by using cluster-thresholding based on Markov Chain Monte Carlo sampling for each region of interest. Finally, for post hoc analyses and graphing, raw mean z-scores were extracted for each individual from several significant clusters identified in the analyses above.
2. Results
2.1. Twin participants
Participants were 10 pairs of female MZ twins with a mean age of 30.6±16 years (range 20–65). Mean BMI was 23.9±3.4 kg/m2 (range 19–32). All twins for whom data was available were right-handed (17 of 20). Twin characteristics, identified by restraint status, are shown in Table 1. Restrained-eating twins were taller than their co-twins and tended to have lower BMIs, with some variation. The mean within-pair difference in height (heightrestrained–heightunrestrained) was 1.4±1.9 cm. Within-pair differences in BMI (BMIrestrained–BMIunrestrained) ranged from −2.5 to 1.7 kg/m2 with a mean of 1.3±0.6 kg/m2. In the preload study conducted separately from the fMRI scans, we served milkshakes identical to the ones in the fMRI study and monitored the amount of ice cream consumed after the milkshake, replicating prior studies among restrained eaters [17]. On average, the amount of ice cream (in calories) consumed by restrained eaters was similar to the amount consumed by their unrestrained co-twins, again with some variation. Within-pair differences (Kcalsrestrained–Kcalsunrestrained) ranged from −206 to 168.
Table 1.
Characteristics of 10 female monozygotic twin pairs discordant for restrained eating based on Restraint Scale scores.
| Restrained Mean (SD) | Unrestrained Mean (SD) | P value* | |
|---|---|---|---|
| Age, years | 31 (16) | 31 (16) | – |
| Restraint Scale score | 18 (2) | 10 (2) | <0.0001 |
| Body mass index, kg/m2 | 23.6 (4) | 24.3 (3) | 0.1 |
| Weight, kg | 65.0 (10.8) | 66.4 (8.3) | 0.3 |
| Height, cm | 166.5 (6.3) | 165.1 (6.7) | <0.05 |
| Lifetime maximum weight, kg | 68.3 (10.2) | 68.6 (6.7) | 0.7 |
| TFEQ-R18 subscale scores† | |||
| Emotional eating | 35 (34) | 21 (20) | 0.07 |
| Uncontrolled eating | 33 (16) | 20 (8) | <0.05 |
| Percent correct on behavioral post-test pre-milkshake | 90 (6) | 90 (4) | 0.9 |
| Percent correct on behavioral post-test post-milkshake | 90 (7) | 90 (5) | 0.8 |
| Post-milkshake appeal rating for fattening food | 4.6 (1.2) | 4.5 (1.7) | 0.9 |
| Post-milkshake appeal rating for non-fattening food | 6.6 (0.6) | 6.0 (1.8) | 0.3 |
| Amount of ice cream eaten after a milkshake, kcals | 188 (39) | 225 (45) | 0.4 |
| Time elapsed between breakfast and fMRI, min† | 203 (74) | 194 (32) | 0.7 |
P values based on paired t-tests.
Data available for 9 pairs. TFEQ-R18=Three Factor Eating Questionnaire-Revised 18-item.
2.2. Behavioral post-test and appeal ratings
On the behavioral post-test, all twins correctly identified previously viewed photographs with an accuracy of 90±5% (see Table 1). After drinking a milkshake and completing all fMRI procedures, both restrained and unrestrained twins gave similar ratings for the appeal of fattening and non-fattening food photographs (Table 1); both groups found the fattening foods less appealing (fattening food mean±SD=4.5±1.4 vs. non-fattening=6.3±1.3; P<0.001). Among restrained eaters, appeal ratings were positively correlated with activation (fattening food>objects) in the hypothalamus, left and right amygdala, right striatum, and occipital lobe (Supplemental Table 1). Among unrestrained eaters, positive correlations were also present in the hypothalamus and occipital lobe.
2.3. Baseline differences in response to food cues among restrained and unrestrained twins
At baseline, before consuming a milkshake, restrained-eating twins showed significantly greater activation in the left amygdala and the right thalamus than their unrestrained co-twins in response to fattening food images as compared to object images (Table 2). In addition, restrained eaters also had greater activation in the occipital lobe than their unrestrained co-twins for the comparison of fattening vs. non-fattening food images. Unrestrained eaters showed stronger responses to non-fattening food images in the medial OFC than did their restrained co-twins for the comparison of non-fattening food to object images.
Table 2.
Clusters of activation in regions of interest that differed significantly between restrained eaters and their unrestrained co-twins at baseline, before a milkshake.
| Number of voxels | P-value | Max. z-score | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|
| Restrained>Unrestrained | ||||||
| Fattening>Object | ||||||
| L amygdala | 29 | 0.031 | 3.12 | −20 | −6 | −12 |
| R thalamus | 117 | 0.048 | 3.16 | 2 | −12 | 0 |
| Non-fattening>Object | ||||||
| None | ||||||
| Fattening>Non-fattening | ||||||
| Occipital lobe (BA 18, cuneus, lingual gyrus) | 866 | 0.0004 | 3.76 | −8 | −70 | 4 |
| Unrestrained>Restrained | ||||||
| Fattening>Object | ||||||
| None | ||||||
| Non-fattening>Object | ||||||
| Medial OFC (BA 11, 32) | 172 | 0.032 | 3.68 | −2 | 38 | −10 |
OFC=orbitofrontal cortex; BA=Brodmann area; L=left; R=right. Coordinates are in MNI space.
2.4. Change in brain response to food cues elicited by drinking a milkshake
Among restrained-eating twins, activation by fattening food images, as compared to both non-fattening food and object images, was significantly greater before drinking the milkshake than afterward within multiple brain regions of interest (Table 3). Clusters in the brainstem were located in the pons, abutting the fourth ventricle in the region of the nucleus of the solitary tract, and in the bilateral midbrain encompassing the ventral tegmental area. Two diminutive clusters in the left hypothalamus were located at the lateral margin of the region of interest. The pattern differed for activation in response to non-fattening food images in that activation was greater after the milkshake. Significant clusters were located in the medial OFC and occipital cortex. Among unrestrained eaters, activation in response to fattening food images was greater before consuming the milkshake in the right inferior frontal cortex (Table 4).
Table 3.
Clusters within regions of interest that demonstrated significant changes in activation due to drinking a milkshake among restrained eaters.
| Number of voxels | P-value | Max. z-score | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|
| Restrained—Activation greater before milkshake | ||||||
| Fattening>Object | ||||||
| Hypothalamus | 2 | 0.038 | 2.46 | −8 | −10 | −8 |
| L amygdala | 3 | 0.032 | 2.72 | −24 | 0 | −12 |
| R insula | 134 | 0.040 | 3.4 | 36 | 22 | −2 |
| L thalamus | 169 | 0.034 | 3.48 | −20 | −18 | 2 |
| Occipital lobe (BA 18, 19, cuneus, lingual gyrus) | 777 | 0.004 | 4.43 | 20 | −80 | 22 |
| Occipital lobe (BA 19) | 567 | 0.014 | 4.92 | −42 | −74 | 18 |
| Non-fattening>Object | ||||||
| None | ||||||
| Fattening>Non-fattening | ||||||
| Brainstem | 212 | 0.035 | 3.71 | 0 | −42 | −50 |
| Brainstem | 261 | 0.023 | 3.79 | −10 | −16 | −14 |
| Hypothalamus | 3 | 0.036 | 2.61 | 8 | −6 | −12 |
| Hypothalamus | 2 | 0.038 | 2.85 | −8 | −10 | −8 |
| L amygdala | 6 | 0.042 | 2.61 | −24 | −8 | −26 |
| R inferior frontal | 405 | 0.009 | 4.62 | 36 | 24 | −16 |
| R inferior frontal | 246 | 0.032 | 4.01 | 52 | 14 | −2 |
| Medial OFC (BA 10, 32) | 189 | 0.044 | 3.97 | −14 | 18 | −22 |
| R insula | 124 | 0.044 | 4.26 | 34 | 22 | −16 |
| L thalamus | 289 | 0.012 | 3.1 | −16 | −6 | 0 |
| R thalamus | 294 | 0.011 | 3.94 | 10 | −8 | 4 |
| Occipital lobe (BA 17, 18, 19, cuneus, lingual gyrus) | 5603 | 3.67E-011 | 4.52 | 10 | −78 | −8 |
| Restrained—Activation greater after milkshake | ||||||
| Fattening>Object | ||||||
| None | ||||||
| Non-fattening>Object | ||||||
| Medial OFC (BA 10, 32) | 201 | 0.034 | 3.55 | −10 | 36 | −16 |
| Occipital lobe (BA 17, 18, R BA 19, middle occipital gyrus, lingual gyrus) | 2744 | 1.2E-007 | 4.67 | 10 | −90 | −4 |
| Fattening>Non-fattening | ||||||
| None | ||||||
OFC=orbitofrontal cortex; BA=Brodmann area; L=left; R=right. Coordinates are in MNI space.
Table 4.
Clusters within regions of interest that demonstrated significant changes in activation due to drinking a milkshake among unrestrained co-twins.
| Number of voxels | P-value | Max. z-score | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|
| Unrestrained—Activation greater before milkshake | ||||||
| Fattening>Object | ||||||
| None | ||||||
| Non-fattening>Object | ||||||
| None | ||||||
| Fattening>Non-fattening | ||||||
| R inferior frontal | 200 | 0.048 | 3.76 | 48 | 44 | −10 |
| Unrestrained—Activation greater after milkshake | ||||||
| Fattening>Object | ||||||
| L Occipital lobe (BA 17, 18, cuneus, lingual gyrus) | 638 | 0.009 | 4.82 | −10 | −88 | 2 |
| Non-fattening>Object | ||||||
| Occipital lobe (BA 17, 18, lingual gyrus) | 718 | 0.003 | 4.17 | −4 | −88 | −6 |
| Fattening>Non-fattening | ||||||
| None | ||||||
BA=Brodmann area; L=left; R=right. Coordinates are in MNI space.
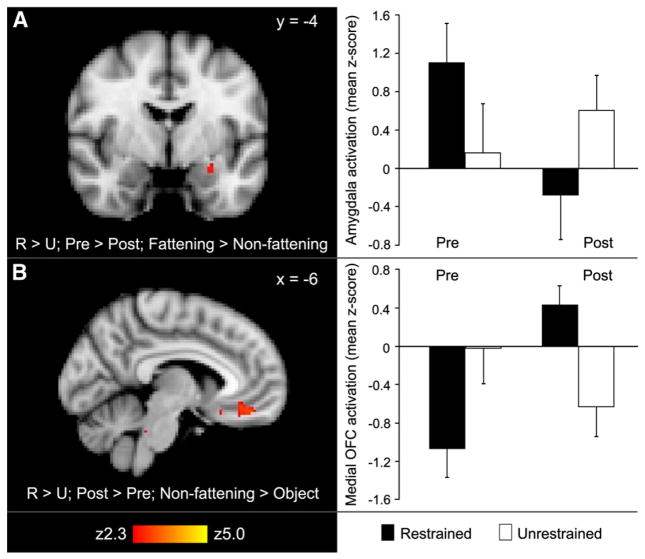
We then directly compared restrained eaters and their unrestrained co-twins on the change in brain response while viewing food images from before to after the milkshake (Table 5). In the left amygdala and the occipital cortex, restrained eaters showed greater decreases in activation after drinking the milkshake than did their unrestrained co-twins for the contrast of fattening vs. non-fattening food cues. Fig. 1 (Panel A) shows the cluster located in the region of the central nucleus of the left amygdala. To demonstrate the direction of change, the bar graph shows mean z-scores extracted from this cluster and separated into the pre- and post-milkshake MR scans for each group. By contrast, in the medial OFC and occipital cortex, increases in response to non-fattening food cues after drinking the milkshake were greater among restrained eaters than among their unrestrained co-twins (Fig. 1, Panel B). In post-hoc correlation analyses using data from the left amygdala cluster, a trend was present toward an association between higher emotional eating scores and higher baseline activation (β=6.0; 95% confidence interval −0.9, 13; P=0.089; n=18; 1 pair missing).
Table 5.
Clusters in regions of interest that differed significantly between restrained eaters and their unrestrained co-twins for the change in activation from pre- to post-milkshake.
| Number of voxels | P-value | Max. z-score | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|
| Restrained eaters showed greater decrease after milkshake | ||||||
| Fattening>Object | ||||||
| L amygdala | 9 | 0.039 | 2.57 | −26 | −8 | −16 |
| Non-fattening>Object | ||||||
| None | ||||||
| Fattening>Non-fattening | ||||||
| L amygdala | 10 | 0.038 | 2.7 | −26 | −2 | −16 |
| Occipital lobe (BA 17, 18, 19, cuneus, superior occipital gyrus, lingual gyrus, middle occipital gyrus) | 1184 | 0.0004 | 3.46 | −28 | −84 | 32 |
| Occipital lobe (BA 17, 18, 19, cuneus) | 630 | 0.009 | 3.63 | 26 | −66 | 6 |
| Restrained eaters showed greater increase after milkshake | ||||||
| Fattening>Object | ||||||
| None | ||||||
| Non-fattening>Object | ||||||
| Medial OFC (BA 10,32) | 295 | 0.014 | 3.75 | −12 | 38 | −16 |
| Occipital lobe (BA 17, 18, 19, cuneus, lingual gyrus) | 498 | 0.014 | 3.43 | −14 | −78 | 14 |
| Fattening>Non-fattening | ||||||
| None | ||||||
OFC=orbitofrontal cortex; BA=Brodmann area; L=left. Coordinates are in MNI space.
Fig. 1.
The change in brain response to visual food cues with food intake differs between monozygotic twins discordant for restrained eating. Panel A: Cluster within the amygdalar region of interest in which the decrease in activation by fattening food cues from before to after a milkshake was significantly greater among restrained eaters than their unrestrained co-twins (P<0.05, corrected). Individual mean z-scores for each twin were also extracted from the amygdala cluster and used to calculate the group means (±SE) shown in the accompanying bar graph. Activation (mean z-score) was based on the contrast of fattening>non-fattening food images. Panel B: Cluster within the medial orbitofrontal cortex (OFC) region of interest in which the increase in activation by non-fattening food cues from before to after a milkshake was significantly greater among restrained eaters than their unrestrained co-twins (P<0.05, corrected). To illustrate the direction of changes, individual mean z-scores for each twin were extracted from the medial OFC cluster and used to calculate the group means (±SE) for both the pre- and post-milkshake fMRI scans (see bar graph). Activation (mean z-score) was based on the contrast of non-fattening food>object images. Restrained eating was measured by the Herman and Polivy Restraint Scale. R=restrained; U=unrestrained.
3. Discussion
Using fMRI, we found evidence in monozygotic twins for differences in brain response to food cues before and after food intake that were associated with self-reported restrained eating. Specifically, female monozygotic twins who differed in restrained eating exhibited distinct patterns of responses to visual food cues. At baseline, images of high-calorie, “fattening” foods elicited greater activation in the left amygdala, right thalamus, and occipital lobe of the restrained twins as compared to their unrestrained co-twins. We did not find evidence of greater prefrontal cortex activation by food cues in the restrained twins. Twins were then challenged by consuming a milkshake, which in the restrained twins triggered significant decreases in response to fattening food photographs across multiple brain areas. Among the restrained twins, consumption of a milkshake also resulted in increases in activation in response to non-fattening food images. This pattern was not observed in their unrestrained co-twins. The change in brain response to food cues from before to after a milkshake differed significantly in the amygdala, OFC, and occipital cortex, which are implicated in emotional processing of cues, behavior modification, and visual perception and attention, respectively. Because our monozygotic twin pairs were raised together and thus matched for age, sex, genetics, family environment, and numerous other unmeasured factors, our findings provide the strongest possible evidence from an observational study [7] for acquired neurobiological changes associated with adopting an intention to restrain eating.
Restrained eating is aimed at cognitive control of food intake and body weight. However, as identified by the Restraint Scale, restrained eaters have been shown to overeat in experimental situations in response to a milkshake preload [17]. This behavior, termed “counterregulatory eating” [34], might be triggered by breaking self-imposed “diet boundaries.” In this view, once a dieting goal is thwarted, restrained eaters eat to satiety. Other triggers, including anxiety, stress, and perceived highly caloric foods, have also been identified. By our psychometric assessments, the restrained twins in our study reported tendencies toward dietary restraint, emotional eating, and uncontrolled eating, but they did not overeat when we observed them surreptitiously. Despite taking pains to separate twins during food intake, we cannot be certain that the presence of their co-twins did not influence the observed behavior of the restrained eaters during the experiment. Thus, in this sample, restrained eating, as determined by self-reported eating and weight concerns, differs between genetically identical twins, while food intake after a preload and body weight were similar.
Among restrained eaters, intake of a milkshake resulted in significant decreases of activation in response to fattening food cues in regions associated with control of food intake (hypothalamus, nucleus of the solitary tract), motivation for and appeal of food (ventral tegmental area), cognitive control of behavior (inferior frontal cortex), visual perception and association (occipital cortex), gustatory [35] and interoceptive cue processing (insula) [36], and emotional and motivational salience of stimuli (amygdala) [37]. In contrast, activation in response to non-fattening food cues increased in the OFC and in occipital cortex visual perception and association areas after drinking the milkshake. These findings suggest that intake of a palatable food decreased attention to and salience of fattening food cues and increased attention to non-fattening foods, perhaps as a result of “top-down” attentional focusing [38]. This reversal may be relevant to counterregulatory eating, as restrained eaters report more hunger and eat more cookies after viewing images of non-fattening foods than after viewing images of non-food objects (unpublished data by V. Provencher and colleagues), and have been shown to respond to food temptations by increasing their focus on dieting [39].
Our findings suggest heightened salience of fattening food cues among restrained eaters that is modified by drinking a milkshake. Dietary restraint has previously been correlated with an orienting bias in visual gaze toward high-calorie food images as compared to neutral images [40]. When compared to their unrestrained co-twins, restrained twins showed greater activation by fattening food cues at baseline in the left amygdala, thalamus, and occipital cortex. Restrained eaters also showed significantly greater decreases in activation by fattening food cues from before to after a milkshake in the amygdala and occipital cortex. We provide preliminary evidence to suggest that heightened amygdalar activation while viewing fattening food cues may be a correlate of emotional eating, in accordance with prior research [41]. This finding also fits with known amygdalar functions, such as encoding the motivational and emotional value of stimuli in initial learning processes and facilitating attention to emotional stimuli [37]. Thus, our findings document non-genetic differences in amygdalar responses to food cues associated with intention to restrain eating.
After drinking the milkshake, restrained twins also showed significantly greater decreases in occipital cortex activation by fattening food cues. The occipital regions implicated are involved in visual perception, object recognition [42], selective visual attention [38], and assigning value and saliency to appetitive visual food cues [43]. Activation in the occipital cortex was positively correlated with the appeal of fattening food photographs among both restrained and unrestrained eaters, implicating extrastriate regions of the occipital cortex in selective attention for and discrimination of appealing, high-energy food in the environment. Decreased activation in response to fattening food cues after a milkshake suggests that these cues are subject to devaluation by intake of palatable food.
In contrast, restrained-eating twins showed increased activation in the OFC and occipital cortex in response to non-fattening food cues after drinking a milkshake. This increase may represent cognitively driven re-direction of attention toward non-fattening stimuli [38]. Other studies have examined brain function in conjunction with exercise of volitional control over appetite. Voluntary inhibition of hunger in men has been shown to suppress activity in the amygdala and OFC [44]. Responses in the OFC have been associated with external eating patterns, whereas responses in the amygdala correlate with emotional eating [41]. Our results reinforce and expand prior findings on the neuroanatomical correlates of eating behavior patterns by showing that both the content of food cues (fattening vs. non-fattening) and the current state of satiety can influence regional brain responses associated with eating behaviors. Most importantly, our findings of non-inherited differences in the response of the amygdala, occipital cortex, and OFC also support the idea of plasticity in brain response to food cues.
Our findings in the left amygdala are very similar to those of a study of chronic dieters as identified by the Restraint Scale. There was an interaction between restraint status and the content of a preload such that dieters who drank water (i.e., diet intentions were unchallenged as in our pre-milkshake scans) showed greater activation in the left amygdala than dieters who drank a milkshake [45]. In contrast, dieters showed greater activity in the nucleus accumbens after drinking a milkshake than did non-dieters; the researchers suggested that this result might represent the neural underpinnings of dietary failure. We did not detect differences in nucleus accumbens activation after consumption of a milkshake. However, the restrained eating twins in our study did not increase intake after a milkshake, an indication that they may have been less susceptible to dietary failure. Another prior study in unrelated, normal-weight women, including both restrained and unrestrained eaters as assessed by the Restraint Scale, tested fMRI responses to food cues before and after a 500 kcal liquid meal [46]. When fasted, unrestrained eaters exhibited greater activation than restrained eaters in response to images of highly palatable food in the superior temporal gyrus, parahippocampal gyrus, putamen, and dorsolateral prefrontal cortex. In contrast, after the meal, restrained eaters exhibited greater activation than unrestrained eaters in the pyramis, OFC, and dorsolateral prefrontal cortex in response to highly palatable food cues. The authors suggested that restrained eaters may paradoxically experience stronger appetitive drive when fed than when fasted. Because fasting evokes distinct responses to visual food cues [47–49], the fact that our observations of twins were performed approximately 3 h after a meal may explain the difference in results between the 2 studies. In addition, the images in the current study were selected based on whether they were perceived as incompatible with weight loss, a potentially important difference. Of note, any neurobiological differences that reflect genetic predispositions toward attempting to restrain eating [50] would not be expected to differ among our twins, but would be found in studies of unrelated individuals.
Our results are similar to a study that failed to identify associations between scores on the Dutch restrained eating scale [51] and activation by appetizing food photographs in the striatum, rolandic operculum, OFC, or prefrontal cortex. However, an association was found between restrained eating and activation in the OFC and dorsolateral prefrontal cortex during the taste of a chocolate milkshake as compared to a tasteless solution [52]. The findings of Burger and Stice (2011) support our interpretation that ingestion of a milkshake elicited cognitive responses in restrained eaters, perhaps underlying the observed shifts in activation by fattening to non-fattening food images.
This study has several limitations. First, the Restraint Scale identifies individuals with a constellation of disinhibitory eating, dieting and restriction of food intake, body dissatisfaction, and drive for thinness [25]. Thus, we cannot be certain that our key findings apply to restrictive behavior rather than to behavior more characteristic of disinhibition and concern with body weight. However, the restrained eaters in this study maintained their restraint when stimulated by the preload milkshake, as they did not differ from their co-twin in subsequent intake of ice cream. If the milkshake was not perceived as sufficiently fattening to warrant abandoning a weight-loss diet, its consumption might actually strengthen dieting intentions. Moreover, typical eating behaviors of restrained eaters identified by the Restraint Scale may have differed in the overweight and obese twins in the study [25], but any resultant variability would have been minimized in group comparisons because pairs were well matched on BMI. Second, in an intricate region such as the amygdala, fMRI is limited in its ability to resolve the anatomic location of activation at the level of particular nuclei; furthermore, opposing functions, such as aversive vs. positive conditioning, may overlap anatomically and/or functionally. These factors complicate interpretation of our findings. Third, susceptibility and motion artifacts may have limited our ability to accurately measure activation within the OFC, hypothalamus, and brainstem. Fourth, twins were not matched for menstrual phase, which is known to alter physiologic regulation of appetite [53]. Fifth, it is possible that study procedures, such as the memory task, elicited differential cognitive effects that may have influenced the results. Finally, zygosity was derived from questionnaire data that could have misclassified physically similar fraternal twins.
Nonetheless, our findings provide strong evidence that intent to exert cognitive control over food intake influences brain response to visual food cues. These findings are neither genetic nor familial, and are plausibly related to environmental effects specific to the individual. Unique environmental factors account for approximately 42–57% of the variance in Restraint Scale scores [50, 54]. Environmental factors specific to individual twins begin early in life, even in utero (e.g., disproportionate placental blood flow). Thus, while we cannot rule out the possibility that an early life environmental exposure induced the observed brain responses and attitudes toward eating restraint, our finding of increased height among restrained twins suggests one way in which individual experience might differ in genetically identical individuals. While the restrained eaters in this study were similar to their co-twins in body weight, they were consistently taller. One interpretation is that the physically larger twin might have been more susceptible to the body image and eating concerns that characterize high scorers on the Restraint Scale.
These fMRI findings are consistent with theories proposing that environmental cues incompatible with weight loss assume enhanced salience for individuals who are highly concerned with weight and dieting, in whom they elicit emotional arousal and increased attention. This association illustrates an apparent conflict between food appeal and attempted restraint that sheds light on overeating behavior. These patterns of activation are altered by food intake in a manner that may be cognitively driven. Future studies should focus on delineating the specific roles that the amygdala, OFC, and occipital cortex may play in volitional control of food consumption. In sum, although body weight is strongly genetically determined, attempting to exert cognitive control over eating may alter responses to food cues at the level of the CNS. Additional research is required to document the long-term impact of these CNS changes on body weight.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (DK070826 to E.S., NS059675 to N.K.) and the University of Washington Institute for Translational Health Sciences (UL1 RR025014, KL2 RR025015, and TL1 RR025016). We are gratefully indebted to the twins who take part in the University of Washington Twin Registry for their time and enthusiasm.
Footnotes
Supplementary materials related to this article can be found online at doi: 10.1016/j.physbeh.2011.09.008.
References
- 1.Barsh GS, Farooqi IS, O’Rahilly S. Genetics of body-weight regulation. Nature. 2000 Apr 6;404(6778):644–51. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- 2.Bosy-Westphal A, Wolf A, Buhrens F, Hitze B, Czech N, Monig H, et al. Familial influences and obesity-associated metabolic risk factors contribute to the variation in resting energy expenditure: the Kiel Obesity Prevention Study. Am J Clin Nutr Jun. 2008;87(6):1695–701. doi: 10.1093/ajcn/87.6.1695. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard C. Heredity and the path to overweight and obesity. Med Sci Sports Exerc Mar. 1991;23(3):285–91. [PubMed] [Google Scholar]
- 4.van den Bree MB, Eaves LJ, Dwyer JT. Genetic and environmental influences on eating patterns of twins aged >/=50 y. Am J Clin Nutr Oct. 1999;70(4):456–65. doi: 10.1093/ajcn/70.4.456. [DOI] [PubMed] [Google Scholar]
- 5.Keller KL, Pietrobelli A, Faith MS. Genetics of food intake and body composition: lessons from twin studies. Acta Diabetol Oct. 2003;40(Suppl 1):S95–S100. doi: 10.1007/s00592-003-0038-6. [DOI] [PubMed] [Google Scholar]
- 6.Pell GS, Briellmann RS, Lawrence KM, Glencross D, Wellard RM, Berkovic SF, et al. Reduced variance in monozygous twins for multiple MR parameters: implications for disease studies and the genetic basis of brain structure. Neuroimage. 2010 Jan 15;49(2):1536–44. doi: 10.1016/j.neuroimage.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspect Psychol Sci. 2010;5(5):546–56. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman CP, Polivy J. Restrained eating. In: Stunkard AJ, editor. Obesity. Philadelphia: W.B. Saunders; 1980. pp. 208–25. [Google Scholar]
- 9.Lowe MR. The effects of dieting on eating behavior: a three-factor model. Psychol Bull Jul. 1993;114(1):100–21. doi: 10.1037/0033-2909.114.1.100. [DOI] [PubMed] [Google Scholar]
- 10.Tepper BJ, Trail AC, Shaffer SE. Diet and physical activity in restrained eaters. Appetite Aug. 1996;27(1):51–64. doi: 10.1006/appe.1996.0033. [DOI] [PubMed] [Google Scholar]
- 11.Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychol Assess Mar. 2004;16(1):51–9. doi: 10.1037/1040-3590.16.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Ogden J. The psychology of eating. Malden, MA: Blackwell Publishers, Ltd; 2003. [Google Scholar]
- 13.Tuschl RJ. From dietary restraint to binge eating: some theoretical considerations. Appetite Apr. 1990;14(2):105–9. doi: 10.1016/0195-6663(90)90004-r. [DOI] [PubMed] [Google Scholar]
- 14.Herman CP, Mack D. Restrained and unrestrained eating. J Pers Dec. 1975;43(4):647–60. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 15.Heatherton TF, Herman CP, Polivy J. Effects of physical threat and ego threat on eating behavior. J Pers Soc Psychol Jan. 1991;60(1):138–43. doi: 10.1037//0022-3514.60.1.138. [DOI] [PubMed] [Google Scholar]
- 16.Polivy J, Herman CP. Distress and eating: why do dieters overeat? Int J Eat Disord Sep. 1999;26(2):153–64. doi: 10.1002/(sici)1098-108x(199909)26:2<153::aid-eat4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Polivy J. Psychological consequences of food restriction. J Am Diet Assoc Jun. 1996;96(6):589–92. doi: 10.1016/S0002-8223(96)00161-7. (quiz 93–4) [DOI] [PubMed] [Google Scholar]
- 18.Del Parigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) Mar. 2007;31(3):440–8. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 19.McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr Oct. 2009;90(4):928–34. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schur EA, Cummings DE, Callahan HS, Foster-Schubert KE. Association of cognitive restraint with ghrelin, leptin, and insulin levels in subjects who are not weight-reduced. Physiol Behav. 2008;93:706–12. doi: 10.1016/j.physbeh.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab May. 2008;7(5):400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007 Sep 7;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afari N, Noonan C, Goldberg J, Edwards K, Gadepalli K, Osterman B, et al. University of Washington Twin Registry: construction and characteristics of a community-based twin registry. Twin Res Hum Genet Dec. 2006;9(6):1023–9. doi: 10.1375/183242706779462543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polivy J, Herman CP, Howard K. The Restraint Scale: Assessment of dieting. In: Hersen M, Bellack AS, editors. Dictionary of behavioral assessment. New York: Pergamon Press; 1988. pp. 377–80. [Google Scholar]
- 25.van Strien T, Herman CP, Engels R, Larsen JK, van Leeuwe JFJ. Construct validation of the Restraint Scale in normal-weight and overweight females. Appetite Jul. 2007;49(1):109–21. doi: 10.1016/j.appet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Polivy J, Heatherton TF, Herman CP. Self-esteem, restraint, and eating behavior. J Abnorm Psychol Aug. 1988;97(3):354–6. doi: 10.1037//0021-843x.97.3.354. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson J, Persson LO, Sjostrom L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord Dec. 2000;24(12):1715–25. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- 28.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr Sep. 2004;134(9):2372–80. doi: 10.1093/jn/134.9.2372. [DOI] [PubMed] [Google Scholar]
- 29.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet Jun. 1989;35(6):423–32. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 30.Magnus P, Berg K, Nance WE. Predicting zygosity in Norwegian twin pairs born 1915–1960. Clin Genet Aug. 1983;24(2):103–12. doi: 10.1111/j.1399-0004.1983.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 31.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979;28(3):225–36. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 32.Reed T, Plassman BL, Tanner CM, Dick DM, Rinehart SA, Nichols WC. Verification of self-report of zygosity determined via DNA testing in a subset of the NAS-NRC twin registry 40 years later. Twin Res Hum Genet Aug. 2005;8(4):362–7. doi: 10.1375/1832427054936763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) Jun. 2009;33(6):653–61. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman CP, Polivy J. A boundary model for the regulation of eating. In: Stunkard AJ, Stellar E, editors. Eating and its Disorders. New York: Raven Press; 1984. pp. 141–56. [PubMed] [Google Scholar]
- 35.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005 May 19;85(1):45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci Feb. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 37.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005 Oct 20;48(2):175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci Mar. 2000;3(3):284–91. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 39.Fishbach A, Friedman RS, Kruglanski AW. Leading us not unto temptation: momentary allurements elicit overriding goal activation. J Pers Soc Psychol Feb. 2003;84(2):296–309. [PubMed] [Google Scholar]
- 40.Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite Apr. 2010;54(2):243–54. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Chechlacz M, Rotshtein P, Klamer S, Porubska K, Higgs S, Booth D, et al. Diabetes dietary management alters responses to food pictures in brain regions associated with motivation and emotion: a functional magnetic resonance imaging study. Diabetologia Mar. 2009;52(3):524–33. doi: 10.1007/s00125-008-1253-z. [DOI] [PubMed] [Google Scholar]
- 42.Davidoff J, De Bleser R. Impaired picture recognition with preserved object naming and reading. Brain Cogn Jan. 1994;24(1):1–23. doi: 10.1006/brcg.1994.1001. [DOI] [PubMed] [Google Scholar]
- 43.Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cereb Cortex Jan. 2011;21(1):95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- 44.Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA. 2009 Jan 27;106(4):1249–54. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demos KE, Kelley WM, Heatherton TF. Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. J Cogn Neurosci Aug. 2011;23(8):1952–63. doi: 10.1162/jocn.2010.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coletta M, Platek S, Mohamed FB, van Steenburgh JJ, Green D, Lowe MR. Brain activation in restrained and unrestrained eaters: an fMRI study. J Abnorm Psychol Aug. 2009;118(3):598–609. doi: 10.1037/a0016201. [DOI] [PubMed] [Google Scholar]
- 47.Goldstone AP, de Hernandez CGP, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci Oct. 2009;30(8):1625–35. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 48.Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) May. 2008;16(5):945–50. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 49.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci Apr. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 50.Schur E, Noonan C, Polivy J, Goldberg J, Buchwald D. Genetic and environmental influences on restrained eating behavior. Int J Eat Disord Dec. 2009;42(8):765–72. doi: 10.1002/eat.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Strien T, Frijters JD, Van Staveren WA, Defares PB, Deurenberg P. The predictive validity of the Dutch restrained eating scale. Int J Eat Disord. 1986;5(4):747–55. [Google Scholar]
- 52.Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 2011 Mar 1;55(1):233–9. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brennan IM, Feltrin KL, Nair NS, Hausken T, Little TJ, Gentilcore D, et al. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol Sep. 2009;297(3):G602–10. doi: 10.1152/ajpgi.00051.2009. [DOI] [PubMed] [Google Scholar]
- 54.de Castro JM, Lilenfeld LR. Influence of heredity on dietary restraint, disinhibition, and perceived hunger in humans. Nutrition Apr. 2005;21(4):446–55. doi: 10.1016/j.nut.2004.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.